Evolocumab has no effects on heart failure with reduced ejection fraction injury biomarkers: The EVO-HF trial
Abstract
Aim
Patients with heart failure with reduced ejection fraction (HFrEF) have not been shown to benefit from statins. We hypothesized that, by limiting disease progression in stable HFrEF of ischaemic etiology, the proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitor evolocumab could reduce circulating troponin levels, a surrogate biomarker of myocyte injury and atherosclerosis progression.
Methods and results
The EVO-HF multicentre prospective randomized trial compared evolocumab (420 mg/month administered subcutaneously) plus guideline-directed medical therapy (GDMT; n = 17) versus GDMT alone (n = 22) for 1 year in patients with stable coronary artery disease and left ventricular ejection fraction (LVEF) <40%, ischaemic aetiology, New York Heart Association class II, N-terminal pro-B-type natriuretic peptide (NT-proBNP) ≥400 pg/ml, high-sensitivity troponin T (hs-TnT) >10 pg/ml, low-density lipoprotein cholesterol (LDL-C) ≥70 mg/dl. The primary endpoint was change in hs-TnT concentration. Secondary endpoints included NT-proBNP, interleukin-1 receptor-like 1 (ST2), high-sensitivity C-reactive protein (hs-CRP), LDL, low-density lipoprotein receptor (LDLR), high-density lipoprotein cholesterol (HDL-C), and PCSK9 levels at 1 year. Patients were mainly Caucasian (71.8%), male (79.5%), relatively young (mean age 68.1 ± 9.4 years), with a mean LVEF of 30.4 ± 6.5%, and managed with contemporary treatments. No significant changes in hs-TnT levels were observed in any group at 1 year. NT-proBNP and ST2 levels decreased in the GDMT plus evolocumab group (p = 0.045 and p = 0.008, respectively), without changes in hs-CRP, HDL-C, or LDLR. Total and LDL-C decreased in both groups, significantly higher in the intervention group (p = 0.003), and PCSK9 levels increased in the intervention group.
Conclusions
This prospective randomized pilot trial, although with the limitation of the small sample size, does not support the benefit of evolocumab in reducing troponin levels in patients with elevated LDL-C levels, history of coronary artery disease, and stable HFrEF.
Introduction
The prevalence of heart failure (HF) keeps growing and though the four pillars for HF with reduced ejection fraction (HFrEF) have shown to reduce morbidity and mortality, it is incorrect to declare victory. One-year mortality in HFrEF remains as high as 8.8%.1
Ischaemic origin remains the main cause of HFrEF, and these patients have not been shown to benefit from statins.2 The recent 2021 ESC HF guidelines do not support the initiation of statins in patients with chronic HFrEF.2 The benefit of treatment with proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitors in HFrEF of ischaemic aetiology remains unknown. However, a biomarker sub-analysis of BIOSTAT-CHF (BIOlogy Study to TAilored Treatment in Chronic Heart Failure) revealed a positive linear association between circulating PCSK9 levels and the risk of mortality and the composite endpoint of death or HF-related hospitalization.3 Likewise, in patients with a first ST-elevation myocardial infarction with reduced ejection fraction, a recent report has shown that PCSK9 was associated with lower left ventricular ejection fraction at 6 months.4
We hypothesized that the PCSK9 inhibitor evolocumab, by limiting disease progression in stable coronary artery disease (CAD)-driven HFrEF, has the potential to reduce circulating levels of high-sensitivity troponin T (hs-TnT), a surrogate biomarker of myocyte injury and atherosclerosis progression. Accordingly, the current study aimed to assess whether reducing low-density lipoprotein cholesterol (LDL-C) adding evolocumab to current guideline-directed medical therapy (GDMT) in patients with stable HFrEF, CAD, and elevated LDL-C results in a significant reduction of hs-TnT compared to GDMT alone.
Methods
Study design
EVO-HF was a multicentre, prospective, electronically randomized, open-label pilot trial comparing evolocumab to GDMT based on a primary endpoint of changes in hs-TnT concentration at 52 weeks. Secondary endpoints included N-terminal pro-B-type natriuretic peptide (NT-proBNP), interleukin-1 receptor-like 1 (ST2), high-sensitivity C-reactive protein (hs-CRP), LDL, LDL receptor (LDLR), high-density lipoprotein cholesterol (HDL-C), and PCSK9 levels, as well as the 6-min walking test and quality of life test (Kansas City Cardiomyopathy Questionnaire [KCCQ]), at 1 year.
From May 2019 to June 2021, patients were randomized 1:1 into two groups to receive GDMT plus evolocumab (420 mg/month administered subcutaneously) or GDMT alone for 1 year with a minimum of three measurements: baseline, 6 months, and 12 months. Clinical and demographic characteristics were recorded at each visit, and a blood sample was taken for biobank storage for future biomarker analysis.
Study subjects
All included patients fulfilled all of the following inclusion criteria and none of the exclusion criteria. Inclusion criteria were signing the informed consent, age ≥18 years, LVEF <40%, ischaemic aetiology (evidence of at least one acute coronary event and CAD by coronary angiography or multislice computed tomography), New York Heart Association (NYHA) class II, NT-proBNP ≥400 pg/ml, hs-TnT >10 pg/ml, LDL-C ≥70 mg/dl, stable CAD (last acute coronary syndrome prior to the last 3 months), GDMT according to the most recent HF guidelines2 for at least the last 3 months, and statin treatment at the same dose as the patient received at the time of enrolment without the need for up-titration.2 Exclusion criteria were extracardiac disease with an estimated life expectancy of less than 1 year, contraindication to evolocumab, pregnant or lactating, age <18 or ≥81 years, liver dysfunction (aspartate aminotransferase or alanine aminotransferase >3 times the upper limit of normal), coronary revascularization in the 3 months prior to randomization or pending coronary revascularization, previous haemorrhagic stroke, or other uncontrolled conditions (hypertension, hyper/hypothyroidism, diabetes, arrhythmia).
Statistical analysis
Categorical variables were expressed as percentages and continuous variables as the mean ± standard deviation or median (interquartile range) according to normal or non-normal distribution as assessed by normal Q–Q plots. Mean ± standard deviation was used only when all of the values for one variable had a normal distribution, including changes between baseline and 1 year. Statistical differences among patients in each treatment group were compared using the paired Student's t-test or Wilcoxon test according to normal or non-normal distribution of the observed changes. Differences between treatment groups were determined by a general linear model for repeated measurements or by a comparison of the median observed changes using the Mann–Whitney U test (hs-CRP and ST2).
Results
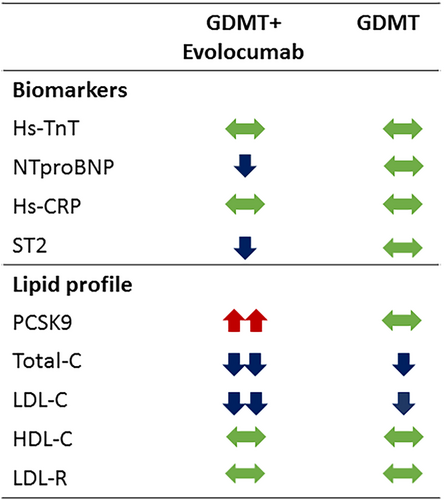
Online supplementary Figure S1 shows the flow-chart of study performance. Table 1 provides the clinical characteristics of the included patients and treatments. The patients were mainly Caucasian (71.8%), male (79.5%), relatively young (mean age 68.1 ± 9.4 years), all in NYHA functional class II, with a mean LVEF of 30.4 ± 6.5%, and managed with contemporary treatments. The study did not reach the primary endpoint. No significant changes in hs-TnT levels were measured at 1 year in either of the two groups (Table 2). Differences between changes observed in both groups were non-significant (Table 2), even after adjusting for age, sex, LVEF, and HF duration (p = 0.539).
| Total (n = 39) | GDMT plus evolocumab (n = 17) | GDMT (n = 22) | p-value | |
|---|---|---|---|---|
| Age, years | 68.1 ± 9.4 | 67.7 ± 9.7 | 68.3 ± 9.4 | 0.843 |
| Male sex | 31 (79.5) | 15 (88.2) | 16 (72.7) | 0.234 |
| Caucasian | 28 (71.8) | 13 (76.5) | 15 (68.2) | 0.568 |
| HF duration, years | 9.9 ± 8.5 | 7.5 ± 7.8 | 11.8 ± 8.7 | 0.119 |
| LVEF, % | 30.4 ± 6.5 | 31.4 ± 5.2 | 29.6 ± 7.3 | 0.383 |
| NYHA class II | 39 (100) | 17 (100) | 39 (100) | |
| AF/Fl | 12 (30.8) | 5 (29.4) | 7 (31.8) | 0.872 |
| Diabetes | 17 (43.6) | 7 (58.8) | 10 (45.5) | 0.789 |
| Peripheral vasculopathy | 3 (7.7) | 2 (11.8) | 1 (4.5) | 0.401 |
| Renal insufficiency | 13 (33.3) | 7 (41.2) | 6 (27.3) | 0.361 |
| COPD | 6 (15.4) | 2 (11.8) | 4 (18.2) | 0.582 |
| Prior STEMI | 19 (48.7) | 7 (41.2) | 12 (54.5) | 0.624 |
| Prior non-STEMI | 11 (28.2) | 7 (41.2) | 4 (18.2 | 0.240 |
| Previous revascularization | 28 (71.8) | 14 (82.4) | 14 (63.3) | 0.198 |
| Percutaneous | 19 (48.7) | 10 (58.8) | 9 (40.9) | 0.267 |
| Surgical | 9 (23.1) | 4 (23.5) | 5 (22.7) | 0.953 |
| Time since prior ACS, years | 6.1 ± 8.0 | 6.7 ± 6.7 | 9.9 ± 8.7 | 0.213 |
| Time since prior revascularization, years | 8.1 ± 8.5 | 7.7 ± 6.5 | 8.5 ± 5.8 | 0.736 |
| Treatment | ||||
| Antiplatelets | 24 (61.5) | 10 (58.8) | 14 (63.6) | 0.759 |
| Anticoagulants | 19 (48.7) | 8 (47.1) | 11 (50) | 0.306 |
| Beta-blockers | 39 (100) | 17 (100) | 22 (100) | |
| ACEI/ARB/ARNI | 39 (100) | 17 (100) | 22 (100) | |
| ARB | 36 (92.3) | 17 (100) | 19 (86.4) | 0.279 |
| SGLT2i | 7 (17.9) | 2 (11.8) | 5 (22.7) | 0.376 |
| Loop diuretics | 30 (76.9) | 15 (88.2) | 15 (68.2) | 0.141 |
| Ivabradine | 10 (25.6) | 4 (23.5) | 6 (27.3) | 0.791 |
| Digoxin | 8 (20.5) | 2 (11.8) | 6 (27.3) | 0.234 |
| MRA | 36 (92.3) | 17 (100) | 19 (86.4 | 0.279 |
| ICD | 24 (61.5) | 11 (64.7) | 13 (59.1) | 0.721 |
| CRT | 3 (7.7) | 2 (11.8) | 1 (4.5) | 1.00 |
| Statins | 38 (97.4) | 16 (94.1) | 22 (100) | 0.249 |
| Atorvastatin | 20 (51.3) | 8 (47.1) | 12 (54.5) | 0.643 |
| Rosuvastatin | 9 (23.1) | 4 (23.5) | 5 (22.7) | 0.953 |
| Simvastatin | 7 (17.9) | 3 (17.6) | 4 (18.2) | 0.966 |
| Pitavastin | 1 (2.6) | 0 | 1 (4.5) | 0.373 |
| Pravastatin | 1 (2.6) | 1 (5.9) | 0 | 0.249 |
| Ezetimibe | 14 (35.9) | 6 (35.3) | 8 (36.4) | 0.945 |
- Values are given as mean ± standard deviation, or n (%).
- ACEI, angiotensin-converting enzyme inhibitor; ACS, acute coronary syndrome; AF/Fl, atrial fibrillation/flutter; ARB, angiotensin receptor blocker; ARNI, angiotensin receptor–neprilysin inhibitor; COPD, chronic obstructive pulmonary disease; CRT, cardiac resynchronization therapy; GDMT, guideline-directed medical therapy; HF, heart failure; ICD, implantable cardioverter-defibrillator; LVEF, left ventricular ejection fraction; MRA, mineralocorticoid receptor antagonist; SGLT2i, sodium–glucose cotransporter 2 inhibitor; STEMI, ST-elevation myocardial infarction.
| Biomarker | Evolocumab plus GDMT (n = 17) | GDMT (n = 22) | p-value between groups | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Baseline | 1 year | Change | p-value | Baseline | 1 year | Change | p-value | ||
| hs-TnT | 24.4 ± 11.9 | 23.8 ± 9.9 | −0.63 ± 8.6 | 0.767 | 19.1 ± 5.9 | 20.5 ± 8.7 | 1.42 ± 7.3 | 0.372 | 0.427 |
| NT-proBNP | 2021 ± 2071 | 1140 ± 842 | −882 ± 1676 | 0.045 | 1495 ± 1113 | 1378 ± 848 | −117 ± 545 | 0.325 | 0.087 |
| hs-CRP | 4 (1.15–6.4) | 1.6 (1.05–3.75) | −0.3 (−3.55 to 0.15) | 0.114 | 2.95 (2.4–6.35) | 2.5 (1.2–6.2) | −0.7 (−3.75 to 0.2.35) | 0.476 | 0.705 |
| ST2 | 26 (16.5–32.5) | 20 (11–27) | −3 (−7.5 to 0) | 0.008 | 20.5 (14.75–27.25) | 18.5 (14–23.25) | 1 (−3 to 2.25) | 0.93 | 0.013 |
| PCSK9 | 1407 ± 838 | 3715 ± 1171 | 2307 ± 1366 | <0.001 | 1035 ± 465 | 1146 ± 667 | 111.6 ± 479 | 0.287 | <0.001 |
| Total-C | 160.1 ± 32.3 | 110.5 ± 32.9 | −51.4 ± 42.5 | <0.001 | 169.6 ± 34.9 | 154.5 ± 23.7 | −15.1 ± 26.9 | 0.015 | 0.003 |
| LDL-C | 90.3 ± 22.5 | 45.1 ± 30.7 | −46.1 ± 35 | <0.001 | 94.9 ± 25.5 | 79.6 ± 15.2 | −15.3 ± 23.7 | 0.006 | 0.003 |
| HDL-C | 45.6 ± 14.1 | 44.5 ± 9.7 | −0.98 ± 9.4 | 0.683 | 47.1 ± 10.9 | 49 ± 15.6 | 1.86 ± 9.1 | 0.348 | 0.358 |
| LDLR | 39.4 ± 13.7 | 48.9 ± 24.1 | 7.5 ± 23.1 | 0.201 | 39.8 ± 18.7 | 40.9 ± 16.5 | 1.04 ± 12.6 | 0.704 | 0.274 |
- Values are given as mean ± standard deviation, or median (interquartile range).
- C, cholesterol; GDMT, guideline-directed medical therapy; HDL-C, high-density lipoprotein cholesterol; hs-CRP, high-sensitivity C-reactive protein; hs-TnT, high-sensitivity troponin T; LDL-C, low-density lipoprotein cholesterol; LDLR, low-density lipoprotein receptor; NT-proBNP, N-terminal pro-B-type natriuretic peptide; PCSK9, proprotein convertase subtilisin/kexin type 9; ST2, interleukin-1 receptor like-1.
Two patients (one of each group) were admitted due to acute pulmonary oedema or acute dyspnoea and one patient was treated with subcutaneous furosemide in the outpatient clinic due to decompensation (intervention group).
The NT-proBNP levels decreased only in the evolocumab plus GDMT group (p = 0.045), but differences between treatment groups were non-significant. ST2 levels decreased significantly in the evolocumab plus GDMT group (p = 0.008) and not in the GDMT alone group, and the difference between groups was significant (p = 0.013). There were no significant changes in hs-CRP, HDL-C, or LDLR in either of the two groups. In contrast, changes in total cholesterol and LDL-C were significantly different between groups, with higher decrease in the GDMT plus evolocumab group (p = 0.003). Notably, PCSK9 blood levels increased only in the intervention group (Table 2, Figure 1).
No significant differences were measured in the distance walked in the 6-min walking test (GDMT plus evolocumab: 376 ± 104 m at baseline to 371 ± 120 m at 1 year, p = 0.790; GDMT alone: from 357 ± 89 m to 367 ± 104 m, p = 0.4; p = 0.489 between groups).
There were also no significant changes in the scoring on the KCCQ, although a non-significant trend for better evolution on quality of life perception in the intervention group was observed (GDMT plus evolocumab: 70.6 ± 24.2 at baseline and 74.9 ± 18.7 at 1 year, p = 0.376; GDMT alone: 84.3 ± 13.6 at baseline and 78.7 ± 18.1 at 1 year, p = 0.075; p = 0.071 between groups).
Discussion
This prospective randomized pilot study examined the potential of evolocumab to reduce hs-TnT levels in stable HFrEF patients with underlying CAD. We hypothesized that limiting atherosclerosis progression with strict LDL-C lowering in HFrEF could lead to lower hs-TnT levels, assuming that at least a portion of this troponin elevation was due to microinjury caused by the progression of small vessel atherosclerosis. Our results do not support the primary endpoint, suggesting that circulating troponin levels in HFrEF are more likely caused by leakage/apoptosis of stressed myocytes and not from infarctlets caused by progression of atherosclerotic microvascular disease.
This study was conducted before considering a clinical endpoint randomized clinical trial with PCSK9 inhibitors in HFrEF of ischaemic origin. However, recently, a post-hoc analysis of the ODYSSEY OUTCOMES (Evaluation of Cardiovascular Outcomes After an Acute Coronary Syndrome During Treatment With Alirocumab) trial showed that patients with a history of HF do not benefit from alirocumab after acute coronary syndrome.5 Therefore, it is unlikely that PCSK9 inhibitors will enter the therapeutic armamentarium of patients with HF.
In BIOSTAT-CHF, which included 2174 patients with worsening HF, multivariable analysis revealed a positive linear association between PCSK9 levels and the risk of mortality, as well as with the composite of mortality and unplanned HF hospitalization.3 This led to the hypothesis that higher PCSK9 levels contribute to worsening HF. Here, we found that PCSK9 inhibition significantly increased circulating levels of PCSK9, likely due to evolocumab-driven upregulation of the PCSK9 gene. The differences observed in the other secondary endpoint biomarkers were not relevant enough in such small groups to deserve further discussion, yet additional pro-inflammatory biomarkers or markers of cell damage (i.e., Klotho) could yield novel insight.
In conclusion, this prospective randomized pilot trial, although with the limitation of the small sample size, does not support the benefit of evolocumab in reducing troponin levels in patients with elevated LDL-C levels, history of CAD, and stable HFrEF. These data, together with the post-hoc analysis of the ODYSSEY OUTCOMES trial, may shut the door for PCSK9 inhibitors in HF.
Conflict of interest: A.B.G. reports participation in advisory boards or lectures for Abbott, AstraZeneca, Boehringer Ingelheim, Novartis, Roche Diagnostics, Vifor. All other authors have nothing to disclose.




