Effects of dapagliflozin on heart failure hospitalizations according to severity of inpatient course: Insights from DELIVER and DAPA-HF
Abstract
Aims
Dapagliflozin resulted in significant and sustained reductions in first and recurrent heart failure (HF) hospitalizations among patients with HF across the spectrum of ejection fraction. How treatment with dapagliflozin differentially impacts hospitalization for HF of varying complexity is not well studied.
Methods and results
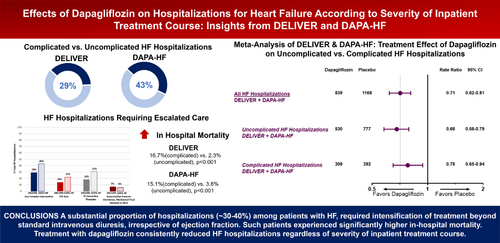
In the DELIVER and DAPA-HF trials, we examined the effects of dapagliflozin on adjudicated HF hospitalizations of varying complexity and hospital length of stay (LOS). HF hospitalizations requiring intensive care unit stay, intravenous vasoactive therapies, invasive/non-invasive ventilation, mechanical fluid removal or mechanical circulatory support were categorized as complicated. The balance was classified as uncomplicated. Of the total 1209 HF hospitalizations reported in DELIVER, 854 (71%) were uncomplicated and 355 (29%) were complicated. Of the total 799 HF hospitalizations reported in DAPA-HF, 453 (57%) were uncomplicated and 346 (43%) were complicated. Relative to patients experiencing a first uncomplicated HF hospitalization, those with complicated HF hospitalizations had a significantly higher in-hospital mortality both in DELIVER (16.7% vs. 2.3%, p < 0.001) and DAPA-HF (15.1% vs. 3.8%, p < 0.001). Dapagliflozin similarly reduced total ‘uncomplicated’ (DELIVER: rate ratio [RR] 0.67, 95% confidence interval [CI] 0.55–0.82 and DAPA-HF: RR 0.69, 95% CI 0.54–0.87) and ‘complicated’ HF hospitalizations (DELIVER: RR 0.82, 95% CI 0.63–1.06 and DAPA-HF: RR 0.75, 95% CI 0.58–0.97). Dapagliflozin consistently reduced hospitalizations irrespective of their LOS: <5 days (DELIVER: RR 0.76, 95% CI 0.58–0.99 and DAPA-HF: RR 0.58, 95% CI 0.42–0.80) or ≥5 days (DELIVER: RR 0.71, 95% CI 0.58–0.86 and DAPA-HF: RR 0.77, 95% CI 0.62–0.94).
Conclusion
A substantial proportion of hospitalizations (∼30–40%) among patients with HF irrespective of ejection fraction required intensification of treatment beyond standard intravenous diuretics. Such patients experienced significantly higher in-hospital mortality. Treatment with dapagliflozin consistently reduced HF hospitalizations regardless of severity of inpatient course or LOS.
Clinical Trial Registration: ClinicalTrials.gov, DELIVER (NCT03619213) and DAPA-HF (NCT03036124).
Graphical Abstract
Background
Hospitalizations for heart failure (HF) are associated with poor prognosis and significant healthcare costs. Efforts to prevent HF hospitalization are a top priority for physicians and payers alike and these events represent an important clinical trial endpoint and quality measure.
Hospitalizations for HF are recognized as a sentinel event in the natural history of disease with mortality rates that are up to three-fold higher in patients experiencing hospitalization compared to those that do not.1, 2 However HF hospitalizations may vary widely with regard to their complexity, length of stay (LOS), and post-discharge trajectory.3 Patients with HF carry a high burden of comorbidities4 including chronic kidney disease and underlying lung disease which enhance the risk of complications with standard therapies and increase the risk for intensive care unit (ICU) needs as well as invasive or non-invasive ventilation. While clinical trials have typically focused on any HF hospitalizations, how chronic HF treatment differentially impacts hospitalizations of varying severity is not as well studied.
Treatment with dapagliflozin resulted in significant reductions in first and recurrent HF hospitalizations in patients with HF across the left ventricular ejection fraction (LVEF) spectrum.5, 6 In this post hoc analysis of the DELIVER and DAPA-HF trials, we further describe the frequency and outcomes of HF hospitalizations requiring management beyond standard intravenous (IV) diuretics and examine the effects of dapagliflozin on HF hospitalizations of varying severity of inpatient treatment course and LOS across the spectrum of ejection fraction.
Methods
Study design
The study designs and primary results of the DELIVER and DAPA-HF trials have previously been reported.5, 6 Both trials were double-blind, randomized controlled trials which assessed the effect of dapagliflozin 10 mg once daily relative to placebo. DELIVER enrolled ambulatory or hospitalized patients aged >40 years with symptomatic HF, LVEF >40%, evidence of structural heart disease, at least intermittent diuretic requirement, and elevated natriuretic peptides. DAPA-HF included patients aged ≥18 years with symptomatic HF, LVEF ≤40%, and elevated natriuretic peptides. The study protocols received ethics committee approval at each study site and participants provided written informed consent.
Statistical analysis
We evaluated total (first and recurrent) HF hospitalizations that were adjudicated by a clinical endpoints committee. ‘Complicated hospitalizations’ were defined, according to investigator reported data as those requiring intensive therapy including ICU stay, IV vasoactive therapies (vasopressor, inotrope or vasodilator), invasive or non-invasive ventilation, mechanical fluid removal, ultrafiltration or mechanical circulatory support (MCS), where data were available. Six HF hospitalizations in DELIVER and 10 HF hospitalizations in DAPA-HF with insufficient data on complexity parameters were excluded. HF hospitalizations not requiring these in-hospital resources were classified as ‘uncomplicated’. Where data on LOS were available, HF hospitalizations were further categorized according to their LOS: longer (≥5 days) or shorter (≥5 days). One thousand one hundred four of the total 1209 (91%) HF hospitalizations in DELIVER and all 798 (100%) of the included HF hospitalizations in DAPA-HF had a known LOS. We evaluated the influence of dapagliflozin relative to placebo on the occurrence of adjudicated HF hospitalizations of varying complexity and LOS. Treatment effects on total HF hospitalizations were analysed using semiparametric proportional rates methods of Lin et al.7 We also conducted a trial-level meta-analysis of treatment effects by HF hospitalization complexity status across DELIVER and DAPA-HF.
For descriptive purposes, baseline characteristics and presenting clinical symptoms and signs of worsening HF of patients experiencing a first HF hospitalization were compared according to HF hospitalization complexity status. Data are reported as mean ± standard deviation, median (interquartile range) for skewed distributions, and frequency (percentage) for categorical variables. Student's t-tests and chi-square tests were used where appropriate.
Since the decision to admit and hospital care practices may vary significantly worldwide, the breakdown of complicated hospitalizations requiring various interventions were calculated as a percentage of total hospitalizations by geographic region. Among the subset of patients with available discharge data, in-hospital mortality was further estimated for first complicated and uncomplicated HF hospitalizations and compared using Pearson chi square tests. Post-discharge mortality was assessed in a time-to-first events analysis using Cox proportional hazards models. Interaction testing was carried out to evaluate for any region-specific differences in associations between complexity of HF hospitalization and outcomes.
All analyses were conducted using STATA 17 (Stata Corp., College Station, TX, USA). A p-value of <0.05 was considered statistically significant.
Results
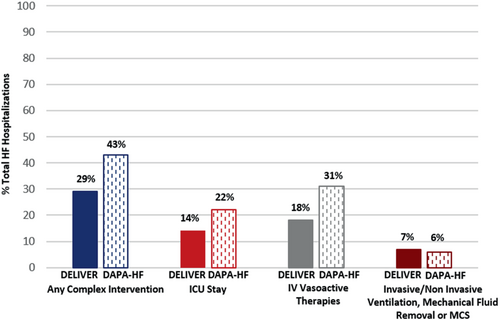
Baseline characteristics
In DELIVER, over a median follow-up of 2.3 years, 6263 participants experienced a total (first and recurrent) of 1209 HF hospitalizations of which 854 (71%) were uncomplicated and 355 (29%) were complicated in that they required at least one intensive intervention. Overall, 14% required an ICU stay, 18% required IV vasoactive medications (vasopressor, inotrope or vasodilator), and 7% required invasive or non-invasive ventilation, mechanical fluid removal or MCS (Figure 1). In DAPA-HF, over a median follow-up time of 1.5 years, 4744 participants experienced a total of 799 HF hospitalizations of which 453 (57%) were uncomplicated and 346 (43%) were complicated. Overall, 22% required an ICU stay, 31% required IV vasoactive medications (vasopressor, inotrope or vasodilator), and 6% required invasive or non-invasive ventilation, mechanical fluid removal or MCS (Figure 1). For descriptive purposes, baseline characteristics (online supplementary Table S1) and clinical symptoms and signs of worsening HF (online supplementary Table S2) are described based on first uncomplicated or complicated HF hospitalization. The relative proportion of uncomplicated versus complicated HF hospitalizations was similar when considering first and total (first and recurrent) HF hospitalizations. In DELIVER, those with a complicated first HF hospitalization were younger, had a slightly lower baseline LVEF, and had lower use of baseline loop diuretics compared with those with uncomplicated HF hospitalizations. In DAPA-HF, participants experiencing first uncomplicated HF hospitalization had a generally lower burden of comorbidities and were less often on renin–angiotensin system inhibitor or angiotensin receptor–neprilysin inhibitor at baseline. In both trials, patients who experienced a complicated HF hospitalization more frequently experienced signs and symptoms consistent with a low output state and high filling pressures. Of the 1104 hospitalizations in DELIVER with available data on LOS, 29% had a LOS <5 days and 71% had a LOS ≥5 days. Of the 798 hospitalizations in DAPA-HF with available data on LOS, 26% had a LOS <5 days and 74% had a LOS ≥5 days.
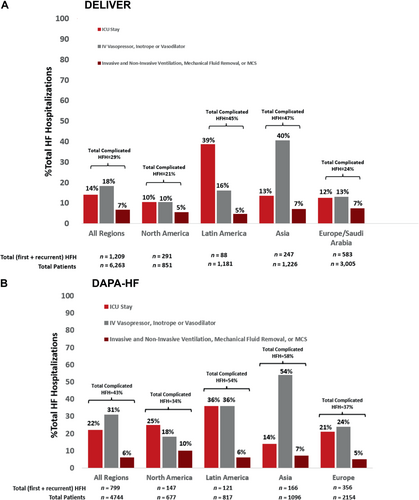
Heart failure hospitalization complexity by geographic region
The geographic distribution of complicated HF hospitalizations was similar for both trials and is displayed in Figure 2. In both trials, complicated HF hospitalizations occurred most frequently in Latin America and Asia, and least frequently in North America and Europe or Saudi Arabia. Complicated hospitalizations requiring ICU stay were most common in Latin America and those requiring IV vasoactive medications were most frequent in Asia.
In-hospital and post-discharge mortality
Among patients enrolled in DELIVER, in-hospital mortality among patients experiencing a first HF hospitalization was highest in Latin America (13.4%) followed by Europe or Saudi Arabia (6.4%), Asia (4.7%), and North America (3.6%) (p = 0.04). In DAPA-HF, although in-hospital mortality was numerically higher in Latin America (11.0%) followed by North America (10.5%), Europe (8.9%), and Asia (3.5%), these regional differences were not statistically significant (p = 0.19). Among patients experiencing a first HF hospitalization, those with a complicated hospitalization experienced significantly higher rates of in-hospital mortality compared with those with uncomplicated hospitalizations in both DELIVER (16.7% vs. 2.3%, p < 0.001) and DAPA-HF (15.1% vs. 3.8%, p < 0.001). Post-discharge mortality was similar among patients surviving first complicated HF hospitalization versus uncomplicated HF hospitalization in DELIVER (hazard ratio [HR] 0.91, 95% confidence interval [CI] 0.64–1.29; p = 0.59), and DAPA-HF (HR 1.14, 95% CI 0.84–1.53; p = 0.40). In DELIVER, interaction testing did not identify any region-specific differences in these associations between HF hospitalization complexity and either in-hospital mortality (pinteraction = 0.40) or post-discharge mortality (pinteraction = 0.21) (online supplementary Table S3). In DAPA-HF, while there were no region specific differences with respect to post-discharge mortality (Pinteraction = 0.20) there was a borderline-significant interaction between HF hospitalization complexity and region for in-hospital mortality (pinteraction = 0.045). In DAPA-HF, hospitalizations requiring more intensive in-hospital interventions were less closely associated with in-hospital mortality in Asia compared with other regions (online supplementary Table S3).
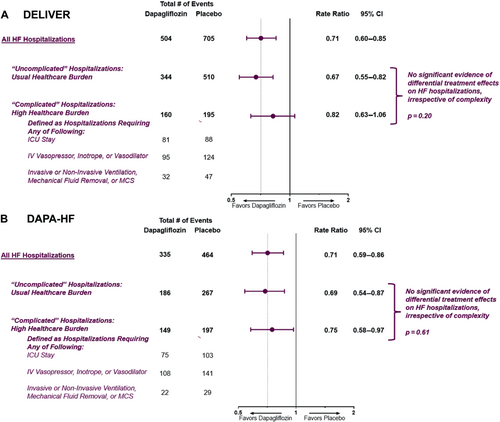
Treatment effect of dapagliflozin on total heart failure hospitalizations according to severity of inpatient course and length of stay
Relative to placebo, treatment with dapagliflozin consistently reduced total uncomplicated and complicated HF hospitalizations in both DELIVER (uncomplicated: RR 0.67, 95% CI 0.55–0.82 and complicated: RR 0.82, 95% CI 0.63–1.06; p for heterogeneity = 0.20) and DAPA-HF (uncomplicated: RR 0.69, 95% CI 0.54–0.87 and complicated: RR 0.75, 95% CI 0.58–0.97; p for heterogeneity = 0.61) (Figure 3). Reductions in complicated HF hospitalizations were driven by lower requirement of ICU stays (DELIVER: 81 vs. 88 and DAPA-HF: 75 vs. 103), IV vasoactive therapies (DELIVER: 95 vs. 124 and DAPA-HF: 108 vs. 141), and invasive or non-invasive ventilation, mechanical fluid removal or MCS (DELIVER: 32 vs. 47 and DAPA-HF 22 vs. 29) with dapagliflozin compared with placebo. Similar results were found when the definition of complicated HF hospitalization excluded the requirement for ICU admission which may be a subjective decision in some regions (online supplementary Table S4).
In a trial level meta-analysis of DELIVER and DAPA-HF, dapagliflozin significantly reduced total uncomplicated (RR 0.68, 95% CI 0.58–0.79) and complicated (RR 0.78, 95% CI 0.65–0.94) HF hospitalizations across the spectrum of ejection fraction (Graphical Abstract).
Among HF hospitalizations with known LOS information, the median LOS was 7 (4–12) days in the placebo arm compared with 7 (4–11) days in the dapagliflozin arm in DELIVER and 7 (4–14) days in the placebo arm compared with 8 (5–14) days in the dapagliflozin arm in DAPA-HF.
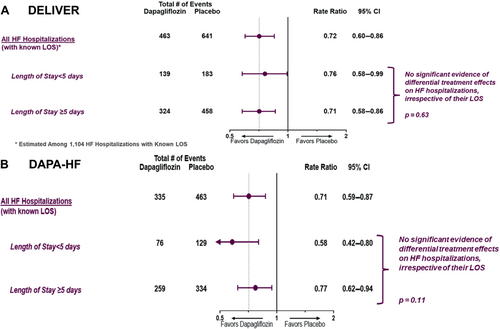
The total number of days spent in the hospital for HF in trial follow-up was significantly lower in the dapagliflozin-treated group compared with placebo in both DELIVER (4087 vs. 6379 days; incidence rate ratio [RR] 0.64, 95% CI 0.49–0.83; p = 0.001) and DAPA-HF (3899 vs. 5048 days; incidence RR 0.76, 95% CI 0.60–0.98; p = 0.034). Dapagliflozin reduced total HF hospitalizations irrespective of their hospital LOS in both DELIVER (LOS <5 days [RR 0.76; 95% CI 0.58–0.99] or LOS ≥5 days [RR 0.71; 95% CI 0.58–0.86]; p for heterogeneity = 0.63) and in DAPA-HF (LOS <5 days [RR 0.58; 95% CI 0.42–0.80] or LOS ≥5 days [RR 0.77; 95% CI 0.62–0.94]; p for heterogeneity = 0.11) (Figure 4).
Discussion
In this post hoc analysis, ∼30% of hospitalizations among patients with HF with mildly reduced or preserved ejection fraction in DELIVER and over 40% of hospitalizations among patients with HF with reduced ejection fraction (HFrEF) in DAPA-HF required escalation of treatment beyond decongestion with IV diuretic. Patients experiencing complicated HF hospitalizations had substantially higher in-hospital mortality irrespective of ejection fraction. Treatment with dapagliflozin consistently reduced HF hospitalizations irrespective of severity of in-hospital course or LOS.
In current clinical practice, patients with HF are generally older with a higher burden of cardiovascular and non-cardiovascular comorbidities which may impact the severity of in-hospital course when decompensated.8 While more intensive use of in-hospital therapies are typically associated with the care of patients with HFrEF, we found a substantial number of hospitalized patients with higher LVEF similarly required therapies beyond standard IV diuretics. In fact, the proportions of ‘complex’ HF hospitalizations in DELIVER mirror proportions needing advanced therapies in patients with HFrEF in the real world.9
Complicated hospitalizations were associated with markedly higher rates of in-hospital mortality irrespective of ejection fraction. No significant differences in the burden of most comorbidities were detected in patients with HF with mildly reduced or preserved ejection fraction and the burden of comorbidities was generally lower in patients with HFrEF with complicated versus uncomplicated HF hospitalizations. Baseline haemodynamic profiles were also similar between patients with and without complicated HF hospitalization in both trials. In DAPA-HF, patients experiencing complicated HF hospitalizations were less well optimized on background HF therapy which may explain a propensity to decompensation and need for higher level of care in patients with HFrEF. Baseline use of loop diuretic was lower in patients with complicated HF hospitalizations in DELIVER; however, it is not clear whether this reflects a lower requirement for decongestive therapy or under optimization of volume status. As such, variability in presenting severity and treatment course may reflect other intrinsic biologic or social differences that impact tolerance of decompensation and requirement for more intensive interventions.10
Treatment patterns among complicated hospitalizations were subject to substantial geographic variation particularly with regard to the need for ICU care and receipt of IV vasoactive medications. This may reflect varying standards of care for HF patients globally, including with respect to thresholds for admission and need for escalation in care. In both trials, regions with the highest proportion of complicated HF hospitalizations requiring ICU stay also had the highest in-hospital mortality, suggesting that geographic variations in this element of in-hospital care may be reflective of greater disease severity on presentation. However, it is noteworthy that in DAPA-HF, more advanced in-hospital interventions in Asia (which were most commonly IV vasoactive therapies) was less associated with in-hospital mortality than in other regions.
In both trials, dapagliflozin demonstrated consistent treatment benefits on preventing HF hospitalizations irrespective of the complexity of inpatient course or hospital LOS. These data are in line with observations from EMPEROR-Preserved and EMPEROR-Reduced which similarly demonstrate the clinical benefits of sodium–glucose cotransporter 2 (SGLT2) inhibitors in preventing inpatient worsening HF events across the spectrum of severity.11, 12 Prevention of HF hospitalizations, including those that require greater in-hospital interventions, with SGLT2 inhibitors may add to the overall value of their use across the spectrum of ejection fraction.
The following limitations should be noted. As this was not a pre-specified analysis, the findings are hypothesis generating. While the definition used to identify complicated and uncomplicated HF hospitalizations has not been prospectively validated, it is based on routinely used markers of clinical severity which have been used in other analyses.9 The criteria used to categorize hospitalizations as complicated and uncomplicated were not formally adjudicated. Components of these criteria including the decision to admit to ICU may be subject to practice variation as opposed to true physiologic requirement. Some hospitalizations lacked clear discharge dates, thus precluding calculation of LOS. LOS is also subject to geographic variation and may reflect factors unrelated to severity of hospital course. No data were available regarding the costs of hospitalizations.
In two contemporary trials, a substantial proportion of hospitalizations (∼30–40%) among patients with HF, irrespective of ejection fraction, required intensification of treatment beyond standard IV diuresis. Such patients experienced significantly higher in-hospital mortality. Treatment with dapagliflozin consistently reduced HF hospitalizations regardless of severity of inpatient course or LOS.
Funding
The DELIVER and DAPA-HF trials were funded by AstraZeneca.
Conflict of interest: S.C. is supported by the Canadian Child's Scholarship from the Libin Institute of Alberta/Cumming School of Medicine. T.K. has received lecture fees from Abbott Medical Japan LLC., Ono Pharmaceutical Co., Ltd., Otsuka Pharmaceutical Co., Ltd., Novartis Pharma K.K., AstraZeneca K.K., Bristol-Myers Squibb Co., and Abiomed Japan K.K. B.L.C. has received consulting fees from Amgen, Cardurion, Corvia, and Novartis. A.S.D. has received research grant support from Abbott, AstraZeneca, Alnylam, Bayer, and Novartis; and has received consulting fees and/or honoraria from Abbott, AstraZeneca, Alnylam, Avidity, Axon Therapeutics, Bayer, Boston Scientific, Biofourmis, Cytokinetics, GlaxoSmithKline, Merck, Novartis, Parxel, Regeneron, Roche, and Verily. P.S.J. reports speakers' fees from AstraZeneca, Novartis, Alkem Metabolics, ProAdWise Communications, Sun Pharmaceuticals; advisory board fees from AstraZeneca, Boehringer Ingelheim, Novartis; research funding from AstraZeneca, Boehringer Ingelheim, Analog Devices Inc, Roche Diagnostics. P.S.J.'s employer the University of Glasgow has been remunerated for clinical trial work from AstraZeneca, Bayer AG, Novartis and Novo Nordisk. Director, Global Clinical Trial Partners (GCTP). R.A.d.B. has received research grants and fees from AstraZeneca, Abbott, Boehringer Ingelheim, Cardio Pharmaceuticals Gmbh, Ionis Pharmaceuticals, Inc, Novo Nordisk, and Roche, outside the submitted work; and has received speaker fees from Abbott, AstraZeneca, Bayer, Novartis, and Roche, outside the submitted work. A.F.H. has received research grant support from American Regent, Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, Merck, Novartis, Somologic, and Verily; and has served as a consultant or on the advisory board for Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, Boston Scientific, Bristol Myers Squibb, Cytokinetics, Eidos, Intercept, Merck, and Novartis. S.E.I. has served on clinical trial committees or as a consultant to AstraZeneca, Boehringer Ingelheim, Novo Nordisk, Merck, Pfizer; and has given lectures sponsored by AstraZeneca and Boehringer Ingelheim. M.N.K. has received research grant support from AstraZeneca, and Boehringer Ingelheim; has served as a consultant or on an advisory board for Alnylam, Amgen, Applied Therapeutics, AstraZeneca, Bayer, Boehringer Ingelheim, Eli Lilly, Esperion Therapeutics, Janssen, Merck (Diabetes and Cardiovascular), Novo Nordisk, Sanofi, Pharmacosmos, and Vifor Pharma; has received other research support from AstraZeneca; and has received honorarium from AstraZeneca, Boehringer Ingelheim, and Novo Nordisk. C.S.P.L. is supported by a Clinician Scientist Award from the National Medical Research Council of Singapore; has received research support from AstraZeneca, Bayer, and Roche Diagnostics, has served as a consultant or on the advisory board/steering committee/executive committee for Actelion, Alleviant Medical, Allysta Pharma, Amgen, AnaCardio AB, Applied Therapeutics, AstraZeneca, Bayer, Boehringer Ingelheim, Boston Scientific, Cytokinetics, Darma Inc, EchoNous Inc, Eli Lilly, Impulse Dynamics, Ionis Pharmaceutical, Us2.ai, Janssen Research & Development LLC, Medscape, Merck, Novartis, Novo Nordisk, Prosciento Inc, Radcliffe Group Ltd, Roche Diagnostics, Sanofi, Siemens Healthcare Diagnostics and WebMD Global LLC; and serves as the cofounder and non-executive director of Us2.ai. F.A.M. has received consultation fees and research grants from AstraZeneca, Baliarda, Bayer, Boehringer Ingelheim, Bristol-Myers Squibb, Gador, Milestone, Novartis, Pfizer, and St Lukes University. S.J.S. has received research grants from the National Institutes of Health (U54 HL160273, R01 HL107577, R01 HL127028, R01 HL140731, R01 HL149423), Actelion, AstraZeneca, Corvia, Novartis, and Pfizer, and has received consulting fees from and consulting fees from Abbott, Actelion, AstraZeneca, Amgen, Aria CV, Axon Therapies, Bayer, Boehringer Ingelheim, Boston Scientific, Bristol-Myers Squibb, Cardiora, Coridea, CVRx, Cyclerion, Cytokinetics, Edwards Lifesciences, Eidos, Eisai, Imara, Impulse Dynamics, GSK, Intellia, Ionis, Ironwood, Lilly, Merck, MyoKardia, Novartis, Novo Nordisk, Pfizer, Prothena, Regeneron, Rivus, Sanofi, Sardocor, Shifamed, Tenax, Tenaya, and United Therapeutics. M.P. is an employee and shareholder of AstraZeneca. A.M.L. is an employee and shareholder of AstraZeneca. J.J.V.M. has received payments through Glasgow University for work on clinical trials, consulting and other activities from Alnylam, Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, BMS, Cardurion, Cytokinetics, Dal-Cor, GSK, Ionis, KBP Biosciences, Novartis, Pfizer, Theracos; personal lecture fees: the Corpus, Abbott, Hikma, Sun Pharmaceuticals, Medscape/Heart.Org, Radcliffe Cardiology, Servier Director, Global Clinical Trial Partners (GCTP). S.D.S. has received research grants from Actelion, Alnylam, Amgen, AstraZeneca, Bellerophon, Bayer, BMS, Celladon, Cytokinetics, Eidos, Gilead, GSK, Ionis, Lilly, Mesoblast, MyoKardia, NIH/NHLBI, Neurotronik, Novartis, NovoNordisk, Respicardia, Sanofi Pasteur, Theracos, US2.AI, and has consulted for Abbott, Action, Akros, Alnylam, Amgen, Arena, AstraZeneca, Bayer, Boehringer Ingelheim, BMS, Cardior, Cardurion, Corvia, Cytokinetics, Daiichi-Sankyo, GSK, Lilly, Merck, Myokardia, Novartis, Roche, Theracos, Quantum Genomics, Cardurion, Janssen, Cardiac Dimensions, Tenaya, Sanofi-Pasteur, Dinaqor, Tremeau, CellProThera, Moderna, American Regent, Sarepta, Lexicon, Anacardio, Akros, Puretech Health. M.V. has received research grant support, served on advisory boards, or had speaker engagements with American Regent, Amgen, AstraZeneca, Bayer AG, Baxter Healthcare, Boehringer Ingelheim, Chiesi, Cytokinetics, Lexicon Pharmaceuticals, Merck, Novartis, Novo Nordisk, Pharmacosmos, Relypsa, Roche Diagnostics, Sanofi, and Tricog Health, and participates on clinical trial committees for studies sponsored by AstraZeneca, Galmed, Novartis, Bayer AG, Occlutech, and Impulse Dynamics. All other authors have nothing to disclose.