European Society of Cardiology quality indicators for the care and outcomes of adults with pulmonary arterial hypertension. Developed in collaboration with the Heart Failure Association of the European Society of Cardiology
Abstract
Aims
To develop a suite of quality indicators (QIs) for the evaluation of the care and outcomes for adults with pulmonary arterial hypertension (PAH).
Methods and results
We followed the European Society of Cardiology (ESC) methodology for the development of QIs. This included (i) the identification of key domains of care for the management of PAH, (ii) the proposal of candidate QIs following systematic review of the literature, and (iii) the selection of a set of QIs using a modified Delphi method. The process was undertaken in parallel with the writing of the 2022 ESC/European Respiratory Society (ERS) guidelines for the diagnosis and treatment of pulmonary hypertension and involved the Task Force chairs, experts in PAH, Heart Failure Association (HFA) members and patient representatives. We identified five domains of care for patients with PAH: structural framework, diagnosis and risk stratification, initial treatment, follow-up, and outcomes. In total, 23 main and one secondary QIs for PAH were selected.
Conclusion
This document presents the ESC QIs for PAH, describes their development process and offers scientific rationale for their selection. The indicators may be used to quantify and improve adherence to guideline-recommended clinical practice and improve patient outcomes.
Introduction
Pulmonary arterial hypertension (PAH) is a relatively rare condition, but confers a considerable burden on healthcare systems worldwide.1 Mortality for patients with PAH remains high with rates reaching over 20% at 3 years.2 Professional societies including the European Society of Cardiology (ESC) and the European Respiratory Society (ERS) have developed comprehensive guidelines outlining the evidence-base for PAH.3, 4 Whilst these guidelines play a major role in translating knowledge into recommendations that guide clinical practice, the implementation of guideline-recommended therapies for PAH remains suboptimal.5
Quality indicators (QIs) help healthcare professionals benchmark clinical practice against standardized measures in order to identify areas for improvement.6 The application of such measures has been shown to influence quality of care and subsequent outcomes.7, 8 For instance, the initiation of a combination therapy for PAH in treatment-naïve patients is known to significantly reduce clinical failure events,9 but data from clinical registries continue to show a room for improvement and disparities in the adherence to combination therapy in eligible patients.2, 5 Besides, the growing awareness of PAH and the establishment of specialized centres have led to an increased recognition of the condition and an improvement in its outcomes.3
As such, there is a need to establish a framework to help standardize the structure and processes of care for PAH and unify the method by which PAH outcomes are captured to offset inequalities and improve patient experience. In parallel with the development of the 2022 ESC/ERS guidelines for the diagnosis and treatment of pulmonary hypertension,3 we convened a group comprising the Task Force chairs, members of the Heart Failure Association (HFA), international experts in PAH and patient representatives to develop QIs for the management of adults with PAH.
Methods
We used the ESC methodology for the development of QIs for the quantification of cardiovascular care and outcomes.10 This methodology comprises (i) the identification of the key domains of care, (ii) the development of candidate QIs by conducting a systematic review of the literature, and (iii) the selection of the final set of QIs using a modified Delphi method.10
According to the Donabedian quality of care assessment model, QIs may capture a structural, process or outcome aspect of care,11 where structural and process indicators evaluate quality at the institutional and the individual patient level, respectively. Outcome QIs define important outcome measures relevant to PAH and specify the methods for their collection, analysis and reporting.10 In addition, the ESC QIs are further classified into main and secondary indicators. Main QIs may be used for performance measurement across regions and over time, whilst secondary QIs may be used for local quality improvement activities.10
Members of the development group
The development group was established to select the 2022 ESC QIs for PAH using a structured methodology.10 The group comprised members of the Task Force of the 2022 ESC/ERS guidelines for the diagnosis and treatment of pulmonary hypertension,3 nominees from the HFA, representatives from the ESC QI Committee, patients and selected international experts with track records in registries and quality improvement projects in PAH.5, 12 In total, the development group comprised 30 experts with a diverse range of clinical and academic backgrounds, including clinical, interventional and imaging cardiologists, as well as respiratory physicians, a specialist nurse, intensivists, researchers, and patients.
Target population
The first stage of the development process involved identifying the target population which is the group of patients for whom the QIs are used.10 This was defined as patients with suspected or confirmed Group 1 pulmonary hypertension according to the 2022 ESC/ERS guidelines for the diagnosis and treatment of pulmonary hypertension (i.e. PAH).3 A consensus was reached amongst the development group members to focus the scope of the current initiative on the diagnosis and management of the various subgroups of PAH, without extending to other forms of pulmonary hypertension (e.g. chronic thromboembolic pulmonary hypertension [CTEPH]) because their management is group-specific. Separate initiatives may be needed for each group of patients with pulmonary hypertension, such as the recently published QIs for heart failure which may be applicable to Group 2 pulmonary hypertension.13
Domains of pulmonary arterial hypertension care
Following the identification of the target population, the development group selected key domains of care for the management of patients with PAH by conceptually illustrating the steps involved in diagnosing, risk stratifying and treating patients with PAH in various clinical settings.10 This aimed to comprehend the continuum of PAH management including infrastructural aspects in specialized centres, key processes of PAH care that span the breadth of patient journey and important outcome measures.
Systematic review
Search strategy
Members of the development group (SA, MB, GG and GK) conducted a systematic review of the literature using the Preferred Reporting Items for Systematic Reviews and Meta-Analyses statement criteria (online supplementary Table S1).14 The aim of the review was to identify key aspects of PAH clinical practice including structural components and processes of care that have strong association with favourable patient outcomes. The selected members constructed a comprehensive search strategy, which comprised a variety of keywords and medical subject headings (MeSH) terms such as ‘PAH’, ‘pulmonary vascular disease’ and ‘pulmonary veno-occlusive disease’ with two separate search strategies developed for MEDLINE® and Embase (online supplementary Table S2).
Eligibility criteria and study selection
The retrieved articles were then screened by at least two independent reviewers (SA, MB, GG, GK) against pre-defined inclusion criteria. Included studies were randomized controlled trials or controlled observational studies (including publications from clinical registries), published in English between 1 January 2015 and 19 November 2021, and enrolled patients ≥18 years of age with a diagnosis of PAH. Studies reporting only surrogate outcomes (e.g. biomarkers) without an associated ‘hard’ endpoint (e.g. mortality) or patient-reported outcome measures (e.g. health-related quality of life) were excluded. We only included the main publications of the major trials and registries from which our search extracted sub-studies. No restrictions were applied on the intervention(s) studied or on the presence or characteristics of the comparison arm. EndNote X9 was used for reference management and duplicate removal.
Quality assessment and data extraction
Data were extracted from all studies that met the eligibility criteria. No quality assessment was carried out because we wanted to include a broad spectrum of PAH literature during the initial phase of the development process. All relevant data variables were extracted to an Excel spreadsheet alongside their respective definitions and collection methods.
Clinical practice guidelines and existing quality indicators
The systematic review team also reviewed relevant clinical practice guidelines15, 16 and QIs12 to assess these recommendations against the ESC criteria for QIs (online supplementary Table S3).10
Data synthesis
Modified Delphi process
The systematic review team developed the candidate QIs from the literature and presented these indicators to the wider group using the modified Delphi method.10 The process was standardized on the basis of the ESC criteria for QI development (online supplementary Table S3), which were shared with the development group members before each voting round. Each candidate QI was voted upon by each member of the wide development group via an online platform. The panellists were asked to vote on both the validity and the feasibility using a 9-point ordinal scale.10 In total, two voting rounds and five meetings were conducted between May 2021 and June 2022. The initial meetings aimed to introduce the development process, whilst the subsequent meetings were focused on discussing the characteristics and formulation of the candidate QIs in line with the voting results. When concerns were raised about any of the indicators, they were included in the subsequent voting rounds.
Analysis of the voting results
Voting with ratings between 1 and 3 were classified as the QI being not valid/feasible; ratings between 4 and 6 that the QI was of uncertain validity/feasibility; and ratings between 7 and 9 that the QI was valid/feasible. We then calculated the median and the mean deviation from the median for each QI and included those with median scores ≥7 for validity, ≥4 for feasibility, and had minimal dispersion across the panellists.10 The wide development group then discussed the included QIs and determined the main from the secondary ones based on the voting results. Potential secondary QIs were then voted upon in a second Delphi round to determine their inclusion in the final set of QIs.
Results
Domains of pulmonary arterial hypertension care
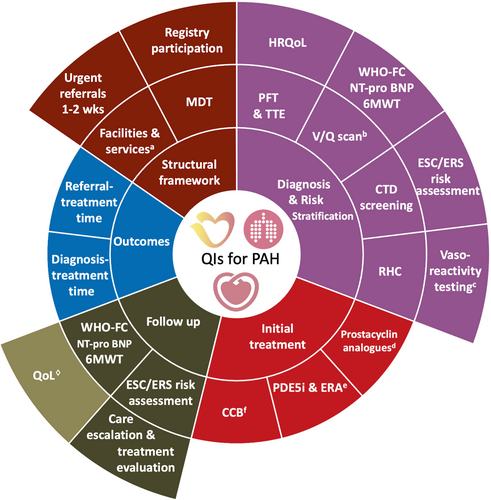
Five domains of care for the management of adults with PAH were selected. These domains included: (1) structural framework, (2) diagnosis and risk stratification, (3) initial treatment, (4) follow-up, and (5) outcomes (Figure 1).
Systematic review and voting results
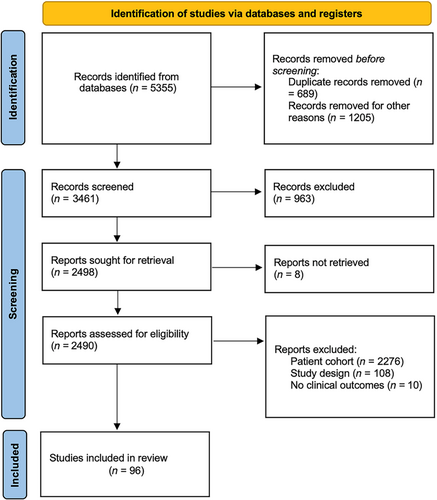
The literature search retrieved 3461 articles, of which 96 (3%) met the inclusion criteria (Figure 2). In total, 42 candidate QIs were extracted from the literature and included in the first voting round. Of these, 23 (55%) met the inclusion criteria and were included as main indicators, 10 (24%) QIs were excluded, and 9 (21%) were included as potential secondary QIs. After the second voting round, one (11%) secondary QI was included.
Quality indicators
The final list of the 2022 ESC QIs for PAH are presented in Table 1, with the definitions of the numerators and denominators for each QI shown in online supplementary Table S4.
| Domain 1. Structural framework |
|---|
| Main 1.1 Pulmonary hypertension centres that have a specialized MDTa responsible for the management of patients with PAH |
Main 1.2 Pulmonary hypertension centres that have the following facilities and skills:
|
| Main 1.3 Pulmonary hypertension centres that participate in a national or an international PAH registry |
| Main 1.4 Pulmonary hypertension centres that have a fast-track policy to reviewb urgent referrals within 1–2 weeks |
| Domain 2. Diagnosis and risk stratification |
|---|
| Main 2.1 Proportion of patients with suspected PAH who undergo pulmonary function test (including lung volumes and DLCO) at the time of diagnostic work-up |
| Main 2.2 Proportion of patients with suspected PAH who have an echocardiography at the time of diagnostic work-up |
| Main 2.3 Proportion of patients with suspected PAH who have a RHC at the time of diagnostic work-up |
| Main 2.4 Proportion of patients with suspected PAH who have perfusion imaging (V/Q scan or new modalityc) to exclude CTEPH at the time of diagnostic work-up |
| Main 2.5 Proportion of patients with suspected PAH who have been screened for CTD at the time of diagnostic work-up |
| Main 2.6 Proportion of patients with a diagnosis of idiopathic, heritable, or drug-induced PAH who have RHC with acute vasodilator testing at the time of diagnostic work-up |
| Main 2.7 Proportion of patients with a diagnosis of PAH who have their WHO-FC, NT-proBNP and 6MWT assessed at the time of PAH diagnosis |
| Main 2.8 Proportion of patients with a diagnosis of PAH who have their risk assessed using a validated tool (e.g. ESC/ERS guidelines) at the time of PAH diagnosis |
| Main 2.9 Proportion of patients with a diagnosis of PAH who have their quality of life assessed using a validated tool at the time of PAH diagnosis |
| Domain 3. Initial treatment |
|---|
| Main 3.1 Proportion of patients with a diagnosis of non-vasoreactive idiopathic, heritable, or drug-associated PAH and at high risk without significant cardiopulmonary comorbidities who are prescribed i.v./s.c. prostacyclin analogues |
| Main 3.2 Proportion of patients with a diagnosis of non-vasoreactive idiopathic, heritable, drug-associated or CTD-associated PAH and at low or intermediate risk without significant cardiopulmonary comorbidities who are prescribed initial combination therapy with a PDE5i and an ERA |
| Main 3.3 Proportion of patients with a diagnosis of vasoreactive idiopathic, heritable, or drug-associated PAH and acute vasodilator response who are prescribed high doses of calcium channel blockers |
| Domain 4. Follow-up |
|---|
| Main 4.1 Proportion of patients with a diagnosis of PAH who have their risk assessed using a validated tool (e.g. ESC/ERS guidelines) at least every 6 months |
| Main 4.2 Proportion of patients with a diagnosis of PAH who have been informed about available patient association/support group(s) |
| Main 4.3 Proportion of patients with a diagnosis of PAH who have their WHO-FC, NT-proBNP and 6MWT assessed at least every 6 months |
| Main 4.4 Proportion of patients with a diagnosis of PAH in whom low risk is not achieved who have a discussion with a member of the MDT on treatment strategy |
| Main 4.5 Proportion of patients with a diagnosis of PAH and at intermediate-high or high riskd who are evaluated for lung transplantation |
| Secondary 4 Proportion of patients with a diagnosis of PAH who have their quality of life assessed using a validated tool at least every 6 months |
| Domain 5. Outcomes |
|---|
| Main 5.1 The median time between establishing the diagnosis of PAHe and commencing PAH therapy |
| Main 5.2 The median time between referralf and commencing PAH therapy |
- 6MWT, 6-min walking test; CT, computed tomography; CTD, connective tissue disease; CTEPH, chronic thromboembolic pulmonary hypertension; DLCO, diffusing capacity of lung for carbon monoxide; ECMO, extracorporeal membrane oxygenation; ERA, endothelin receptor antagonist; ERS, European Respiratory Society; ESC, European Society of Cardiology; MDT, multidisciplinary team; MRI, magnetic resonance imaging; NT-proBNP, N-terminal pro-brain natriuretic peptide; PAH, pulmonary arterial hypertension; PDE5i, phosphodiesterase 5 inhibitor; PFT, pulmonary function test; RHC, right heart catheterization; V/Q, ventilation–perfusion; WHO-FC, World Health Organization functional class.
- a MDT comprises at least a cardiologist, pneumologist, and nurse specialist. Formal collaboration should be established with a rheumatologist, interventional radiologist/cardiologist, cardio-thoracic surgeon, social worker, and psychologist.
- b Review includes virtual and/or in-person visit.
- c Alternative perfusion imaging techniques include iodine subtraction mapping, dual-energy CT, or MRI perfusion.
- d Who are eligible for lung transplantation (based on age and comorbidities) and have been established on a combination therapy.
- e Date of diagnosis is date of diagnostic RHC.
- f Referral time is date of the receipt by the specialist PAH centre of the referral request.
Domain 1: Structural framework
The group selected indicators that form key components of PAH care, but may be difficult to capture on the individual patient level,10 such as the availability of a specialized multidisciplinary team for the management of patients with PAH (Main 1.1), the availability of necessary services for PAH care including high dependency units and diagnostic tools (e.g. cardiac catheterization) (Main 1.2), the participation in a national or an international registry for PAH (Main 1.3) and the establishment of a local policy to facilitate a clinical review for urgent referrals with suspected PAH within 1–2 weeks (Main 1.4).3
These measures may help address the potential delays in the diagnosis and management of patients with suspected PAH.17 Besides, they aim to provide a framework that stimulates a holistic evaluation for patients presenting with symptoms and signs suggestive of PAH to ensure the accessibility to required investigations, as well as appropriate phenotyping and multidisciplinary management (Table 1).18
Domain 2: Diagnosis and risk stratification
The QIs in this domain capture key components of PAH diagnosis and risk stratification. They also capture the evaluation of other potential causes for pulmonary hypertension (e.g. CTEPH) and concomitant conditions (e.g. connective tissue disease [CTD]). As such, Main 2.1 evaluates the assessment of pulmonary function test, Main 2.2 the performance of transthoracic echocardiogram and Main 2.3 the invasive assessment of pulmonary pressures for patients with suspected PAH. Furthermore, Main 2.4 captures the exclusion of CTEPH, whilst Main 2.5 the screening for CTD for the same cohort of patients (Table 1).3
For patients with an established diagnosis of PAH, Main 2.6 covers the assessment of vasoreactivity during right heart catheterization for those with idiopathic, heritable, or drug-induced PAH, and Main 2.7 the evaluation of prognostic functional and biochemical parameters (Table 1).19, 20 The other two QIs in this domain capture the systematic assessment of patient estimated risk of mortality at 1 year (Main 2.8) and their quality of life (Main 2.9) at the time of diagnosis (Table 1).3
Domain 3: Initial treatment
The three QIs in this domain pertain to specific subgroups of PAH patients. Main 3.1 evaluates the prescription of parenteral prostacyclin analogues for patients with non-vasoreactive idiopathic, heritable, or drug-associated PAH that are high risk without significant cardiopulmonary comorbidities, while Main 3.2 the use of an initial combination therapy of phosphodiesterase type 5 inhibitor and endothelin receptor antagonist in those with non-vasoreactive idiopathic, heritable, drug- or CTD-associated PAH that are low or intermediate risk without significant cardiopulmonary comorbidities.3 For patients with vasoreactive idiopathic, heritable or drug-associated PAH, high doses of calcium channel blockers have been shown to improve outcomes (Main 3.3) (Table 1).
Domain 4: Follow-up
Given PAH is a progressive condition in which symptoms, treatment and prognosis evolve over time, follow-up is a key component of PAH care. As such, a number of QIs have been selected in this domain including assessing risk on regular basis (Main 4.1), informing patients about support groups (Main 4.2), evaluating prognostic parameters (Main 4.3), escalating to the multidisciplinary team when low risk is not achieved (Main 4.4), referring to lung transplantation when indicated (Main 4.5) and monitoring quality of life (Secondary 4) (Table 1).
Domain 5: Outcomes
Whilst time point measurements may be determined by several factors, some of which beyond the control of healthcare professional, they may highlight potential areas for improvement.17 To that end, the development group reached consensus on two intervals as indicators of care quality for patients with PAH. These are the time between the establishment of PAH diagnosis and the initiation of PAH targeted therapy (Main 5.1) and the time between referral to specialist centre and the time of therapy commencement (Main 5.2) to evaluate the process by which patients with PAH are optimally treated (Table 1).17
Discussion
This document presents the first suite of ESC QIs for the evaluation of care and outcomes for adults with PAH. In total, 23 main and one secondary QIs have been selected across five domains of care which span the breadth of PAH processes of care and outcomes and help standardize the methods by which PAH care delivery is measured, analysed and reported to allow international comparative analyses. These QIs were constructed using the ESC methodology,10 combining existing evidence with expert consensus. The development of these QIs was in parallel the writing of the 2022 ESC Clinical Practice Guidelines for pulmonary hypertension and with the involvement of patient representatives.3
Despite the growing evidence base and the established prognostic measures for PAH,3 there is heterogeneity in the adherence to these measures across regions creating a missed opportunity to improve patient outcomes.2, 5 In contrasts to other cardiovascular diseases in which suites of QIs exist to address the so called ‘evidence-practice gap’, there is to date no set of QIs that standardizes the diagnostic and therapeutic strategies for PAH, and enable teams to benchmark their practice against these agreed standards. Such QIs may harmonize the methods by which PAH care is measured and its outcomes captured, providing a means for a unified international registry for PAH that uses the same standards and help generate knowledge, address disparities in care and improve patient outcomes.21
We formed an international group of domain experts and combined available evidence with expert consensus using a structured approach.10 The candidate QIs were extracted from the literature following a systematic review that aimed to identify existing gaps in PAH care and evidence-based measures that improve patient outcomes. A diverse working group took part in a modified Delphi process to select the final set of indicators based on their perceived validity and feasibility for each of the candidate QIs creating a balance between what is supported by evidence and can practically be captured in daily practice.10
The identification of key domains of care was sought to encapsulate the multifaceted nature of PAH processes of care which spreads across a number of clinical settings. The main challenge was selecting domains of care that are of the highest importance to patients, have strong association with outcomes, but can also be measured with reasonable costs and efforts.10 However, it is fundamental to recognize that the group prioritized the identification of the aspects of PAH care that affects outcomes the most, even if the measurement of these aspects were aspirational in some healthcare systems. As such, efforts may be advocated to systematically collect data variables that allow the operationalization of the developed QIs.
The working group anticipate that the QIs presented in this document will have a number of applications in clinical practice. First, they may be used as standards for quality improvement projects on a local, regional or national level to assess the quality of PAH care. Second, the developed QIs may stimulate the efforts to systematically collect structured data for patients with PAH to allow the seamless calculation of these QIs using registries or electronic health records. Third, the attainment of these QIs may be evaluated in observational cohort studies which aim to investigate the association between these indicators and clinical outcomes (i.e. external validation studies). Such effort help disseminate the implementation of these QIs, but also guide their future iterations.
We recognize the limitations of our study. First, the reliance on experts to select the QIs following the systematic review is prone to subjectivity. However, the modified Delphi method and the use of the ESC criteria ensured the process was standardized, and consensus development was important to ensure that the final set of indicators is relevant to clinical practice and feasible. Second, whilst alignment with the 2022 ESC/ERS guidelines for pulmonary hypertension was sought throughout the process, some QIs may not have a corresponding guideline recommendation. The QIs presented in this document reflects the findings of the literature review and the consensus of the development group. Other aspects of PAH care (e.g. palliative care for high-risk patients) is also important, but were not selected because of the complexity of such a decision which make it less feasible to be captured in practice. Third, the composition of the working group determined the selection of the final set of QIs. Our group comprised clinical, interventional and imaging cardiologists, as well as respiratory physicians, a specialist nurse, intensivists, researchers, and patients. Collaborating with other members of the multidisciplinary team (e.g. psychologists, physiotherapists, etc.) may have added a wider perspective to the project. Finally, there is a need to assess the feasibility of the developed QIs in existing registries for PAH to identify areas for improvement. Such assessment help evaluate the proportion of QIs that can be directly measured from these registries and investigate their association with patient outcomes.
Conclusion
This document defines 23 main and one secondary QIs across five domains of care for the management of PAH. The domains comprise: (1) structural framework, (2) diagnosis and risk stratification, (3) initial treatment, (4) follow-up, and (5) outcomes. The developed QIs provide standards for the measurement of PAH care through local, regional or national quality improvement projects which may help reduce missed opportunities and address disparities in care delivery. The integration of the developed QIs into routine data collection systems (e.g. electronic health records, clinical registries) allows the seamless and the continuous assessment of PAH care.
Funding
Suleman Aktaa is funded by the ESC.
Conflict of interest: C.P.G. chairs the Data Science Group of EuroHeart, Deputy Editor of EHJ Quality of Care and Clinical Outcomes; research grants from Abbott, BMS, BHF, Horizon 2020, NIHR; speaker's honoraria from AstraZeneca, Raisio Group, Wondr Medical; consulting from Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, Daiichi Sankyo, Ely-Lilly, Menarini, Vifor. M.B. is supported by the Deutsche Forschungsgemeinschaft (German Research Foundation; TTR 219, project number 322900939) and reports personal fees from Abbott, Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, Cytokinetics, Edwards, Medtronic, Novartis, Recor, Servier and Vifor during the conduct of the study. G.G. is a member of European Journal of Preventive Cardiology Editorial Board; lecturing fees and/or research grants from MSD, Janssen, Genesis Pharma, Novartis, Boehringer Ingelheim, AstraZeneca, Menarini, Roche Diagnostics, Bayer Healthcare, ELPEN Pharmaceuticals, Pfizer, Amgen, Unipharma, Galenica, Ferrer, GlaxoSmithKline, Sanofi, Servier. M.D.A. has served on the advisory board/provided consulting for Janssen, Merck Sharp and Dohme, United Therapeutics, Dompè, Ferrer. P.E.S.: unrelated to the present work: speaker's honoraria from Janssen, MSD, Bayer, Gossamer BIO, Ferrer; consulting from Gossamer BIO, Janssen, MSD, Ferrer, AOP Orphan Pharmaceuticals. J.S.R.G. receives honoraria for consultancy with Acceleron, Actelion/Janssen, Aerovate, Bial, Gossamer Bio, Merck, Pfizer. W.G.S.: speaker's honoraria from Janssen-Cilag Ltd; consulting from Gossamer Bio, Janssen-Cilag Ltd Merck Sharp & Dohme (UK) Ltd. M.M.H. has received honoraria for consultations and/or lectures from Acceleron, Actelion, AOP Health, Bayer, Ferrer, Janssen and MSD. M.H. is coordinator of the French Pulmonary Hypertension Reference Center (PulmoTension), coordinator of the French Reference Network on Rare Respiratory Diseases (RespiFIL), vice coordinator of the European Reference Network on Rare Respiratory Diseases (ERN-LUNG); and is supported by the Investissement d'Avenir programme managed by the French National Research Agency (grant contract ANR-18-RHUS-0006 [DESTINATION 2024]). B.A.M.: Actelion Pharmaceuticals, Deerfield Company, Tenax Therapeutics, outside the scope of the current work. A.V.N.: unrelated to this work is supported by the Netherlands CardioVascular Research Initiative (CVON-2012-08 PHAEDRA, CVON-2017-10 DOLPHIN-GENESIS) and the Netherlands Organization for Scientific Research (NWO-VICI: 918.16.610); institutional receipt of speakers money from Johnson & Johnson, MSD, Actelion, Bayer and Ferrer in the past 3 years; he served as a member of the scientific advisory board of Morphogen-X, Ferrer, Gosammer Bio Services Inc and Johnson & Johnson. L.C.P.: none relevant to the work; educational and research grants from Janssen, Ferrer, Altavant. A.R.: unrelated to the present work: research grants and speakers honorary from Actelion/Janssen. A.T.: consultations and/or speakers' honoraria for AOP, Bayer, Janssen, MSD, Pfizer. S.U. receives research grants from the Swiss National Science Foundation, Zurich and Swiss Lung League and received grants, travel support and consultancy fees from Orpha Swiss, Janssen SA, MSD SA and Novartis, all unrelated to the present work. M.D. reports research grants from Actelion/Janssen, speaker and consultant fees from Altavant, Acceleron, AOP, Daiichi Sankyo, Bayer, Ferrer, Gossamer, MSD, outside the submitted work, and all paid to her institution; holder of the Janssen Chair for Pulmonary Hypertension at the KU Leuven. All other authors have nothing to disclose.




