Inotropic therapy in patients with advanced heart failure. A clinical consensus statement from the Heart Failure Association of the European Society of Cardiology
Abstract
This clinical consensus statement reviews the use of inotropic support in patients with advanced heart failure. The current guidelines only support use of inotropes in the setting of acute decompensated heart failure with evidence of organ malperfusion or shock. However, inotropic support may be reasonable in other patients with advanced heart failure without acute severe decompensation. The clinical evidence supporting use of inotropes in these situations is reviewed. Particularly, patients with persistent congestion, systemic hypoperfusion, or advanced heart failure with need for palliation, and specific situations relevant to implantation of left ventricular assist devices or heart transplantation are discussed. Traditional and novel drugs with inotropic effects are discussed and use of guideline-directed therapy during inotropic support is reviewed. Finally, home inotropic therapy is described, and palliative care and end-of-life aspects are reviewed in relation to management of ongoing inotropic support (including guidance for maintenance and weaning of chronic inotropic therapy support).
Introduction
Inotropic agents are currently recommended in patients in the inpatient setting presenting with acute heart failure (AHF) with low cardiac output and hypotension (systolic blood pressure [SBP] <90 mmHg).1 This restricted recommendation is provided because of lack of quality evidence supporting other indications for use of inotropes for the majority of patients with AHF or in many other heart failure (HF) clinical settings. However, a group of patients with advanced stages of chronic HF exists, whose symptoms cannot be well managed with current guideline-directed medical therapy and in whom inotropic support may be appropriate in selected instances. Generally, these patients will have advanced HF with severely reduced ejection fraction. The clinical scenarios where inotropes may be considered in this setting have not been well described, nor has the choice of inotropic agent or subsequent clinical management of such patients. The aim of this scientific consensus statement is to provide a review of the literature related to inotropic therapy in patients with advanced chronic HF with reduced ejection fraction (HFrEF) and to describe a strategy for inotrope use in the care of this population.
Advanced chronic heart failure – definitions and clinical scenarios
A list of definitions related to inotropic therapy in patients with chronic HF is given in online supplementary Table S1.1-8 Some scenarios of advanced HF outside the setting of cardiogenic shock where inotropic support could be considered are described below (Figure 1). The clinical conditions are dynamic and can be overlapping in some instances. Also, they may incorporate patients recovered from cardiogenic shock, where inotropes may have already been used. Patients with temporary mechanical circulatory support (MCS) are not covered in this scientific consensus report statement.
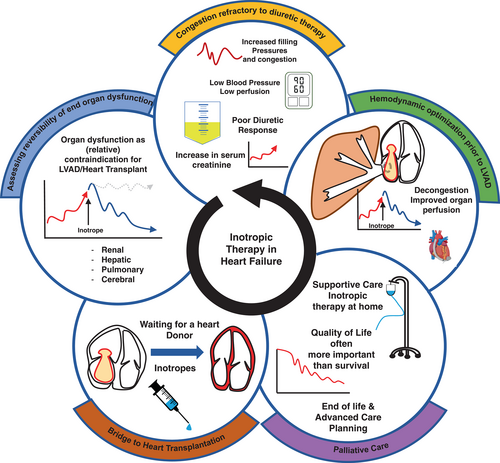
- Persistent congestion refractory to loop or combinational diuretic therapy
- Evaluation of reversibility of end-organ dysfunction prior to a decision about durable left ventricular assist device (LVAD) implantation or heart transplant listing
- Inotropic support to stabilize clinical condition prior to heart transplantation (HTx) or LVAD implantation
- Palliative or supportive therapy for advanced HF in patients not eligible for HTx or LVAD implantation
Pharmacology of β-adrenergic drugs (dobutamine, epinephrine, norepinephrine, dopamine)
Dobutamine, epinephrine, norepinephrine and dopamine are sympathomimetic agents that act directly through different adrenoreceptors: the α1, β1 and β2 receptor.9 Activation of the α1 receptors, that are located in the vascular wall, results in vasoconstriction. Stimulation of the myocardial β1 (and to some extent β2) receptors increases inotropy and chronotropy, whereas stimulation of vascular β2 receptors results in vasodilatation. The stimulation of β1 adrenergic receptors results in increased intracellular level of cyclic adenosine monophosphate (cAMP), changing calcium handling pathways including increased influx of calcium into the cardiomyocyte. This induces improved contractility, however, at the cost of increased oxygen consumption and arrhythmogenicity.
When addressing the clinical and haemodynamic effects of the different adrenergic drugs, it should be kept in mind that: (i) one drug may act on several different receptors, (ii) the drugs have different affinities for different receptors, and (iii) adrenergic drugs can act both directly on the different receptors and may induce reflex reactions by the autonomic nervous system (e.g. reflex vasodilatation after β1 stimulation).
The clinical and haemodynamic effects of the different sympathomimetic drugs are summarized in Table 1.
| Drug | Main mode of action | Main clinical effect | Bolus | Infusion rate | Elimination t½ |
|---|---|---|---|---|---|
| Dobutamine |
β1 (β2) |
Inotropy Chronotropy Vasodilatation |
− | 2–20 μg/kg/min | ± 2 min |
| Epinephrine/adrenaline |
A-1 B-1 B-2 |
Inotropy Chronotropy Vasoconstriction |
+ | 0.05–0.5 μg/kg/min | ± 2.5 min |
| Norepinephrine/noradrenaline |
A-1 (B-1) |
Vasoconstriction | − | 0.2–1.0 μg/kg/min | ± 1–2 min |
| Dopamine |
Low dose: Dopamine receptors High dose: B-1 and A-1 |
Low dose: selective vasodilatation High dose: vasoconstriction and inotropy |
− |
Low dose: <3 μg/kg/min High dose: >5 μg/kg/min |
± 2 min |
Dobutamine
Dobutamine is a synthetic catecholamine that acts primarily as a β1 agonist, resulting in increased inotropy and chronotropy and reduction in left ventricular filling pressures. The additional β2 receptor effect and the reflex vasodilatation due to increased cardiac output may result in a decrease in blood pressure especially with lower doses.
Dopamine
Dopamine is an endogenous precursor of norepinephrine. At moderate to higher dose (>3–5 μg/kg/min) dopamine has β1 adrenergic affects, as well as α1 adrenergic effects, the latter resulting in vasoconstriction. At lower dose (1–3 μg/kg/min), dopamine acts predominantly through dopamine receptors, resulting in selective vasodilatation (e.g. in the renal beds).
Norepinephrine (also called noradrenaline)
Norepinephrine is an endogenous catecholamine that acts on both α1 and β1 receptors, resulting mainly in vasoconstriction and a modest increase in cardiac output. Therefore, its use is limited for the management of significantly reduced blood pressure, and only rarely in chronic HF patients.
Epinephrine (also called adrenaline)
Epinephrine is a potent α1, β1 and β2 receptor agonist. At low doses, it results in an increase in cardiac output because of the predominant β1 agonist effect (increase in inotropy and chronotropy), whereas at higher doses, the α1 receptor agonist effect results in vasoconstriction.
A large proportion of advanced HF patients in whom inotropic support is considered, are on chronic β-blocker therapy. For example, in the LION-HEART study up to 81% of the patients received β-blockers at baseline, although this was a study using levosimendan.10 The use of β-blockers (in particular carvedilol, bisoprolol, and to a lesser extent metoprolol) may inhibit the favourable haemodynamic effects of dobutamine.11, 12 As β-blockers may not influence the haemodynamic effects of phosphodiesterase (PDE) inhibitors (e.g. milrinone) or calcium sensitizers (e.g. levosimendan), these drugs may be preferred over β-adrenergic drugs in patients treated with and tolerating (high-dose) β-blockers.12, 13
Pharmacology of phosphodiesterase III inhibitors
The 3′, 5′ cyclic nucleotide PDEs are a major class of enzymes that play a vital part of this intracellular signalling mechanism. They degrade cAMP or cyclic guanosine monophosphate (cGMP) to their respective 5′ nucleotides, thereby providing a key brake on cyclic nucleotide signalling.13 Within the cardiovascular system, numerous functional PDEs have been identified including PDE1–5, PDE8 and PDE9. PDE3 was identified in the cardiovascular system several decades ago. PDE3 inhibitors, most notably including milrinone which was developed in the 1980s, have been consistently demonstrated to exert positive inotropic, vasodilator and positive lusitropic properties in both animal studies and patients with HF. These physiologic actions result in dose-dependent, clinically meaningful improvements in cardiac output, filling pressures and pulmonary arterial pressures. Plasma levels are not usually monitored in clinical practice. It is recommended that dose adjustments should be considered in the setting of an estimated glomerular filtration rate <50 ml/min/1.73 m2 as milrinone is renally cleared, although studies suggest that the exposure remains within the therapeutic range in most cases.14 Adverse effects of milrinone are well recognized including systemic hypotension and arrhythmia.
Haemodynamic and functional studies showed that orally administered milrinone improved cardiac index and reduced pulmonary capillary wedge pressure, however these effects were relatively transient given its pharmacokinetic profile. Based on experience with long-term use of lower dose intravenous milrinone therapy in a bridge to transplant patient cohort,15 an extended release milrinone formulation that yields a stable plasma milrinone profile with twice daily dosing has been developed. In preliminary open-label studies in patients with advanced HFrEF, evidence of improved functional capacity without evidence of increased arrhythmia has been demonstrated16 and the drug has also shown promise in advanced HF with preserved ejection fraction (HFpEF).17 Larger trials are required to establish the potential for the selected use of oral PDE3 inhibitors in advanced HF.
Pharmacology of calcium sensitizers
Calcium sensitizers such as levosimendan enhance the sensitivity of cardiac myofilaments to calcium, without influencing intracellular calcium transients. Levosimendan acts through two main mechanisms: (i) calcium sensitization by binding troponin C, changing the conformation of troponin I and reducing its inhibition on actin–myosin bridges formation; and (ii) opening of adenosine triphosphate (ATP)-dependent potassium channels in vascular smooth muscle cells, favouring coronary, pulmonary and systemic vasodilatation and in mitochondria, with cardioprotective effects in ischaemic settings. Levosimendan also holds PDE3 inhibitory effects and increases intracellular cAMP.9
Despite levosimendan's short half-life of 1 h, its effect can last for weeks due to the longer (70–90 h) half-life of its active metabolite (OR-1896).18 This makes levosimendan an option for periodic administrations. Its high oral bioavailability (85%) has stimulated trials on an oral formulation, so far without favourable results. Levosimendan reduces pulmonary artery wedge pressure and increases cardiac output compared to placebo or dobutamine.19 The use of bolus should be avoided, as studies where bolus was used reported more initial hypotension20 than studies without bolus use.10, 21 The dose of 0.1 μg/kg/min could be respectively modified to 0.05 or 0.2 μg/kg/min based on blood pressure and clinical response.
Istaroxime
Istaroxime inhibits Na/Ca exchange at the sarcolemmal level and activates sarcoplasmic reticulum calcium ATPase. It can be administered intravenously.
Myotropic drugs
Omecamtiv mecarbil is the first oral drug in this class.22 It is a direct cardiac myosin activator, improving cardiac function through an increase in actin–myosin interaction without affecting calcium transients. The drug increases stroke volume and ejection time in healthy volunteers23 and HF patients24 dependent on plasma concentration. The drug can be administered orally.
New and emerging inotropic therapies
Novel developments in biotechnology hold great promise for future inotropic HF therapies (online supplementary Table S2).22, 25-43 Technical novelties that are now available have inspired whole new areas of research, including next-level gene therapies and cell reprogramming. Translational research is more important than ever, and basic scientists work together with translational and clinical scientists to identify the most promising therapeutic targets, now that delivery and modification are so much more feasible than before.
Inotropes in advanced chronic heart failure – evidence from clinical trials
Inotropic agents have been tested in several clinical trials that included patients with varying degree of circulatory failure. The main trials are briefly presented below.
β-agonists
Dobutamine's safety and efficacy were extrapolated from a subgroup analysis of the Flolan International Randomized Survival Trial (FIRST) that randomized 471 patients with New York Heart Association (NYHA) class IIIb–IV symptoms to epoprostenol (prostacyclin) infusion versus standard care with unfavourable results in the epoprostenol group. A post-hoc analysis of this study showed that dobutamine use was associated with a higher 6-month mortality rate. The dobutamine group, however, included a sicker population, which may have influenced the results.44 A trend to excess mortality with intermittent dobutamine therapy was also observed in a meta-regression analysis along with trends to improved symptoms.45
Similarly, the addition of low-dose dopamine infusion to low-dose furosemide in the Efficacy and safety of high dose versus low dose furosemide with or without dopamine infusion: the Dopamine in Acute Decompensated Heart Failure II (DAD-HF II) and Renal Optimization Strategies Evaluation in Acute Heart Failure (ROSE-AHF) trials failed to show a favourable effect on renal function and diuresis and any beneficial effects in the in-hospital and post-discharge outcomes.46, 47 In some studies dopamine has been associated with an increased risk of tachycardia or atrial arrythmias.47, 48 Lack of improvement in renal function with dopamine was also recently reported in patients with HFpEF.49
Phosphodiesterase III inhibitors
Phosphodiesterase inhibitors in two landmark studies failed to offer additional benefit in patients with severe or acutely decompensated HF (ADHF). In the Prospective Randomized Milrinone Survival Evaluation (PROMISE) trial, oral milrinone therapy was not only associated with more frequent hospitalizations, and more hypotensive and syncopal episodes, but also with a significant increase in mortality.50 In the Outcomes of a Prospective Trial of Intravenous Milrinone for Exacerbations of Chronic Heart Failure (OPTIME-CHF) trial, where 951 patients with acute exacerbations of chronic HF were randomized to either milrinone (0.375–0.75 μg/kg/min) or saline placebo, the PDE inhibitor offered no benefit on either mortality or readmission endpoints. Those randomized to milrinone experienced more clinically relevant hypotension (possibly related to the loading dose) and atrial arrhythmias and patients with ischaemic heart disease had significantly greater in-hospital mortality with milrinone. In contrast, outcomes in non-ischaemic patients tended to be improved in terms of the primary endpoint (number of days hospitalized due to cardiovascular causes within 60 days) and the composite of death or rehospitalization implying a rather bidirectional effect based on aetiology in decompensated HF.51
Low-dose oral enoximone, compared with placebo, in 1854 patients with advanced HF had neutral effects on the combined endpoint of all-cause or cardiovascular hospitalization and did not change exercise capacity, assessed by the 6 min walking distance.52
Calcium sensitizers
Levosimendan has been tested in several trials including patients with advanced HF, both in the acute and more chronic settings. Despite the favourable haemodynamic results of the phase II Levosimendan Infusion versus Dobutamine (LIDO) trial,19 levosimendan in the Survival of Patients With Acute Heart Failure in Need of Intravenous Inotropic Support (SURVIVE) randomized trial offered no advantage in 6-month mortality when compared to dobutamine in patients with acute decompensated HF.53 Similar findings were reported from two US trials comparing levosimendan to placebo in AHF.20 Several smaller prospective, randomized trials have evaluated the effect of repeated doses of levosimendan in the setting of advanced chronic HF (LevoRep, LAICA and LION-HEART). Levosimendan was used repetitively with cycles every 2–4 weeks in similar patient populations with different dose schedules. In the LevoRep study the primary endpoint comprised improvement in 6-min walking distance and quality of life assessed by Kansas City Cardiomyopathy Questionnaire, which were not significantly improved with levosimendan.21, 54 In the Levosimendan® Intermittent administration in Outpatients: effects on Natriuretic peptides in advanced chronic HEART failure (LION-HEART) study, the primary endpoint of change in N-terminal pro-B-type natriuretic peptide was significantly improved with levosimendan treatment. A significant reduction in combined incidence of all-cause mortality and hospitalization for patients receiving levosimendan was observed, but importantly the study was small and not powered to assess effect on hard outcomes.10 In the Long-Term Intermittent Administration of Levosimendan in Patients with Advanced Heart Failure (LAICA) study, efficacy and safety of long-term intermittent use of levosimendan to reduce the incidence of hospital admissions was evaluated, however the study was stopped prematurely for lack of funding and slow patient recruitment. Although the reduction in the incidence of hospital readmissions for ADHF did not reach statistical significance, there was a significantly lower cumulative incidence of ADHF and mortality during short (1 and 3 months) and long-term (1 year) levosimendan treatment.55 Results from a larger randomized study in patients with advanced HF symptoms after an admission for ADHF are underway but the study has been terminated early because of slow recruitment related to the COVID-19 pandemic.56 In all, despite clear haemodynamic effects, no adequately powered study on levosimendan has demonstrated effect on mortality or hospitalization in chronic advanced HF.
Myotropic drugs
In a large placebo-controlled trial conducted in patients with HFrEF in NYHA class II–IV enrolled either during a hospitalization for HF (inpatients, 25.3%) or as outpatients with a HF event in the previous year (outpatients), omecamtiv mecarbil reduced the primary endpoint of cardiovascular mortality or HF hospitalization.26 Its beneficial effects on the risk of cardiovascular death and HF hospitalizations were greater in the patients with the lower left ventricular ejection fraction (LVEF) or lower blood pressure as well as in the subgroup (n = 2258) with advanced HF according to the Heart Failure Association (HFA) criteria.57, 58 In another trial it did not increase exercise capacity compared with placebo.59 Currently, this drug is undergoing evaluation by the regulatory agencies.
Selection of patients for inotropic support: clinical scenarios
Inotropes may be an appropriate therapy for patients with chronic HF who present with volume overload resistant to diuretic therapy or with organ malperfusion. Their use should be limited to the lowest clinically effective dose administered for the shortest period of time.
Clinical condition 1: Congestion refractory to diuretic therapy
Congestion resistant to diuretic therapy frequently accompanies advanced HF, and patients, while not in cardiogenic shock, but with persistent congestion despite several days of aggressive diuretic therapy may be candidates for short-term inotropic support. Haemodynamically these patients are characterized by elevated filling pressures, and cardiac index may be low or normal. SBP is often low but may be >90 mmHg. Often renal function is compromised due to reduced renal perfusion and venous congestion or by concomitant kidney disease. These advanced HF patients are often diuretic resistant due to their haemodynamic profile, tubular hypertrophy due to long-term use of loop diuretics and renal congestion. Diuretic resistance further deteriorates persistent congestion, which will detrimentally impact left- and right-sided filling pressures and reduce cardiac output and renal perfusion. In parallel, high right-sided filling pressures, increased congestion and preload to the right ventricle can also lead to worsening tricuspid regurgitation, all of which are associated with deteriorating renal function.60-62
Short-term inotropic support may be useful to overcome this vicious circle of low perfusion, persistent congestion and diuretic resistance. As discussed above, dopamine, which has previously been used often in this setting, has no role in treating these patients considering neutral results of randomized controlled trials46, 47 Milrinone has been shown to increase renal blood flow in a small study of postoperative HF.63 Levosimendan increased estimated glomerular filtration rate and urine output in a small randomized study in 21 patients with advanced HF64 likely by improving intrarenal haemodynamics.65 After decongestion, inotropic support should be discontinued and guideline-directed medical therapy resumed.1, 8 Need for inotropic support to overcome diuretic resistance in congested patients should always prompt considerations for referral of the patient to an advanced HF centre for advanced HF therapies such as LVAD implantation or HTx.3
Clinical condition 2: Evaluation of reversibility of end-organ dysfunction prior to decision on durable LVAD implantation or heart transplant listing (renal, hepatic, pulmonary)
Severe, irreversible renal or hepatic dysfunction is a contraindication to LVAD implantation and HTx.1, 66 However, commonly, end-organ dysfunction is a consequence of worsening HF, either due to congestion or inadequate perfusion, rather than an intrinsic organ disease per se.60, 67, 68 A period of haemodynamic optimization using inotropic support may in some instances revert end-organ dysfunction (cardio-renal, cardio-hepatic syndrome or elevated pulmonary pressures), proving that the dysfunction was caused by HF and enabling listing for transplantation or LVAD implantation. Although elevated pulmonary vascular resistance is a contraindication to HTx,69 a challenge using inotropic support is generally recommended if pulmonary vascular resistance remains fixed after inhalation of nitric oxide or acute vasodilator infusion. Hence, inotropic support could occasionally be used as ‘bridge to candidacy’ either for LVAD or HTx, where it may then be continued as described under ‘Clinical condition 3’.
Clinical condition 3: Haemodynamic optimization of patients prior to LVAD implantation
Pulmonary congestion and high central venous pressures increase the risk of early right ventricular failure post-LVAD implantation. Further, hepatic congestion or hypoperfusion is associated with coagulopathy and increased bleeding risk. Consequently, pre-LVAD optimization of congestion and organ perfusion is often pursued using inotropic support. If inotropes are administered for extended periods and attempts to wean have resulted in recurrence of end-organ dysfunction, the patient is designated inotrope-dependent. A new definition of inotrope dependency with specific criteria and taking into consideration non-continuous inotropic support is given in Table 2. In Europe, approximately 40% of LVAD implantations are performed in this group of patients (referred to as INTERMACS level 3).70 Few studies have described strategies for haemodynamic optimization, but improved outcomes, compared with registry data after initiating systematic preoperative inotrope management in non-inotrope-dependent patients have been demonstrated.71 Compliance during a period of intermittent intravenous inotropic therapy may also suggest acceptance of long-term LVAD therapy. Efficacy and safety of a strategy of intravenous inotropic therapy before either as ‘destination therapy’ or as ‘bridge to LVAD’ both require final data through randomized trials.
|
FAILURE to wean intravenous inotropic supporta within 72 hb WITHOUT:
The diagnosis of inotrope dependence should not be made during simultaneous introduction or up-titration of β-blockers or RAS inhibitors. |
- eGFR, estimated glomerular filtration rate; INR, international normalized ratio; NYHA, New York Heart Association; RAS, renin–angiotensin system.
- a Epinephrine, norepinephrine, dopamine, dobutamine, milrinone.
- b If the patient has received continuous intravenous inotropic support >7 days, inotrope dependence is defined as failure to reduce infusion rate at 72 h after each attempt to reduce. In the case of levosimendan infusion, dependence is defined as need for new infusion <10 days after the former.
- c In the absence of reduction in loop diuretic dose.
Clinical condition 4: Inotropic support as bridge to heart transplantation
In the most recent International Society for Heart and Lung Transplantation registry analysis, 36% of patients undergoing HTx were treated with inotropic support72 and in some series a substantial proportion of patients bridged with inotropic support can reach transplantation.73 This proportion has decreased in parallel to an increased use of MCS as bridge to HTx. If sufficient, inotropic support is often preferred over MCS in heart transplant candidates with anticipated short waiting time and always preferred where LVADs are likely ineffective, such as patients with severe or isolated right ventricular failure. In a large transplant registry analysis, inotropic support was not associated with inferior outcomes after HTx.72
Clinical condition 5: Palliative therapy for advanced heart failure in patients not candidates for heart transplantation or LVAD implantation
Patients with advanced HF not eligible for HTx or LVAD implantation may be treated with extended inotropic support either as continuous intravenous infusion of dobutamine or milrinone or intermittent infusions of dobutamine or levosimendan. Years of inotropic support can occasionally be relevant. Treatment may also be offered in the home setting in some countries. Clear indications and protocols should be followed even if they may vary from institution to institution, and treatment goals should be set with plans for re-evaluation at regular intervals. If adverse events occur or the treatment is no longer effective, as per the pre-defined goals, it should be terminated. The palliative use of inotropes in end-of-life care is discussed below.
Management of guideline-directed therapy and diuretics during inotropic support
There is only scarce evidence on the safety and efficacy of guideline-directed therapy in chronic HF patients already on inotropic support, as these patients have routinely been excluded from randomized clinical trials. Often, such patients are unable to tolerate neurohormonal-directed therapy due to circulatory insufficiency or co-existing end-organ dysfunction such as chronic kidney disease not caused by cardiac failure. In the recent LCZ696 in Advanced Heart Failure (LIFE) study in patients with advanced HFrEF where 20% used ambulatory inotropic support, sacubitril/valsartan therapy did not impact on the clinical composite endpoint.74 In a subsequent analysis, these investigators identified reasons for intolerance to sacubitril/valsartan therapy and noted that a lower mean arterial pressure, lower serum chloride, moderate or greater mitral regurgitation, were factors associated with inability to tolerate the drug, among other factors.75 In the chronic outpatient setting, the use of guideline-directed therapy should remain the cornerstone of HF treatment even if the patient is on inotropic support. However, tolerability of these therapies is more challenging, especially those that impact blood pressure which is often lower in advanced HF patients. It may therefore sometimes be necessary to down-titrate or even (temporarily) discontinue these therapies to be able to initiate or continue inotropic support. In patients treated with repeated levosimendan infusion interruption of β-blockade is seldom necessary and β-blockade may even enhance the effect of levosimendan.76
In the clinical setting of low cardiac output and elevated filling pressure, combination of inotropic support and diuretic treatment is often required. The focus in these patients should be adequate decongestion by whatever means necessary. After decongestion inotropes should be weaned and guideline-directed therapy reinitiated. As these patients are often haemodynamically fragile, it is often advisable to restart standard therapy while still in hospital with subsequent outpatient up-titration. In some cases it may be possible to initiate low-dose guideline-directed therapy while the patient is still on inotropes, followed by down-titration and discontinuation of inotropic therapy if possible.77
Oral inotropic agents for the long-term treatment of patients with advanced heart failure
Safe and effective oral inotropes would be highly attractive for the treatment of advanced HF, but at this time no oral drugs have been approved for this indication. Digoxin has inotropic effect and may be helpful in patients with atrial fibrillation but likely has little effect in advanced HF in the presence of sinus rhythm. Omecamtiv mecarbil reduced the primary endpoint of cardiovascular death and HF hospitalizations in the Global Approach to Lowering Adverse Cardiac Outcomes Through Improving Contractility in Heart Failure (GALACTIC-HF) trial enrolling 8256 patients with HFrEF (hazard ratio [HR] 0.92; 95% confidence interval [CI] 0.86–0.99; p = 0.03).26 The effects were larger in the patients with lower LVEF as well as in those with criteria for severe HF.58 Amongst the pre-specified subgroups, LVEF was the strongest modifier of the treatment effect of omecamtiv mecarbil on the primary outcomes.78 In addition, omecamtiv mecarbil was well tolerated, with no significant changes in blood pressure or kidney function. In another subgroup analysis, omecamtiv mecarbil, compared with placebo, appeared to be more effective in reducing the primary composite endpoint in patients with SBP ≤100 mmHg (HR 0.81; 95% CI 0.70–0.94) compared with those with SBP >100 mmHg (HR 0.95; 95% CI 0.88–1.03; p-value for interaction = 0.051).57 Thus, if approved by regulatory bodies, omecamtiv mecarbil will be a treatment option that may be considered in patients with advanced HF.1, 79
Monitoring of patients on inotropic support and practical aspects of administration (ward/intensive care unit)
The monitoring needs of patients receiving inotropic support vary depending on several factors including the inotrope used, the patient's clinical status and medical history, the aim of inotropic support as well as local protocols and care level at different ward types. This is reflected by the fact that some patients receiving inotropes are treated in the intensive care unit (ICU) while others are managed at home (see below). Patients treated with multiple inotropes or requiring norepinephrine or epinephrine need admission to an ICU/cardiac care unit and placement of a pulmonary artery catheter will likely be helpful in most cases.80 Patients treated with inodilators or low-dose dopamine may in many instances be treated in the cardiology/internal medicine ward. Thresholds to accept non-ICU administration of inotropes may be higher in patients where a clear palliative strategy has been decided upon and in patients with an implantable cardioverter-defibrillator (ICD). In recent years, levosimendan in particular, has been used in non-ICU settings. Levosimendan can be administered in hospital, in the ward or in a day hospital (for 6 h infusions) with electrocardiogram and non-invasive blood pressure monitoring during the drug infusion. Electrolyte monitoring is advisable.81, 82 Patients with severe hypotension (SBP <90 mmHg) should be admitted to the ICU for the levosimendan infusion to enable vasopressor support if needed. In some centres low-dose dobutamine, dopamine or milrinone is also used outside the ICU setting with precautions as described above.
Administration of inotropes in the ambulatory setting (‘home inotropes’)
Among the growing population of patients refractory to conventional HF therapy, only a minority are candidates for HTx or MCS1 whereas the majority of this group, either due to advanced biological age, various comorbidities or psychosocial factors, is not. The latter patients require palliative care mainly for improvement in quality of life.3, 83 Advanced HF patients referred to specialized HF centres are commonly hospitalized, optimally treated and re-assessed for their potential eligibility for HTx or MCS. Frequently, candidates for those two procedures require long-term inotropic support. The HTx or MCS un-eligible patients may also benefit from inotropic therapy, in terms of quality of life.84, 85 The safety concerns of inotropes described above are based on studies mostly published before the ICD era and the broad use of β-blockers and were largely disproved by more contemporary studies.85, 86 This has opened a possibility for longer-term home treatment with inotropes. This practice has been extensively used in the United States, potentially reflecting prior organ heart transplant allocation rules, but until recently less frequently in Europe.
When home inotropic support is considered indicated, several factors including different pharmacological properties of the inotropic agents used affect the decision between which of the different agents to be used. Dobutamine is less expensive than milrinone but there are no head-to-head studies comparing the safety and efficacy of those two agents in the home setting. Compared with dobutamine, more patients treated in their home with milrinone eventually underwent HTx or LVAD implantation (30% vs. 10%) suggesting more frequent use of milrinone in the HTx or MCS eligible population and dobutamine use in the non HTx or MCS eligible palliative care population.22
Because of a very high risk of phlebitis,87 longer-term administration of dobutamine and milrinone requires an indwelling catheter such as peripherally inserted central catheter and bears the potential for various adverse events including arrhythmias and line infections.85 The doses used, mandated by the safety concerns, are lower than those used in the monitored setting and are typically in the range 0.125–0.325 μg/kg body weight/min of milrinone and 1–5 μg/kg body weight/min of dobutamine.88 Because of the long half-life of its pharmacodynamically active metabolite, levosimendan administered intermittently in hospital or in an outpatient setting will also have pharmacological effects after the patient is sent home but is not considered a ‘home inotrope’. The tolerability and safety of the repetitive use of this agent was discussed above. There are no head-to-head studies comparing the safety and efficacy of levosimendan and milrinone in the home inotrope setting.
It appears rational to apply home inotropic support in a hierarchical order: start and continue with dobutamine if still effective (because of lowest cost). In cases of reduced inotropic effectiveness of dobutamine, switch to milrinone. When prolonged support is expected or if the patient is intolerant to insertion of an indwelling venous catheter, consider switching to levosimendan if available in the geo-region. Repeated doses of levosimendan may be the first choice in those patients with history of positive clinical response to this agent.
As a rule, monitor for adverse effects and avoid hypokalaemia: keep potassium levels above 4.0 mmol/L. Consider modifying the diuretic dose and guideline-directed medical therapy, and in the palliative group, discuss with the patient turning off the shock therapy if the patient has an ICD or a cardiac resynchronization therapy-defibrillator device. In patients treated with home inotropes with a non-palliative target, appropriate measures (typically an implantable defibrillator) to avoid sudden arrhythmic death must be taken.
Use of home inotropic support with dobutamine, milrinone and levosimendan as bridge to advanced HF therapy or, in selected cases, as palliative therapy is a viable option. Repetitive home infusions of levosimendan in advanced HF seem to be feasible and a more practical approach although systematic studies are lacking. Evidence-based protocols for continuous intravenous inotropic support are needed.
End-of-life considerations for patients with advanced chronic heart failure on inotropic support
American and European guidelines suggest inotropes may prove effective as a palliative therapy for symptomatic relief in patients with advanced HF who have no further treatment options.1, 89 Patients with advanced HF can experience multiple symptoms, including physical, psychological or social in nature. Several studies illustrate the beneficial outcome of inotropic therapy in terms of improving functional class for this group of patients.90 The impetus and understanding to integrate palliative care early into HF management would support the consideration of inotropic therapy for symptomatic relief,8 however with the caveat that its initiation follows open communication and an informed decision shared with the patient and close family members. When death is imminent, cessation of inotropic therapy, like deactivation of ICD should be discussed.
For most patients, their preferred place of care is home. The provision of ambulatory or home-based inotropic therapy is appealing, however this is frequently determined by healthcare organizational factors, socio-demographic characteristics, the patient's level of social support and living arrangements.91 A small study (n = 21) conducted in Belgium found that when intravenous inotropic support was provided at home to patients recently discharged with advanced HF, there was an improved quality of life and 75% (9 out of 12 patients) of the patients died in their preferred place of care – that being at home.84 Results from this study are supported by a previous report from the US showing that patients (n = 217) who received inotropic support at home were more likely to die at home, as compared to national sample of patients with HF.92 This choice and control regarding place of death is an important factor in what patients perceive as a ‘good death’.93 However, this should not be the only factor, rather the patient's clinical status including intravenous access, as well as the treatment expectations and concerns expressed by the patient and family members. Such should be carefully considered to ensure adverse effects associated with current therapy do not outweigh its benefits. A pilot of a cardiac home hospice programme has shown promising results within the United States, however transferability to other countries and healthcare systems may be limited.94
All too often patients with complex HF symptoms do not or are unable to avail themselves of hospice services. Increased education on how to best manage complex patients with HF at the end of life is recognized as an essential missing component within the HF specialist training curricula for both cardiologists, nurses as well as that of the hospice staff.8, 95 Since the first palliative care position paper published by the HFA over a decade ago,96 there is greater awareness of the need for sensitive discussions and advanced planning concerning complex therapies such as ICDs and LVADs. For the successful instigation of the use of inotropes in advanced HF, informing the patient that cessation of the treatment may be warranted as the disease progresses, is vital.
Gaps in evidence and directions for future research
As evident from the discussion above, there are several gaps in our knowledge about how to use inotropic support in chronic HF. First, although progress has been made with respect to the documentation of safety of long-term use of inotropes, evidence for efficacy in terms of improved survival is lacking. Unfortunately, existing trials have not been adequately powered and recently an ongoing trial has been stopped due to slow inclusion.56 This leaves uncertainty regarding the use of these drugs even in situations where they appear necessary including in the scenarios described above. New drugs affecting cardiac function offer promise for long-term use in patients with advanced HF, but we are still awaiting evidence from clinical trials. More knowledge about the integration of new and conventional inotropes with guideline-directed medical therapy for chronic HF is clearly needed. As the prognosis in non-advanced chronic HF has improved tremendously with the development of modern drug therapy, better tools and a better understanding of how to treat advanced HF in patients not eligible for MCS or HTx are clearly needed to improve overall well-being and survival of the entire HF population.
Conflict of interest: none declared.




