Association of time-to-intravenous furosemide with mortality in acute heart failure: data from REPORT-HF
Abstract
Aim
Acute heart failure can be a life-threatening medical condition. Delaying administration of intravenous furosemide (time-to-diuretics) has been postulated to increase mortality, but prior reports have been inconclusive. We aimed to evaluate the association between time-to-diuretics and mortality in the international REPORT-HF registry.
Methods and results
We assessed the association of time-to-diuretics within the first 24 h with in-hospital and 30-day post-discharge mortality in 15 078 patients from seven world regions in the REPORT-HF registry. We further tested for effect modification by baseline mortality risk (ADHERE risk score), left ventricular ejection fraction (LVEF) and region. The median time-to-diuretics was 67 (25th–75th percentiles 17–190) min. Women, patients with more signs and symptoms of heart failure, and patients from Eastern Europe or Southeast Asia had shorter time-to-diuretics. There was no significant association between time-to-diuretics and in-hospital mortality (p > 0.1). The 30-day mortality risk increased linearly with longer time-to-diuretics (administered between hospital arrival and 8 h post-hospital arrival) (p = 0.016). This increase was more significant in patients with a higher ADHERE risk score (pinteraction = 0.008), and not modified by LVEF or geographic region (pinteraction > 0.1 for both).
Conclusion
In REPORT-HF, longer time-to-diuretics was not associated with higher in-hospital mortality. However, we did found an association with increased 30-day mortality, particularly in high-risk patients, and irrespective of LVEF or geographic region.
Clinical Trial Registration: ClinicalTrials.gov Identifier NCT02595814.
Introduction
Acute heart failure (AHF) can be life-threatening, requiring immediate diagnosis and medical intervention.1-4 Analogous to door-to-balloon time in patients with acute ST-elevation myocardial infarction, the concept of shorter time-to-diuretics was proposed as imperative to improve outcomes.5-9 However, unlike door-to-balloon time, data on the association between time-to-diuretics and patients outcomes have been conflicting.5-9
Two studies suggested that a delay in initiating intravenous (IV) diuretics was associated with higher in-hospital and post-discharge mortality.7, 9 Conversely, a single study from South Korea showed no significant association between time-to-diuretics and mortality.8 Unfortunately, these previous studies were limited to either single centres,9 or countries,7, 8 or had a retrospective study design.9 Differences in local practices, time of presentation and diagnosis, and quality of data collection can introduce bias in the association between time-to-diuretics and mortality,10 which might explain these conflicting results.
Accordingly, we evaluated the association between time-to-diuretic treatment and mortality in the International Registry to Assess Medical Practice with Longitudinal Observation for Treatment of Heart Failure (REPORT-HF).11
Methods
Study design, and participant selection and procedures
The design of REPORT-HF was reported previously.11-13 In short, REPORT-HF was a prospective observational cohort study designed to investigate global differences in AHF presentation, treatment, and outcomes.11 Patients with a primary diagnosis of AHF, as assessed by the clinician investigator, were prospectively enrolled in 358 centres from 44 countries between 23 July 2014 and 24 March 2017. This study was conducted in accordance with the Declaration of Helsinki,14 and the protocol received institutional review board and/or ethics committee approval at each participating centre. Patients provided written informed consent.
Heart failure with reduced ejection fraction (HFrEF) was defined as a left ventricular ejection fraction (LVEF) ≤40%, heart failure with mid-range ejection fraction (HFmrEF) was defined as an LVEF between 41–50%, and heart failure with preserved ejection fraction (HFpEF) was defined as an LVEF ≥50% according to current guidelines.15 Medication data were captured at discharge, including doses and units.
The time of first administration of IV diuretics for AHF and dose were recorded; the time of arrival at the hospital was similarly captured. The difference between the time of arrival in the hospital and time of administration was used to calculate time-to-diuretics. Patients who received only oral diuretics or lacked information on the timing of IV treatment were excluded from this analysis. We only included patients with time-to-diuretics from time of admission up to 24 h for the primary analysis. In secondary analyses, we investigated the association between receiving IV furosemide before admission to the hospital (presented as a negative time-to-diuretics) and receiving IV furosemide >24 h after hospital admission with outcomes.
Outcomes
The primary outcomes of this study were in-hospital and 30-day all-cause mortality. Mortality was prospectively captured during the index hospitalization and during the 30-day follow-up period from clinic visits, phone follow-up visits or death records, as described previously.13 Patients were considered lost to follow-up if no information could be obtained on vital status.
Statistical analysis
Data were expressed as median with quartiles for all data. Categorical data were expressed as numbers with percentage. Group differences were evaluated using analysis of variance and Kruskal–Wallis test for continuous variables and Chi-square or Fisher exact tests for categorical variables. We compared clinical characteristics and outcomes related to time-to-diuretics from arrival to the hospital to 30 min, 30 min–1 h; 1–6 h, 6–12 h, 12–24 h. In secondary analysis, we included patients receiving IV diuretics; prior to hospital arrival, at 24–48 h, and ≥48 h. The association between time-to-diuretics and in-hospital or 30-day all-cause mortality was analysed using generalized estimating equation (GEE) models accounting for intra-facility correlations.
First, we modelled the association between time-to-diuretics and mortality on a continuous scale by applying GEE models to natural cubic splines of 1 to 5 knots to model the possible non-linear association of time-to-diuretics with the outcome. The optimal number of knots was determined by the lowest value of the quasi-likelihood information criterion (QIC), a modification of the Akaike information criterion designed for GEE models.16 We corrected for treatment indication bias using established methods including multivariable analyses, propensity score matching (PS)17-19 and inverse probability weighting (IPW)20 in our models. The multivariable models consisted of age, sex, ischaemic aetiology, valvular heart disease, chronic obstructive pulmonary disease/asthma, smoking, cardiac resynchronization therapy, LVEF category (HFrEF/HFmrEF/HFpEF), IV inotropes and IV vasodilators. IV inotropes and IV vasodilators were recorded as not administered, administered before or after IV diuretic administration. IPW weights and propensity scores were determined by performing LASSO penalized logistic regression for patients receiving IV diuretics within and after 1 h using all variables presented in online supplementary Table S1. For the LASSO regression analyses, independent continuous variables were transformed into tertiles. Because REPORT-HF was designed to assess differences in global practice patterns, missingness was often not at random. Therefore, if variables had missing data, a separate factor level with ‘missing’ was included. We used a 10-fold cross validation approach with 1000 bootstrap samples. Because results of the multivariable, PS and IPW models were similar (online supplementary Figure S1), we only report the IPW results. To assess how well our models for treatment indication bias correction predicted time-to-diuretics, we calculated the C-index.
Second, we stratified patients according to treatment intervals (0–30 min, 30 min–1 h; 1–6 h, 6–12 h and 12–24 h). In secondary analyses, we included patients receiving IV diuretics prior to hospital arrival or >24 h. The reference group in all analyses was the group who received IV diuretics within 0–30 min, according to the recommendations of the European Society of Cardiology regarding early and pre-hospital management of AHF.1
Because a previous publication found a modifying effect of baseline patient mortality risk,9 we tested whether the ADHERE risk score21 or MAGGIC risk score22 modified the association between time-to-diuretics and mortality. We combined patients with intermediate-2, intermediate-1, and high ADHERE scores because of the small sample size (n = 1657, n = 303 and n = 467, respectively). In addition, we tested whether LVEF and geographic region modified the association between time-to-diuretics and mortality using an interaction test. We tested for interaction by comparing the goodness-of-fit between models with and without the interaction term to determine the overall p-value for interaction. We considered a two-tailed p-value <0.05 as statistically significant. Statistical analyses were performed using R, version 4.0.2 (R Foundation for Statistical Computing, Vienna, Austria).
Results
Baseline characteristics
Figure 1 shows that out of 18 553 patients included in REPORT-HF, 1571 (8.5%) patients receiving only oral diuretics and 665 (3.6%) patients missing time-to-diuretic data were excluded from further analyses. Overall, 15 078 (92%) patients receiving IV diuretics between hospital arrival and 24 h after hospital arrival were considered in the primary analysis. Out of a total of 15 078 patients with data on time-to-diuretics, 367 died in-hospital. Among 14 711 discharged alive, 364 (2.5%) had missing data on 30-day mortality.
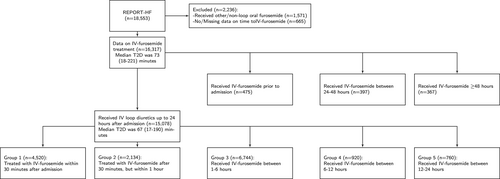
The median time-to-first IV diuretics was 67 (25th–75th percentiles 17–190) min. Online supplementary Figure S2 shows no differences in furosemide dose among time-to-diuretic groups. The model to predict time-to-diuretics had a C-index of 0.68. The median dose was 40 (25th–75th percentiles 40–80) mg in all five diuretic groups mentioned in Figure 1. There was a small but significant negative correlation between time-to-diuretics and total daily dose (Spearman's ρ = −0.039, p < 0.0001).
The in-hospital time-to-diuretics ranged from 28 (25th–75th percentiles 1–99.5) min in Eastern Europe to 217 (25th–75th percentiles 119–383) min in North America (online supplementary Table S2). When correcting for differences in age, sex, comorbidities and signs and symptoms, differences between regions remained significant. Table 1 shows that patients with shorter time-to-diuretics were more often women, had worse signs and symptoms at hospital arrival, more often HFpEF, had diabetes and a lower estimated glomerular filtration rate. Online supplementary Table S3 shows the differences between patients included and excluded from analyses. Online supplementary Table S4 show the differences between patients with time-to-diuretics prior to hospital arrival, between 0–24 h and 24 h after hospital arrival.
| 0–30 m | 30 m-1 h | 1–6 h | 6–12 h | 12–24 h | p-value | |
|---|---|---|---|---|---|---|
| n | 4520 | 2134 | 6744 | 920 | 760 | |
| Female sex | 1842 (40.8%) | 855 (40.1%) | 2533 (37.6%) | 386 (42%) | 276 (36.3%) | 0.001 |
| Age (years) | 67 (58–76) | 67 (57–77) | 67 (57–77) | 68 (58–78) | 68 (57–78) | 0.029 |
| Race | <0.0001 | |||||
| White | 2470 (54.6%) | 1046 (49%) | 3471 (51.5%) | 505 (54.9%) | 415 (54.6%) | |
| Black | 58 (1.3%) | 61 (2.9%) | 506 (7.5%) | 94 (10.2%) | 40 (5.3%) | |
| Asian | 1271 (28.1%) | 755 (35.4%) | 1957 (29%) | 202 (22%) | 213 (28%) | |
| Native American | 139 (3.1%) | 33 (1.5%) | 123 (1.8%) | 16 (1.7%) | 16 (2.1%) | |
| Pacific Islander | 2 (0%) | 0 (0%) | 4 (0.1%) | 0 (0%) | 1 (0.1%) | |
| Other | 580 (12.8%) | 239 (11.2%) | 683 (10.1%) | 103 (11.2%) | 75 (9.9%) | |
| Region | <0.0001 | |||||
| Central and South America | 769 (17%) | 317 (14.9%) | 937 (13.9%) | 150 (16.3%) | 121 (15.9%) | |
| Eastern Europe | 1259 (27.9%) | 359 (16.8%) | 780 (11.6%) | 51 (5.5%) | 71 (9.3%) | |
| Eastern Mediterranean region and Africa | 658 (14.6%) | 308 (14.4%) | 847 (12.6%) | 75 (8.2%) | 36 (4.7%) | |
| North America | 45 (1%) | 78 (3.7%) | 966 (14.3%) | 228 (24.8%) | 98 (12.9%) | |
| South East Asia | 730 (16.2%) | 327 (15.3%) | 762 (11.3%) | 59 (6.4%) | 66 (8.7%) | |
| Western Europe | 534 (11.8%) | 339 (15.9%) | 1294 (19.2%) | 213 (23.2%) | 207 (27.2%) | |
| Western Pacific | 525 (11.6%) | 406 (19%) | 1158 (17.2%) | 144 (15.7%) | 161 (21.2%) | |
| BMI (kg/m2) | 27.1 (23.8–31.24) | 26.82 (23.44–31.11) | 27.45 (23.81–32.65) | 27.67 (23.72–32.93) | 27.63 (23.88–32.28) | <0.0001 |
| NYHA class | <0.0001 | |||||
| I | 230 (7.4%) | 75 (5.6%) | 284 (7.1%) | 29 (6.4%) | 32 (8%) | |
| II | 999 (32.1%) | 347 (26%) | 1055 (26.3%) | 147 (32.3%) | 98 (24.5%) | |
| III | 1274 (40.9%) | 614 (46.1%) | 1876 (46.8%) | 199 (43.7%) | 191 (47.8%) | |
| IV | 611 (19.6%) | 297 (22.3%) | 790 (19.7%) | 80 (17.6%) | 79 (19.8%) | |
| HF category | <0.0001 | |||||
| HFrEF | 1988 (47.3%) | 1003 (52%) | 3472 (56.2%) | 442 (53.7%) | 374 (54.8%) | |
| HFmrEF | 808 (19.2%) | 345 (17.9%) | 955 (15.5%) | 117 (14.2%) | 119 (17.4%) | |
| HFpEF | 1405 (33.4%) | 580 (30.1%) | 1753 (28.4%) | 264 (32.1%) | 189 (27.7%) | |
| SBP (mmHg) | 130 (115–152) | 130 (112–152) | 130 (111–150) | 130 (112–150) | 126 (109–145) | <0.0001 |
| % with values | <0.0001 | |||||
| <100 mmHg | 277 (6.7%) | 136 (6.8%) | 501 (7.9%) | 84 (10.2%) | 77 (11.5%) | |
| 100–120 mmHg | 867 (21.1%) | 463 (23.2%) | 1543 (24.4%) | 188 (22.8%) | 188 (28.1%) | |
| 120–140 mmHg | 1184 (28.8%) | 559 (28%) | 1780 (28.2%) | 246 (29.8%) | 188 (28.1%) | |
| >140 mmHg | 1779 (43.3%) | 838 (42%) | 2488 (39.4%) | 307 (37.2%) | 216 (32.3%) | |
| Ischaemic aetiology | 1696 (37.5%) | 701 (32.8%) | 2133 (31.6%) | 249 (27.1%) | 251 (33%) | <0.0001 |
| Hypertension | 3038 (67.3%) | 1333 (62.5%) | 4298 (63.8%) | 603 (65.9%) | 470 (61.9%) | 0.0001 |
| CKD | 917 (20.3%) | 385 (18%) | 1464 (21.7%) | 220 (23.9%) | 175 (23%) | 3e-04 |
| Diabetes | 1823 (40.4%) | 789 (37%) | 2647 (39.3%) | 341 (37.1%) | 287 (37.8%) | 0.05 |
| COPD | 586 (13%) | 311 (14.6%) | 1057 (15.7%) | 158 (17.2%) | 103 (13.6%) | 3e-04 |
| Precipitant | <0.0001 | |||||
| ACS/MI | 693 (15.9%) | 251 (12.2%) | 673 (10.3%) | 89 (10.3%) | 80 (11.2%) | |
| Arrhythmia | 413 (9.5%) | 210 (10.2%) | 667 (10.2%) | 84 (9.7%) | 71 (10%) | |
| Uncontrolled hypertension | 355 (8.1%) | 152 (7.4%) | 385 (5.9%) | 52 (6%) | 18 (2.5%) | |
| Non-adherence diet/meds | 377 (8.6%) | 210 (10.2%) | 756 (11.6%) | 74 (8.5%) | 48 (6.7%) | |
| Medication WHF | 22 (0.5%) | 16 (0.8%) | 38 (0.6%) | 11 (1.3%) | 8 (1.1%) | |
| Pneumonia/infection | 392 (9%) | 234 (11.4%) | 703 (10.8%) | 85 (9.8%) | 63 (8.8%) | |
| Pulmonary embolism | 23 (0.5%) | 8 (0.4%) | 32 (0.5%) | 3 (0.3%) | 2 (0.3%) | |
| Worsening renal function | 95 (2.2%) | 40 (1.9%) | 196 (3%) | 21 (2.4%) | 11 (1.5%) | |
| Signs and symptoms | ||||||
| Fatigue | 3496 (89.4%) | 1533 (84.5%) | 4202 (80.3%) | 479 (76.4%) | 444 (78.9%) | <0.0001 |
| Pulmonary rales | 3144 (78.2%) | 1351 (73.1%) | 3711 (68.2%) | 405 (59.6%) | 332 (57.9%) | <0.0001 |
| Dyspnoea at rest | 3797 (89.8%) | 1662 (84.8%) | 4882 (83.8%) | 590 (81%) | 506 (77.8%) | <0.0001 |
| Orthopnoea | 3241 (83.9%) | 1466 (80.8%) | 4347 (80.7%) | 469 (73.9%) | 408 (71.8%) | <0.0001 |
| Peripheral oedema | 3030 (72.2%) | 1410 (72.3%) | 4402 (73%) | 565 (69.5%) | 457 (67.9%) | 0.024 |
| Elevated JVP | 1702 (61.6%) | 762 (59%) | 2554 (64.3%) | 308 (60.4%) | 247 (56.7%) | 0.001 |
| Hepatomegaly | 1120 (29.8%) | 397 (23.5%) | 1060 (21.1%) | 74 (12.1%) | 96 (18.6%) | <0.0001 |
| Medication | ||||||
| IV inotropes | 671 (14.8%) | 330 (15.5%) | 959 (14.2%) | 132 (14.3%) | 128 (16.8%) | 0.27 |
| Time to inotropes (min) | 26 (0–1252) | 81 (41–1483) | 298 (112–2580) | 492 (268–2120) | 1160 (199–4061) | <0.0001 |
| IV vasodilators | 1068 (23.6%) | 454 (21.3%) | 1164 (17.3%) | 140 (15.2%) | 103 (13.6%) | <0.0001 |
| Time to vasodilators (min) | 10 (0–27) | 46 (35–74) | 125 (70–269) | 387 (126–719) | 202 (54–1184) | <0.0001 |
| ADHERE | 0.0001 | |||||
| Low risk | 2878 (63.7%) | 1352 (63.4%) | 4113 (61%) | 553 (60.1%) | 418 (55%) | |
| Intermediate risk 3 | 1013 (22.4%) | 492 (23.1%) | 1578 (23.4%) | 213 (23.2%) | 206 (27.1%) | |
| Intermediate risk 2 | 475 (10.5%) | 199 (9.3%) | 707 (10.5%) | 101 (11%) | 96 (12.6%) | |
| Intermediate risk 1 | 54 (1.2%) | 36 (1.7%) | 147 (2.2%) | 23 (2.5%) | 17 (2.2%) | |
| High risk | 100 (2.2%) | 55 (2.6%) | 199 (3%) | 30 (3.3%) | 23 (3%) | |
| MAGGIC | 23.4 (19–27.8) | 23.6 (19.4–27.8) | 23.6 (19.2–28) | 24 (19.2–28.4) | 24.2 (19.75–28.4) | 0.001 |
| In-hospital stay (days) | 8 (5–12) | 8 (5–12) | 8 (5–12) | 8 (5–13) | 9 (6–14) | <0.0001 |
| In-hospital mortality | 109 (2.4%) | 57 (2.7%) | 159 (2.4%) | 18 (2%) | 24 (3.2%) | 0.52 |
| 30-day mortality | 119 (2.7%) | 69 (3.3%) | 222 (3.4%) | 37 (4.1%) | 16 (2.2%) | 0.06 |
- ACS, acute coronary syndrome; BMI, body mass index; CKD, chronic kidney disease; COPD, chronic obstructive pulmonary disease; HF, heart failure; HFmrEF, heart failure with mid-ranged ejection fraction; HFpEF, heart failure with preserved ejection fraction; HFrEF, heart failure with reduced ejection fraction; IV, intravenous; JVP, jugular venous pressure; MAGGIC, Meta-Analysis Global Group in Chronic Heart Failure; MI, myocardial infarction; NYHA, New York Heart Association; SBP, systolic blood pressure; WHF, who likely to worsen heart failure.
Association of time-to-first intravenous diuretics and in-hospital and 30-day mortality
During the index hospitalization, 401 (2.5%) patients died. In-hospital mortality was 2.4% in patients with time-to-diuretics <30 min after hospital arrival, rising to 3.2% for patients with time-to-diuretics 12–24 h (ptrend = 0.84). Figure 2 shows that there was no significant association between time-to-diuretics and in-hospital mortality (p = 0.99). QIC was lowest for a linear model without splines.
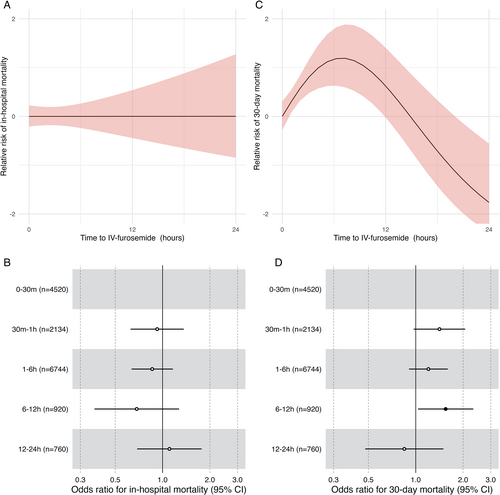
Patients who died in hospital were excluded from the analyses of 30-day post-discharge mortality. After being discharged, 493 (3.3%) died within 30 days. The proportion of patients who died within 30 days after discharge increased from 2.7% in patients with time-to-diuretics <30 min to 4.1% in patients with time-to-diuretics of 6–12 h (ptrend = 0.08). Figure 2 shows a non-linear association between time-to-diuretics and 30-day all-cause mortality. The association between time-to-diuretics and 30-day all-cause mortality increased linearly between hospital arrival and 8-h post-hospital arrival (p = 0.016).
Figure 2 present the association between time-to-diuretics according to groups and mortality outcomes. The relative risk compared to patients receiving IV diuretics 0–30 min for 30-day post-discharge mortality increased within the first 12 h. In secondary analyses, correcting for furosemide dose did not change the association of time-to-diuretics with in-hospital or 30-day post-discharge mortality.
The ADHERE risk score modified (pinteraction = 0.008) the association between time-to-diuretics and 30-day mortality (online supplementary Figure S3), such that a longer time-to-diuretics was associated with a greater relative risk of 30-day mortality in patients with a worse ADHERE risk score. We did not observe a significant interaction between time-to-diuretics and the MAGGIC risk score (pinteraction = 0.09; online supplementary Figure S3 and S4). LVEF and region did not modify the association of time-to-diuretics and 30-day mortality risk (pinteraction >0.1 for all; online supplementary Figure S5 and S5). The ADHERE or MAGGIC risk score, LVEF and region did not modify the association between time-to-diuretics and in-hospital mortality.
Secondary analyses in patients with time-to-diuretics prior to hospital arrival and >24 h after hospital arrival
Online supplementary Figure S7 shows the association of time-to-diuretics prior to hospital arrival and >24 h after hospital arrival relative to patients with time-to-diuretics between 0–30 min prior to hospital arrival. We did not observe an association between patients receiving IV diuretics prior to hospital arrival and any of the mortality outcomes. Patients with IV diuretics after 24 h had a higher relative risk for 30-day all-cause mortality than patients with IV diuretics 0–30 min after hospital arrival.
Discussion
In REPORT-HF, 41% of patients received IV diuretics within 1 h after hospital arrival. Women, patients with more signs and symptoms of heart failure, and more comorbidities had shorter time-to-diuretics. There were notable geographic differences in time-to-diuretics, independent of patient characteristics. Importantly, despite lack of association between time-to-diuretics and in-hospital mortality, we found an association between time-to-diuretics and 30-day mortality, such that longer time-to-diuretics (administered within the first 12 h post hospital arrival window of time) was associated with a higher 30-day mortality risk. This risk was enhanced in high-risk patients and was independent of LVEF or geographic region.
Several previous studies investigated the association of time-to-diuretics with in-hospital and post-discharge outcomes (Table 2).5-9 These studies had different inclusion criteria, were exclusively from single high-income countries6-9 or single centres,9 and were limited by sample size,9 or retrospective design.6, 9 Differences in national healthcare systems and standards for diagnosis of AHF may impact the threshold for hospital arrival23-25 and the effect of time-to-diuretics on mortality,2, 10, 26, 27 limiting the generalizability of single country or single centre studies. The differences in design of previous studies have likely contributed to conflicting results on the association between time-to-diuretics and mortality.6-9 We extend and complement previous work6-9 by investigating the association of time-to-diuretics with mortality in (i) a large multicentre cohort of patients with AHF with global representation, (ii) with a prospective study design, and (iii) comprehensive information on time-to-diuretics according to a single standardized protocol used globally.
| Ward et al.9 | Wong et al.6 | Maisel et al.5 | Matsue et al.7 | Park et al.8 | Ouwerkerk and Tromp et al. | |
|---|---|---|---|---|---|---|
| Country/region | United States | United States | United States | Japan | South Korea | 44 countries |
| n | 695 | 6971 | 58 465 | 1291 | 2761 | |
| Study design | Single centre, observational, retrospective | Multicentre, Medicare claims | Multicentre, Medicare claims | Multicentre, observational, prospective | Multicentre, observational, prospective | Multicentre, observational, prospective |
| Inclusion | Primary diagnosis of AHF with NP measured | Age ≥65 years, diagnosis from ICD coding | Age >18 years, diagnosis from ICD coding admitted through the ED | Diagnosis based on Framingham criteria, BNP ≥100 pg/ml or NT-proBNP ≥300 pg/ml | Signs and symptoms, LV dysfunction or structural heart disease | Age ≥18 years, primary diagnosis of AHF |
| Time-to-diuretics definition | Any | Any | Any | <24 h, time to first therapy | <24 h | Any |
| Patient characteristics | ||||||
| Age (years) | 66 | 81 | 65 | 79 | 70 | 67 |
| Women | 49 | 55 | 53 | 56 | 51 | 61 |
| NYHA class III/IV | NR | NR | NR | 82 | 90 | 40 |
| Peripheral oedema | NR | NR | 66 | 71 | NR | 71 |
| Rales | NR | 65 | 71 | 69 | 87 | 70 |
| ADHERE risk | ||||||
| Low | 66 | NR | NR | NR | NR | 61 |
| Intermediate | 25 | NR | NR | NR | NR | 36 |
| High | 9 | NR | NR | NR | NR | 3 |
| Outcome | ||||||
| Time-to-diuretics (median IQR) | 166 (110–241) | 138 (66–264) | NR | 90 (36–186) | 128 (63–243) | 73 (18–221) |
| In-hospital mortality | 5% | 3.50% | 3.50% | 5% | 5% | 3% |
| 30-day mortality | NR | 9.3% | NR | NR | 3% | 3% |
| Main result | ||||||
| In-hospital | Yes | Yes | Yes | Yes | No | No |
| 30-day post-discharge | NR | No | NR | No | No | Yes |
| Interaction severity | Yes | NR | NR | No | No | Yes |
- AHF, acute heart failure; BNP, B-type natriuretic peptide; ED, emergency department; ICD, International Classification of Diseases; IQR, interquartile range (25th–75th percentiles); LV, left ventricular; NP, natriuretic peptide; NR, not reported; NT-proBNP, N-terminal pro-B-type natriuretic peptide; NYHA, New York Heart Association.
The median time-to-diuretics of patients receiving diuretics between hospital arrival and 24 h was 67 min in REPORT-HF. This was shorter than previously reported.6-9 The median time-to-diuretics in previous studies ranged from 90 min in Japan up to 166 min in the United States, with fewer signs of congestion in patients from the United States than Japan7, 9 (Table 2). More signs of congestion predicted shorter time-to-diuretics in the present and previous studies,7, 9 suggesting that symptom severity might compel physicians to start diuretics earlier. In REPORT-HF, there were notable geographic differences in time-to-diuretics, independent of signs and symptoms, suggesting that the threshold to time-to-diuretics was determined by local healthcare practices more than by patient characteristics.
Longer time-to-diuretics was not associated with an increased risk of in-hospital mortality in our study, consistent with results from a prospective registry from South Korea.8 However, because participants in REPORT-HF had to sign informed consent, the in-hospital mortality rate was relatively low (2.4%).12 The limited number of in-hospital deaths may have contributed to the lack of association between time-to-diuretics and in-hospital mortality. Other studies from the United States and Japan found an association between in-hospital mortality and time-to-diuretics.6, 7, 9, 28 These conflicting results might be explained by the need for patients to sign informed consent in REPORT-HF, leaving some sicker, higher risk patients excluded from the registry. Furthermore, recent evidence suggests that time-to-decongestion rather than time-to-diuretics is associated with mortality.29 Time-to-diuretics, in the present study, was associated with a higher risk for post-discharge 30-day mortality. A previous study in the United States found that each hour of delay in IV administration of furosemide was
associated with an 8% lower chance of being discharged to home, highlighting the important downstream effects of IV diuretics administration in the emergency department.9 However, despite our best efforts to correct for treatment indication bias, there was likely residual confounding. For instance, higher ADHERE scores might reflect more severe underlying patient frailty, and the interaction with time-to-diuretics may reflect adverse effects in frailer patients. Yet in the absence of large scale prospective randomized controlled trials, our results from a large global cohort may be the best available evidence to address the important question of whether timing matters in the administration of diuretics to patients with acute decompensated heart failure. Our findings suggest that the association between earlier time-to-diuretics and mortality likely reflects a combination of early recognition of congestion in those with more severe symptoms/signs, less delay in medical decision-making, and the potential beneficial effects of early decongestion.
Several previous studies did not find an association between IV diuretics and post-discharge mortality.6-8 In REPORT-HF, baseline mortality risk modified this association between time-to-diuretics and 30-day mortality, such that this association was stronger in high-risk than low-risk patients. The shorter time-to-diuretics in REPORT-HF compared to other studies suggests that patients in the present study were more congested. A second reason possibly explaining the conflicting results is the non-linear association of time-to-diuretics with post-discharge mortality in the present study. Previous studies divided patients according to early versus late7, 8 time-to-diuretics or used time-to-diuretics on a linear scale,6 which might have masked an underlying association. The fact that region did not modify the association between time-to-diuretics and 30-day mortality suggests that our results are consistent regardless of geographic region or healthcare system.
Limitations
REPORT-HF is reflective of real-world practice and shows variations determined by locally available resources, skills and practice guidelines. We did not randomly sample countries or clinical sites within a country for practical reasons. Therefore, our results likely represent a best-case scenario of clinical care in many countries. The registry required patients to consent to use their data and follow-up. Patients who could not provide consent could not participate, which likely explains our low index hospitalization mortality. Also, patients who were treated with IV diuretic prior to hospitalization or died during transport, could not be included in the study. These patients were most likely high-risk patients, which further reduced in-hospital mortality. Diuretic administration registration might not reflect the provided dosage and time. Therefore, there might be a difference in the registered diuretic dose and time and those in real-world clinical practice. REPORT-HF did not capture data on the time of rehospitalization, nor signs and symptoms in all patients over time. Therefore, we could not investigate the association between time-to-diuretics and rehospitalization or correct for differences in decongestion due to missing data. Selection bias probably led to younger patients with fewer comorbidities and a better prognosis being enrolled. No time to event data was available for hospitalizations. Therefore, we did not include this outcome in our analyses.
Conclusions
Our findings, obtained in a large prospective observational study with global representation, suggest that there is a window of time-to-diuretics where patients are at increased risk for post-discharge mortality. This association was stronger in patients at a higher baseline mortality risk. We did not see an association between time-to-diuretics and in-hospital mortality.
Funding
Jasper Tromp is supported by the National University of Singapore start-up grant, the tier 1 grant from the Singapore Ministry of Education, and the CS-IRG New Investigator Grant from the National Medical Research.
REPORT-HF was funded by Novartis.
Conflict of interest: J.T. has received personal grants and speaker fees from Roche Diagnostics, Us2.ai, Daiichi Sankyo and Boehringer Ingelheim. C.E.A. reports grants, personal fees and other from Novartis; lecture, advisory board or editorship fees and/or committee memberships in trials and/or registries sponsored by Abbott, Boehringer Ingelheim, Medtronic, ResMed, Servier, Springer, Vifor; she further acknowledges non-financial support from the University Hospital Würzburg, non-financial support from the Comprehensive Heart Failure Center Würzburg and grant support from the German Ministry for Education and Research (BMBF). U.D. reports research support from Astra Zeneca, Pfizer, Boehringer Ingelheim, Vifor, Roche Diagnostics, Boston Scientific and speaker's honoraria and consultancies from AstraZeneca, Novartis and Amgen. M.H. received honoraria as a lecturer from Novartis, Aventis, Amgen, MSD, AstraZeneca and Merck. M.G. was formerly Novartis employee. A.S. and A.O. are employed by Novartis. G.F. reports research grants from the European Union. Committee fees from Novartis related to REPORT-HF; lecture fees and/or committee member in trials and/or registries sponsored by Servier, Boehringer Ingelheim, Medtronic, Vifor, Amgen, Bayer. S.P.C. reports research grants from NIH, AHRQ, AHA, PCORI and consulting fees from Novartis, Medtronic, Vixiar and Ortho Clinical. J.G.F.C. reports grants and personal fees from Abbott, Amgen, Bayer, Bristol Myers Squibb, Stealth Biopharmaceuticals, Torrent Pharmaceuticals, personal fees from AstraZeneca, Myokardia, Sanofi, Servier, grants, personal fees and non-financial support from Medtronic, Novartis, grants and personal fees from Philips, grants and non-financial support from Pharmacosmos, PharmaNord, personal fees and non-financial support from Vifor. C.S.P.L. is supported by a Clinician Scientist Award from the National Medical Research Council of Singapore; has received research support from Bayer and Roche Diagnostics; has served as consultant or on the Advisory Board/Steering Committee/Executive Committee for Actelion, Amgen, AnaCardio AB, Applied Therapeutics, AstraZeneca, Bayer, Boehringer Ingelheim, Boston Scientific, Cytokinetics, Darma Inc., EchoNous Inc, Impulse Dynamics, Ionis Pharmaceutical, Janssen Research & Development LLC, Medscape/WebMD Global LLC, Merck, Novartis, Novo Nordisk, Prosciento Inc, Radcliffe Group Ltd., Roche Diagnostics, Sanofi and Us2.ai; and serves as co-founder & non-executive director of Us2.ai. All other authors have nothing to disclose.




