Trial characteristics associated with under-enrolment of females in randomized controlled trials of heart failure with reduced ejection fraction: a systematic review
Abstract
Aims
To evaluate temporal trends in the enrolment of females in randomized controlled trials (RCTs) of heart failure with reduced ejection fraction (HFrEF) published in high-impact journals, and assess RCT characteristics associated with under-enrolment.
Methods and results
We searched MEDLINE, EMBASE and CINAHL for studies published from January 2000 to May 2019 in journals with impact factor ≥10. We included RCTs that recruited adults with HFrEF. We used a 20% threshold below the sex distribution of HFrEF to define under-enrolment. We used multivariable logistic regression to assess trial characteristics independently associated with under-enrolment. We included 317 RCTs. Among the 183 097 participants, mean (standard deviation) age was 63.0 (7.0) years and 25.5% were female. Females were under-enrolled in 71.6% [95% confidence interval (CI) 66.6–76.6%] of the RCTs; enrolment did not increase significantly between 2000–2019. Sex-related eligibility criteria [odds ratio (OR) 2.05, 95% CI 1.01–4.16; P = 0.046]; recruitment in ambulatory settings (OR 2.56, 95% CI 1.37–4.81; P = 0.003); trial coordination in North America (OR 4.44, 95% CI 1.09–18.07; P = 0.037), Europe (OR 6.79, 95% CI 1.63–27.39; P = 0.018) and Asia (OR 9.33, 95% CI 1.40–12.40; P = 0.033); drug (OR 1.76, 95% CI 1.96–7.36; P < 0.001) and device/surgical interventions (OR 1.69, 95% CI 1.16–9.43; P = 0.002); and men in first and last authorship position (OR 1.32, 95% CI 1.12–3.54; P = 0.047) were associated with under-enrolment of females.
Conclusions
Females were under-enrolled relative to disease distribution in a majority of high-impact HFrEF RCTs, with no change in temporal trends between 2000 and 2019. Trial characteristics and gender of trial leaders were associated with under-enrolment.
Graphical Abstract
Introduction
Well-designed randomized controlled trials (RCTs) are the gold standard for informing clinical practice in heart failure (HF).1 However, RCTs often do not enrol participants that represent the patient population in whom the interventions will be applied.2, 3 The efficacy and safety of an intervention cannot be assumed to apply to populations that are not adequately represented in RCTs. The historical under-enrolment of females in HF RCTs has raised concerns about the generalizability of medical evidence in half the world's population.2-5
There are sex-specific differences in the aetiology, comorbidities, and metabolism of drugs in HF. The optimal dosing of drugs and adverse effects may thus be different in males and females.6-8 While there is no clear evidence from RCTs that there are sex-specific differences in treatment response to therapies used in HF, observational data suggest that this may be the case.9, 10 Interventions that have proven efficacious in trials with primarily male participants have been shown in subsequent observational studies to have unexpected adverse effects in females.11-13 Both sexes must be represented proportionate to sex distribution of the disease to improve RCT generalizability and ensure a sufficient sample size to investigate sex-specific treatment effect and safety.
There has been a revolution of practice changing RCTs in HF with reduced ejection fraction (HFrEF), and this has substantially improved treatment and outcomes in this high-risk condition.14-16 While under-enrolment of females has been reported in these trials,5-13, 17 the reasons for this have not been explored. It is possible that clinical trial design itself may play a role in the disproportionate enrolment of sexes relative to disease distribution, but this has not been investigated.
In this systematic review, we describe temporal trends in the enrolment of females in RCTs of HFrEF published in high-impact journals, determine trial characteristics that are independently associated with under-enrolment of females relative to the sex-specific distribution of HFrEF, and make recommendations to improve the enrolment of females in RCTs of HFrEF.
Methods
Registration
This study is registered in the International Prospective Register of Systematic Reviews (PROSPERO). Our study and the reporting followed Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines.18
Information sources and search strategy
Guided by a professional information specialist, we (S.W. and H.V.). developed our main search strategy in MEDLINE. The search strategy for MEDLINE is available in the online supplementary Appendix S1. In collaboration with the professional information specialist, one of the authors (S.W.) conducted a systematic search of the literature in MEDLINE, EMBASE, and CINAHL. We (S.W. and K.S.) hand searched the reference lists of included articles and relevant systematic reviews to ensure literature saturation.
Eligibility criteria
We included RCTs published in the English language between 1 January 2000 and 7 May 2019. We included studies that recruited adult patients (≥18 years old) with HFrEF. To identify studies more likely to inform clinical practice, we included full-text manuscripts reporting primary results that were published in journals that received an impact factor of ≥10 in the 2019 report.19 The impact factor threshold of 10 was empirically chosen. We included RCTs in which the median ejection fraction was in the reduced range (≤40%),20 and in which greater than 50% of patients had an ejection fraction ≤40%. We did not include trials with HF with preserved ejection fraction (HFpEF) as the sex distribution in this condition is different from HFrEF and trial publications pertaining to HFpEF were clustered in the last quarter of the study period (two-thirds were published in 2015–2019). We excluded studies with methodological designs other than RCTs, those with sex-specific interventions, those that did not report ejection fraction, and protocols. We excluded secondary publications subsequent to the one that described the RCT's primary outcomes; thus, we excluded publications on secondary, subgroup, or exploratory analyses.
Four authors (S.W., K.S., M.A., and Y.E.) independently screened all titles and abstracts from the original search against the predefined eligibility criteria, and reviewed full-text versions of studies that either appeared to meet the inclusion criteria or had insufficient information in the title and abstract to make a decision. Screening and decision-making for inclusion were performed in duplicate (S.W., K.S., M.A., and Y.E.). Disagreements were resolved through discussion, and when required, by consulting a third author (H.V.). We recorded the rationale for excluding studies that underwent full-text screening.
Data abstraction and management
Two authors (S.W. and H.V.) selected variables for extraction. Four authors (S.W., K.S., M.A., and Y.E.) independently extracted the following information in duplicate: year of publication, journal impact factor, region of trial coordination centre (i.e. where critical functions such as coordination of participant accrual sites, randomization, and data processing occur),21 sample size, average age of participants, number and percentage of females, sex-related eligibility criteria such as child-bearing potential or menopausal status, location of recruitment, type of consent, type of intervention, level of randomization, type of follow-up, sex-specific reporting of study flow, scope of trial, number of centers, funding type, and gender of first author, last author, and corresponding author. Any disagreements in data extraction were resolved by discussion and consultation with a third reviewer resolved any discrepancies.
Analysis
We presented continuous variables as mean and standard deviation (SD), and categorical variables as numbers and percentages. Using the Framingham cohort, the Get With the Guidelines HF registry, Cardiology Practice Quality Project registry, and the Change the Management of Patients with Heart Failure registry, we estimated the male:female distribution of HFrEF to be 60:40.22-27 We defined under-enrolment of females as a trial participation to proportion of females with HFrEF <0.8.22, 23, 28 Thus, trials that enrolled <32% females were classified as having under-enrolled females. We used logistic regression to determine independent factors associated with under-enrolment of females. The factors under consideration included region, location of recruitment, number of centres, eligibility criteria, type of intervention, type of funding, and gender of first and last authors. We reported odds ratios (ORs), corresponding 95% confidence intervals (CIs), and associated P-values. All P-values were two-tailed, and the level of significance was set at alpha = 0.05. Data were analysed using SPSS (version 23; IBM Corporation, Armonk, NY, USA).
Results
Identification, screening and selection of studies
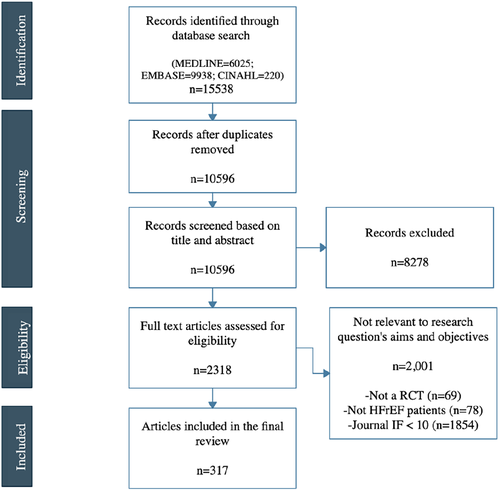
Our systematic search produced 10 596 unique articles, of which 8278 were excluded on the basis of title and/or abstract review. We assessed 2318 full-text articles, of which 317 met eligibility criteria (Figure 1).
Characteristics of included randomized controlled trials
Among 183 097 participants represented in the 317 RCTs, mean (SD) age was 63.0 (7.0) years, and 25.5% were female. The median number of trial participants was 130 (interquartile range 40–407). Most RCTs were conducted in Europe (54.3%), limited to a single country (69.4%), and multi-centre (57.1%). A majority recruited patients in the ambulatory setting (78.2%) and tested drug interventions (66.9%). All (100%) RCTs obtained informed consent and reported eligibility criteria; none (0.0%) recorded the sex breakdown of patients screened, excluded, consented, withdrawn, or lost to follow-up. A vast majority of trials randomized patients at the individual level (99.1%) and required face-to-face follow-up (98.4%). The first authors (84.2%), last authors (88.3%) and corresponding authors (89.2%) were commonly men (Table 1).
| Clinical trial characteristic | No. (%) of trials |
|---|---|
| Unit of randomization | |
| Individual | 314 (99.1) |
| Cluster | 3 (1.0) |
| Type of consent, informed | 317 (100) |
| Region of the coordinating centre | |
| North America | 122 (38.5) |
| Central and South America | 10 (3.2) |
| Australia | 3 (1.0) |
| Asia | 10 (3.2) |
| Europe | 172 (54.3) |
| Eligibility criteria reported | 317 (100) |
| Sex-specific eligibility criteria | |
| Present | 81 (25.6) |
| Absent | 236 (74.4) |
| Recruitment | |
| Inpatient | 69 (21.8) |
| Ambulatory | 248 (78.2) |
| Type of intervention | |
| Health service | 27 (8.5) |
| Drug | 212 (66.9) |
| Device | 46 (14.5) |
| Surgery | 8 (2.5) |
| Exercise/rehabilitation | 24 (7.6) |
| No. of centres | |
| Single-centre | 136 (42.9) |
| Multi-centre | 181 (57.1) |
| Type of follow-up | |
| Face-to-face | 312 (98.4) |
| Database | 5 (1.6) |
| Scope of the trial | |
| National | 220 (69.4) |
| International | 97 (30.6) |
| Type of funding | |
| Public | 138 (43.5) |
| Industry | 131 (41.3) |
| Public and Industry | 48 (15.1) |
| No. of participants | |
| <100 | 149 (47.0) |
| 100–500 | 107 (33.8) |
| >500 | 61 (19.2) |
| Gender of first author | |
| Man | 267 (84.2) |
| Woman | 50 (15.8) |
| Gender of last author | |
| Man | 280 (88.3) |
| Woman | 37 (11.7) |
| Gender of corresponding author | |
| Man | 283 (89.2) |
| Woman | 34 (10.7) |
| Year of publication | |
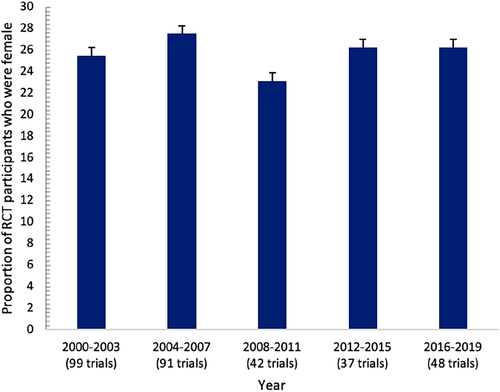
| 2000–2003 | 99 (31.2) |
| 2004–2007 | 91 (28.7) |
| 2008–2011 | 42 (13.2) |
| 2012–2015 | 37 (11.7) |
| 2016–2019 | 48 (15.1) |
As many as 47 RCTs (14.8%) used gender (man/woman, a psychosocial construct) rather than biological sex (male/female) terminology; this included 14.8% of health service, 15.6% of drug, 10.9% of device, 25.0% of surgery, and 12.5% of exercise/rehabilitation trials.
Enrolment of females
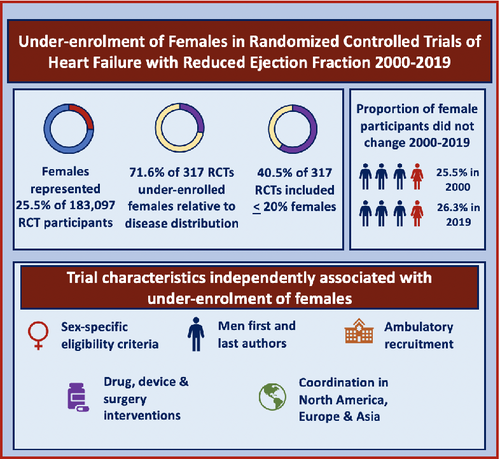
Females represented 25.5% of the enrolled participants with HFrEF (n = 46 657 of 183 097, ranging from 4% to 68% in each trial) in the 317 RCTs. As many as 227 of the 317 RCTs (71.6%; 95% CI 66.6–76.6%) under-enrolled females and 128 (40.5%) RCTs enrolled 20% or fewer females. The proportion of trials that under-enrolled females remained similar from 2000–2003 (25.5%) through to 2016–2019 (26.3%) (Figure 2).
Sex-related eligibility criteria
Of the 317 included RCTs, 81 (25.6%) used sex-related eligibility criteria; among these, none (0.0%) provided a rationale for the sex-related eligibility criteria and 66 (81.5%) under-enrolled females. Mean enrolment of females in RCTs with sex-related eligibility criteria was 23.3% (ranging from 4.2% to 66.1% in each RCT). The sex-related eligibility criteria included in the trials typically related to childbearing, lactation, or menopausal status (Table 2).
| Sex-related eligibility criteria reported in 81 RCTs | No. (%) of 81 trials reporting the sex-related criterion | No. (%) of trials that under-enrolled females/number reporting the criterion |
|---|---|---|
| Must be confirmed post-menopausal | 16 (19.8) | 14/16 (87.5) |
| Must be without childbearing potential based on surgical treatment | 17 (21.0) | 16/17 (94.1) |
| Must not be pregnant | 61 (75.3) | 51/61 (83.6) |
| Must not be lactating or nursing | 26 (32.1) | 18/26 (69.2) |
| Must not have a desire to become pregnant during the study period | 8 (9.9) | 6/8 (75.0) |
| Must be on a scientifically accepted method of contraception | 35 (43.2) | 29/35 (83.0) |
| Must not be of childbearing age | 4 (4.9) | 3/4 (75.0) |
- RCT, randomized controlled trial.
Multivariable analysis of trial characteristics associated with under-enrolment of females
Sex-related eligibility criteria (OR 2.05, 95% CI 1.01–4.16; P = 0.046); recruitment in ambulatory settings (OR 2.56, 95% CI 1.37–4.81; P = 0.003); trial coordination in North America (OR 4.44, 95% CI 1.09–18.07; P = 0.037), Europe (OR 6.79, 95% CI 1.63–27.39; P = 0.018) and Asia (OR 9.33, 95% CI 1.40–12.40; P = 0.033); drug (OR 1.76, 95% CI 1.96–7.36; P < 0.001) and device/surgery interventions (OR 1.69, 95% CI 1.16–9.43; P = 0.002); and men in first and last authorship position (OR 1.32, 95% CI 1.12–3.54; P = 0.047) were independently associated with under-enrolment of females.
Number of centres (OR multi-centre vs. single-centre: 1.17, 95% CI 0.62–2.20; P = 0.640) and type of funding (OR industry vs. public funding: 0.89, 95% CI 0.49–1.87; P = 0.890) were not associated with under-enrolment of females (Table 3).
| Variable | OR (95% CI) | P-value |
|---|---|---|
| Region | ||
| Other | 1.00 (Reference) | – |
| North America | 4.44 (1.09–18.07) | 0.037 |
| Europe | 6.79 (1.63–27.39) | 0.018 |
| Asia | 9.33 (1.40–12.40) | 0.033 |
| Sex-specific eligibility criteria | ||
| Absence | 1.00 (Reference) | – |
| Presence | 2.05 (1.01–4.16) | 0.046 |
| Recruitment location | ||
| Inpatient | 1.00 (Reference) | – |
| Ambulatory | 2.56 (1.37–4.81) | 0.003 |
| Type of intervention | ||
| Other | 1.00 (Reference) | – |
| Drug | 1.76 (1.96–7.36) | <0.001 |
| Device/surgery | 1.69 (1.16–9.43) | 0.002 |
| No. of centers | ||
| Single-centre | 1.00 (Reference) | – |
| Multi-centre | 1.17 (0.62–2.20) | 0.640 |
| Type of funding | ||
| Public | 1.00 (Reference) | – |
| Industry | 0.89 (0.49–1.87) | 0.890 |
| Gender of author | ||
| Woman first or last author | 1.00 (Reference) | – |
| Man first and last author | 1.32 (1.12–3.54) | 0.048 |
- CI, confidence interval; OR, odds ratio.
Discussion
In this systematic review of 317 RCTs of HFrEF published in high-impact journals, only 25.5% of the 183 097 trial participants were female (Graphical abstract). As many as 71.6% of trials under-enrolled females, with less than 80% enrolment relative to the proportion of patients with HFrEF who are female. More than 40% of trials enrolled 20% or fewer females. The proportion of enrolled females did not increase significantly between 2000 and 2019. None of the trials reported the sex breakdown of potential participants who were screened, excluded, and consented, making it difficult to establish patient-level reasons for under-enrolment. Among trial characteristics, sex-related eligibility criteria, recruitment in ambulatory settings, trial coordination in North America, Europe and Asia, drug and device/surgery interventions, and trial leadership by men were independently associated with under-enrolment of females. Number of centres and source of funding were not associated with under-enrolment of females.
Females remain under-enrolled in RCTs of HFrEF with no improvement over time despite recommendations for their inclusion by the National Institutes of Health Revitalization Act in 1992,29 the US Food and Drug Administration guideline in 1993,6 the European Medicines Agency guideline in 2005,30 and the Canadian Institutes of Health Research guideline in 2010.31 Our results are consistent with prior publications that demonstrated <25% enrolment of females in trials of HFrEF.5, 32 Under-enrolment deprives females of the benefits of clinical trial participation. Trial participants who are assigned to either intervention or placebo groups have fewer adverse effects and lower mortality rates than those of eligible non-participants.33-39 There are sex-related differences in presentation and treatment response in many conditions, but without a sufficient number of females in RCTs, trials are underpowered to detect sex interactions.9-14 We are left to rely on hypothesis-generating observational studies to generate sex-related data.40
Sex-related exclusion criteria – associated with twice the odds of under-enrolment of females in our study – were originally formulated to protect pregnant females and fetuses from the potential harms of early-phase drug trials.41 While these criteria are justified for some interventions, they have often been applied broadly and without justification in the context of individual trials.6, 42, 43 The decision to exclude pregnant or lactating females from RCTs should involve careful consideration of the intervention and comparator groups, with risk assessment informed by biological plausibility and preceding research data.23, 29-31 Excluding patients who represent the population treated in clinical settings may leave future patients susceptible to unintended harm from inappropriate generalization of trial results. Many interventions can be safely studied within the monitored setting of an RCT, which typically involves closer follow-up than in routine clinical settings. To reduce the unjustified exclusion of females, we suggest that sex-related eligibility criteria be restricted to interventions that are likely to produce harm to the mother or fetus based on scientific rationale, biological plausibility, or preliminary data.22, 23, 29 We recommend that Methods sections provide the rationale for sex-related eligibility criteria. When justifiable eligibility criteria disproportionately exclude females relative to males, we recommend targeted efforts directed towards female patients, their support networks, and research personnel to engage patients that do satisfy eligibility criteria.44
Ambulatory settings may present barriers to trial participation, although the reason for this is not clear and merits further study. Possibilities include sex-related referral bias for trial participation in ambulatory settings, or lack of consent from female participants due to logistical challenges in accessing study sites.45-49 The country or continent in which a trial is coordinated is another factor that must be considered. With the rapid globalization of HF clinical trials,50-52 one must consider the local context, cultural norms, employment status of the participant, caregiver responsibilities, and cost of trial participation to optimize the enrolment of females in clinical trials. A recent analysis of 740 cardiovascular disease trials published between 2010 and 2017 found no significant difference between the enrolment of males and females based on geographic regions.53 This was inconsistent with our findings that trials coordinated in North America, Europe and Asia were associated with higher odds of under-enrolment of females. The prior study was not limited to HFrEF, encompassed all forms of cardiovascular disease, and included both RCTs and other trial designs, which may explain the difference in findings.53
The association between type of intervention and under-enrolment of female participants has not been thoroughly investigated. The differences may be multi-factorial. There is evidence that females are referred for invasive cardiac procedures less commonly than males even when indications are present54-56; this may decrease the pool from which to recruit female trial participants. Females are often more reluctant to take risks than males, and may be less likely to provide informed consent for participation in trials testing interventions that are perceived to be high-risk.45, 57, 58
We could not assess the role of consent in the under-enrolment of females as none of the trials reported the sex breakdown of participants screened and consented. It is possible that females as a group may consent less frequently to trial participation overall.57, 58 A survey of 270 post-menopausal females found that females declined study participation due to fear of adverse health effects, fear of experimental treatments, and negative experiences of other research studies.58 A multi-centre RCT of 783 participants evaluated sex-differences in willingness to participate in cardiovascular prevention trials and found that females perceived greater risk of harm from trial participation than males.59 Females with HFrEF tend to be older than their male counterparts, which may also be a contributing factor as older age is associated with a lower likelihood of informed consent.30, 58, 59 Ensuring participants are aware that clinical trials are conducted with methodological rigor and are closely monitored for safety may alleviate fears and improve enrolment.
We found an independent association between leadership of trials by men – as measured by men in first and last authorship positions – and under-enrolment of female participants. This is consistent with a recent review of 118 HF clinical trials that reported that trials authored by women enrolled higher proportions of female participants.60 It is possible women leaders of clinical trials direct more effort towards recruiting and retaining female participants via the study protocol, consent process, and follow-up plan. It is also possible that female participants are more inclined to enrol in RCTs that are known to be led by women. Our findings give pause to consider the importance of creating capacity for clinical trial leadership among women, a recognized gap in HF trials.65
Future research should assess the role of implicit bias among research personnel during recruitment. Trials should report sex-specific data on patients screened, consented, excluded, withdrawn from participation, and lost to follow-up. Reasons why males and females decline consent should be recorded so that efforts can be directed towards developing solutions to overcome barriers once they are identified. Funding agencies and journals should consider benchmarks for the enrolment of females based on the sex-distribution of diseases to qualify for funding and publication. It may be useful to engage patient partners of both sexes in research trials to help address these barriers in a patient-centred manner (Table 4).
| Title and abstract | If only one sex is included in the study, or if the results of the study are to be applied to only one sex, the title and the abstract should specify the sex of participants. |
| Introduction | Authors should report whether sex differences may be expected. |
| Methods | Authors should describe incorporation of sex into the study design, justify any sex-specific exclusion criteria of males or females, and describe sex-specific analysis. |
| Results | Reporting should include the sex-specific breakdown of patients approached, eligible, consented, and included. The sex-specific breakdown of withdrawals and losses to follow-up should be included. The sex distribution of study participants and sex-specific results should be reported. Interaction between sex and the intervention should be tested. |
| Discussion | Discussion should include the implications of sex on the results and the extent to which the results are generalizable to broader populations. If no sex-specific analyses were conducted, the rationale for the absence of analyses and the implications on generalizability should be addressed. |
| Funding and publication | Funding agencies and journals should consider benchmarks for the enrolment of females based on the sex-distribution of diseases in order to award funding and publish research. |
The Sex and Gender Equity in Research (SAGER) guidelines can be used as a framework to improve the reporting of sex-specific outcomes in clinicals trials.61 The title and abstract should indicate whether the study included only males or only females. If sex differences in enrolment are expected due to differences in disease prevalence, this should be acknowledged. The implications of sex enrolment on the results and the extent to which the results are generalizable should be described. If the sample size is large enough to achieve adequate power, sex interaction tests and subgroup analyses should be completed to assess the effect of the intervention on both sexes.62 Studies that are underpowered to test interaction should report the main effects by sex and provide sex-specific data to contribute to meta-analyses of sex differences.62 If no sex-based analyses are conducted, the reasons for the absence of analyses and implications on external validity should be addressed.
Strength and limitations
The strengths of this meta-analysis include the systematic literature search and the inclusion of a large number of RCTs published in high-impact journals,18 which minimized the potential for bias caused by the omission of relevant trials. The number of RCTs included in this review exceeds the scope of previous reviews. There was high agreement between reviewers across all stages of the study, which minimized the likelihood that the findings of our review were due to chance or single-reviewer bias.
This meta-analysis has limitations that should be acknowledged. Our review was restricted to the English language and relied primarily on published studies. We investigated the enrolment of females in RCTs in high-impact medical journals.1 The enrolment of females and associations described in this study may not apply to RCTs that were excluded from this review. It is possible that the enrolment rates of females and clinical trial characteristics in lower-impact journals do not follow the trends identified within this study. We assumed that the sex distribution of HFrEF was constant over time. However, a recent analysis demonstrated that the prevalence of HFrEF in females has declined in the United States.63 There has been limited published data on both the prevalence and sex distribution of HFrEF in different regions. It is possible that our assumptions do not adequately reflect the trends in all regions. Thus, the selection of a single proportion to define under-enrolment may not be generalizable to patients with HFrEF across the world. We were not able to account for patient-level characteristics, such as consent, age, and disease severity, and trial factors such as recruitment strategies, and recognize that these factors may play a role in the under-enrolment of females. We focussed on broad clinical trial characteristics that impact the design of RCTs and did not account for the risk of bias of individual studies. Furthermore, the multivariable analysis for identifying characteristics associated with under-enrolment of females is exploratory in nature, and we could only assess variables that were reported in the publication. We were limited in the number of variables we could include in the regression model due to the ratio of events to the degrees of freedom (to avoid overfitting).64
Conclusion
In this study, we demonstrate that females are under-enrolled in RCTs of HFrEF relative to sex distribution of the disease. Sex-specific gaps in enrolment have not improved over the last 19 years. Trials neither report the sex-specific breakdown of patients screened, excluded, and consented, nor provide justification for sex-related eligibility criteria. Sex-related eligibility criteria, ambulatory settings, drug and device/surgery interventions, region of trial coordination, and trial leadership by men are important factors in the under-enrolment of females in HFrEF RCTs. Addressing these factors may facilitate greater sex balance in RCTs, improve the generalizability of results, and provide important data about sex-specific treatment effects.
Funding
Dr Van Spall receives research support from the Women as One Escalator award and the Department of Medicine at McMaster University, and research funding from the Canadian Institutes of Health Research.
Conflict of interest: none declared.