Prediction of 90 day mortality in elderly patients with acute HF from e-health records using artificial intelligence
All the authors take responsibility for all aspects of the reliability and freedom from bias of the data presented and their discussed interpretation.
Abstract
Aims
Mortality risk after hospitalization for heart failure (HF) is high, especially in the first 90 days. This study aimed to construct a model automatically predicting 90 day post-discharge mortality using electronic health record (EHR) data 48 h after admission and artificial intelligence.
Methods
All HF-related admissions from 2015 to 2020 in a single hospital were included in the model training. Comprehensive EHR data were collected 48 h after admission. Natural language processing was applied to textual information. Deaths were identified from the French national database. After variable selection with least absolute shrinkage and selection operator, a logistic regression model was trained. Model performance [area under the receiver operating characteristic curve (AUC)] was tested in two independent cohorts of patients admitted to two hospitals between March and December 2021.
Results
The derivation cohort included 2257 admissions (248 deaths after hospitalization). The evaluation cohorts included 348 and 388 admissions (34 and 38 deaths, respectively). Forty-two independent variables were selected. The model performed well in the derivation cohort [AUC: 0.817; 95% confidence interval (CI) (0.789–0.845)] and in both evaluation cohorts [AUC: 0.750; 95% CI (0.672–0.829) and AUC: 0.723; 95% CI (0.644–0.803]), with better performance than previous models in the literature. Calibration was good: ‘low-risk’ (predicted mortality ≤8%), ‘intermediate-risk’ (8–12.5%) and ‘high-risk’ (>12.5%) patients had an observed 90 day mortality rate of 3.8%, 8.4% and 19.4%, respectively.
Conclusions
The study proposed a robust model for the automatic prediction of 90 day mortality risk 48 h after hospitalization for decompensated HF. This could be used to identify high-risk patients for intensification of therapeutic management.
Introduction
Heart failure (HF) is a common medical condition associated with high mortality and rehospitalization rates. In 2019, there were 56.2 million prevalent cases of HF worldwide, with an age-standardized rate of 7.12 per 1000 individuals.1 In Europe, the prevalence rate of HF in 2019 was approximately 17 per 1000.2 In the United States, according to data from the National Health and Nutrition Examination Survey (NHANES), the prevalence rate was 23 per 1000 between 2017 and 2020 and the average incidence of hospitalized HF was 11.6 per 1000 patient years with a readmission rate of 6.6 per 1000 patient years for patients aged 55 years and older.3
Rates of death and rehospitalization in the first 60 to 90 days after discharge from a hospitalization for decompensated HF are high, and this period has been referred to as the ‘vulnerable phase’.4 Over this period, rates of readmission and death range from 15% to 30% and 7% to 11%, respectively.5-8 Although advances in medical therapy for HF have had a beneficial impact on mortality rates, the number of HF-related deaths and readmissions remains high.9
Various approaches have been studied to reduce early readmission and/or mortality rates in patients with HF, including a personalized medical journey with coordination of care, early visits by specialist nurse, general practitioners or cardiologists and/or remote monitoring, but results are inconsistent and inconclusive.10-13 An alternative approach would be to identify individuals at high risk of early readmission and/or death before they are discharged from hospital to adapt their care management.
Several risk scores using a combination of clinical and laboratory data and/or other parameters have been developed14 in different types of HF (chronic, acute and advanced) such as the PROTECT (Placebo-Controlled Randomized Study of the Selective A1 Adenosine Receptor Antagonist Rolofylline for Patients Hospitalized with Acute Decompensated Heart Failure and Volume Overload to Assess Treatment Effect on Congestion and Renal Function) score,15 the Seattle Heart Failure Model (SHFM),16 the Heart Failure Survival Score (HFSS),17 the MAGGIC (Meta-Analysis Global Group in Chronic Heart Failure) score18 and the Acute Decompensated Heart Failure National Registry (ADHERE) model19 (Table S1). However, despite their potential to help identify high-risk individuals, existing scores usually require manual calculation, which limits their usefulness in clinical practice. In addition, currently available options are not very accurate in determining the risk of readmission or 1 year mortality.20 Furthermore, there is a lack of data on risk prediction during the vulnerable phase after HF-related hospitalization.
Therefore, there is an unmet need for a new approach to automatically identify high-risk individuals at the beginning of their HF-related hospitalization. One approach would involve exploiting the wealth of data available in the electronic health record (EHR) without a priori to automatically predict 90 day mortality. Based on routine care data, this model could be implemented in the hospital information system and calculated automatically, without the need for re-entry or additional data collection. Such an approach is now possible by artificial intelligence (AI) methods. AI can exploit these large amounts of data to capture complex relationships and select relevant variables.
The objective of this study was to develop and validate a model for the automatic prediction of mortality during the vulnerable phase—90 day post-discharge—in patients hospitalized for decompensated HF using data available in the EHR up to 48 h after admission.
Methods
Study design
This study was conducted over the period from 1 January 2015 to 31 December 2021. The research was conducted in two hospitals on retrospective data collected during hospital stays: Paris Saint-Joseph Hospital and Lille University Hospital. The study followed the French National Commission for Information Technology and Civil Liberties (CNIL) reference methodology MR004 and was approved by the institutional ethics committee (institutional review board number IRB00012157). Patients were informed of the study by mail and given the opportunity to refuse to participate.
Study populations
The derivation cohort included all individuals hospitalized for HF at Paris Saint-Joseph Hospital between 1 January 2015 and 31 December 2020. The evaluation cohorts included all individuals hospitalized for HF at Paris Saint-Joseph Hospital (evaluation Cohort A) and Lille University Hospital (evaluation Cohort B) between 1 March 2021 and 31 December 2021. HF hospitalizations were identified by the International Classification of Diseases, 10th Revision (ICD-10) HF codes (I500–I501) and by HF keywords in the discharge summary. Keywords were searched with a natural language processing (NLP) tool considering misspellings and negations. The NLP tool was validated on 100 randomly selected discharge summaries of patients hospitalized at Paris Saint-Joseph with an ICD-10 code for HF and verified by two HF specialists. The NLP tool confirmed HF hospitalization with 97% specificity. Patients with at least one of the following criteria were excluded: aged over 95 years, receiving palliative care, with documented cardiac amyloidosis, who died during the hospitalization or who expressed their opposition to the study (Figure S1). Every admission meeting the inclusion criteria was included, meaning that a given patient could contribute more than one admission to the analysis.
Data extraction and management
Data available up to 48 h after admission in the EHRs of both hospitals were extracted. Two cardiologists selected an initial set of 151 clinical and biological variables considered relevant and obtainable routinely by the two hospitals for the management of HF. A least absolute shrinkage and selection operator21 (LASSO) regression was then used to select the final 42 variables in the model. For example, calcaemia, phosphoraemia and eosinophil count were not extracted. One hundred and fifty-one variables from 11 categories were extracted: demographics; hospital admission mode; month of hospitalization; medications (based on the anatomical therapeutic chemical classification); biology; comorbidities; electrocardiogram (EKG); left ventricular ejection fraction (LVEF) determined by echocardiography; vital signs; clinical signs of HF; and HF aetiology (Figure S2).
Structured data were preferred but, when necessary, NLP tools were used to search for keywords. For discrete variables, the first values were retained and for continuous variables, the mean values were used. A programme was developed to automate the extraction and cleaning of the data and to ensure the homogeneity of the data between the derivation and the evaluation cohorts, and between the two hospitals calculated (Supporting information Methods). Only variables with less than 25% missing data were retained. Missing values were imputed using the variable-specific statistics: for continuous variables, missing values were substituted with the mean value, and for categorical variables, missing values were replaced with the most frequent value.
The 90 day post-discharge vital status was obtained from the hospital information system, supplemented by the database of the French National Institute of Statistics and Economic Studies (INSEE). Matching between the study population and the INSEE database was performed using the INSEEHOP22 open-source tool.
Model development and testing
Logistic regression was used to predict the probability of 90 day post-discharge mortality by creating a model that correlates EHR variables and mortality with an output between 0 and 1. The model was developed in the derivation cohort and then independently tested on the two evaluation cohorts. Learning was performed using five-fold cross-evaluation.
Variables significantly associated with 90 day post-discharge mortality in a univariate model were included in a multivariate logistic regression. Given the large number of variables and the resulting risk of multicollinearity and overfitting, a LASSO21 regression was applied, with a threshold of 0.08, set to maximize cross-validation performance. LASSO is a regularization technique that promotes sparse patterns and limits overfitting, by automatically selecting variables on the basis of a penalty term derived from the absolute values of the coefficients. The variables selected by LASSO were then validated by clinical experts (M. K., P. d. G.). The final model was built with 42 variables (Table S3). This selection of variables not only improved the robustness of the model, but also facilitated its integration into the hospital information system.
The performance of the logistic regressions was evaluated with the area under the curve (AUC) of the receiver operating characteristics (ROC) curve for each of the three cohorts. AUC was also determined in subgroups: according to LVEF (<50% vs. ≥50%), and according to the number of medications recommended for HF (0 or 1 medication vs. ≥2 medications). Pharmacological therapies included at least diuretics, renin–angiotensin system (RAS) inhibitors, beta-blockers, mineralocorticoid receptor antagonists (MRAs) (Table 1).
| Derivation cohort (n = 1733) | Outcome after hospitalization for decompensated HF | Evaluation Cohort A (n = 312) | Evaluation Cohort B (n = 353) | P value | |||
|---|---|---|---|---|---|---|---|
| Survived to 90 days | Death within 90 days | P value | |||||
| (n = 1568) | (n = 223) | ||||||
| Number of hospitalization for HF | 2257 | 2009 | 248 | 348 | 388 | ||
| Agea, years | 81 [73–87] | 81 [72–87] | 85 [79–89] | <0.001 | 80 [71–86] | 72 [61–79] | <0.001 |
| Male sex, n (%) | 1251 (55.4) | 1203 (55.4) | 138 (55.6) | 1.000 | 189 (54.3) | 231 (59.5) | 0.301 |
| HF aetiology, n (%) | |||||||
| Ischaemic | 1014 (44.9) | 885 (44.1) | 129 (52.0) | 0.056 | 143 (41.1) | 122 (31.4) | 0.021 |
| Non-ischaemic | 747 (33.1) | 674 (33.5) | 73 (29.4) | 107 (30.7) | 132 (34.0) | ||
| Other | 496 (22.0) | 450 (22.4) | 46 (18.5) | 98 (28.2) | 134 (34.5) | ||
| LVEF, % | 46.9 ± 15.4 | 47.2 ± 15.4 | 44.8 ± 15.3 | 0.020 | 44.3 ± 19.5 | 43.7 ± 21.0 | 0.682 |
| LVEF category, n (%) | |||||||
| ≥50% | 1102 (48.8) | 993 (49.4) | 109 (44.0) | 138 (39.7) | 173 (44.6) | 0.051 | |
| 40% to <50% | 485 (21.5) | 429 (21.4) | 56 (22.6) | 0.240 | 64 (18.4) | 47 (12.1) | |
| <40% | 670 (29.7) | 587 (29.2) | 83 (33.5) | 146 (42.0) | 168 (43.3) | ||
| BNPa, pg/mL | 836.0 [441.0–1532.0] | 808.0 [431.0–1438.5] | 1262.0 [696.5–2192.0] | <0.001 | 879.5 [409.5–1739.8] | 952.9 [481.5–1879.8] | 0.001 |
| Serum creatinine, μmol/L | 125.0 ± 67.5 | 122.1 ± 65.9 | 148.5 ± 76.0 | <0.001 | 122.1 ± 63.9 | 124.2 ± 58.6 | 0.643 |
| Comorbidities, n (%) | |||||||
| Hypertension | 1723 (76.3) | 1534 (76.4) | 189 (76.2) | 0.937 | 233 (67.0) | 267 (68.8) | 0.635 |
| Atrial fibrillation | 1301 (57.6) | 1155 (57.5) | 146 (58.9) | 0.734 | 201 (57.8) | 217 (55.9) | 0.541 |
| Diabetes | 820 (36.3) | 732 (36.4) | 88 (35.5) | 0.834 | 133 (38.2) | 153 (39.4) | 0.762 |
| Cancer | 473 (21.0) | 417 (20.8) | 56 (22.6) | 0.509 | 75 (21.6) | 31 (8.0) | <0.001 |
| COPD | 518 (23.0) | 459 (22.8) | 59 (23.8) | 0.749 | 83 (23.9) | 81 (20.9) | 0.375 |
| Pacemaker, n (%) | 621 (27.5) | 531 (26.4) | 90 (36.3) | 0.001 | 116 (33.3) | 59 (15.2) | <0.001 |
| Automatic implantable defibrillator, n (%) | 281 (12.5) | 248 (12.3) | 33 (13.3) | 0.683 | 51 (14.7) | 88 (22.7) | 0.006 |
| Medications, n (%) | |||||||
| Diuretics | 1797 (79.6) | 1596 (79.4) | 201 (81.0) | 0.616 | 287 (82.5) | 358 (92.3) | <0.001 |
| Beta-blocker | 1266 (56.1) | 1146 (57.0) | 120 (48.4) | 0.118 | 216 (62.1) | 207 (53.4) | 0.021 |
| RAS inhibitor | 977 (43.3) | 903 (44.9) | 74 (29.8) | <0.001 | 191 (54.9) | 193 (49.7) | 0.237 |
| MR antagonist | 344 (15.2) | 310 (15.4) | 34 (13.7) | 0.513 | 61 (17.5) | 99 (25.5) | 0.009 |
| CCB | 329 (14.6) | 301 (15.0) | 28 (11.3) | 0.128 | 86 (24.7) | 101 (26.0) | 0.735 |
| Cardiac glycosides | 204 (4.6) | 97 (4.8) | 7 (2.8) | 0.198 | 13 (3.7) | 26 (6.7) | 0.098 |
| SGLT2i | 1 (0.0) | 1 (0.0) | 0 (0.0) | 28 (8.0) | 47 (12.1) | 0.087 | |
| Anticoagulants | 1123 (49.8) | 1005 (50.0) | 118 (47.6) | 0.501 | 225 (64.7) | 190 (49.0) | <0.001 |
| Antiplatelets | 887 (39.3) | 788 (39.2) | 99 (39.9) | 0.836 | 112 (32.2) | 118 (30.4) | 0.633 |
| Amiodarone | 547 (24.2) | 488 (24.3) | 59 (23.8) | 0.937 | 42 (12.1) | 60 (15.5) | 0.200 |
| Number of recommended HF medications, n (%) | |||||||
| 0 or 1 | 1483 (65.7) | 1299 (64.7) | 184 (74.2) | 0.003 | 198 (56.9) | 227 (58.5) | 0.659 |
| ≥2 | 774 (34.3) | 710 (35.3) | 64 (25.8) | 150 (43.1) | 161 (41.5) | ||
- Note: Values are mean ± standard deviation, median [interquartile range], or number of hospitalizations (%).
- Abbreviations: BNP, B-type natriuretic peptide; CCB, calcium channel blocker; COPD, chronic obstructive pulmonary disease; HF, heart failure; LVEF, left ventricular ejection fraction; MR, mineralocorticoid receptor; RAS, renin–angiotensin system; SGLT2i, sodium-glucose cotransporter 2 inhibitor.
- a BNP and age values do not follow a normalized distribution.
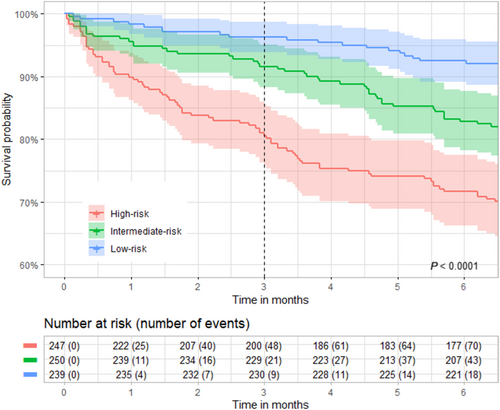
The coherence of predictions obtained with logistic regression was verified with calibration and Kaplan–Meier curves. To plot the Kaplan–Meier curves, the evaluation population of both cohorts was pooled and then divided into three subgroups according to the terciles of 90 day mortality prediction [≤8.0%; (8.0%–12.5%); >12.5%]. Patients with a 90 day mortality probability of less than or equal to 8.0% were classified in the ‘low-risk’ subgroup, those with a probability between 8.0% and 12.5% were classified to the ‘intermediate-risk’ subgroup, and those with a probability greater than 12.5% were classified to the ‘high-risk’ subgroup. Kaplan–Meier curves were plotted up to 6 months of follow-up to assess the consistency of the risk groups over time.
Statistical analysis
After testing for normality of distribution, data were presented as mean ± standard deviation (normal distribution) or median [interquartile range] (non-normal distribution); categorical variables were presented as number (percentage). Characteristics of the derivation and of the two evaluation cohorts were compared using unpaired Student's t-test or Mann–Whitney test for continuous variables and the χ2 test for categorical variables. Performance of the prediction model was determined by calculating the AUC; 95% confidence intervals (CIs) for AUC values were calculated by performing bootstrapping resampling. Differences in survival curves were tested using log-rank. A P value ≤0.05 was considered statistically significant. All experiments were performed using the Python package Scikit-learn version 1.3.0.
The AUC of our model was compared with that of the PROTECT score to predict the 90 day mortality post-discharge in the study population. The PROTECT score was calculated without the uric acid variable, as it was not available in the study population.
Results
Characteristics of the derivation cohort
The derivation cohort included 1733 patients (2257 hospitalizations) (Table 1). Mean age was 81 years. Half of the cohort had an ischaemic HF (44.9%) and nearly half of the cohort had a preserved LVEF (48.8%). Almost half of the cohort (44.0%) had been admitted to intensive care. The most common HF medications were diuretics (79.6% of hospitalizations), followed by beta-blockers (56.1%) and RAS inhibitors (43.3%). MRAs were used in 15% of patients. Due to the recruitment period of this cohort, only one patient was treated with a Sodium Glucose Cotransporter 2 Inhibitor (SGLT2i). The rate of comorbidities was high, especially hypertension (76.3%), atrial fibrillation (57.6%) and diabetes mellitus (36.3%). Patients in the ‘0 or 1 medication recommended for HF’ subgroup were more likely to have preserved LVEF than those in the ‘≥2 medications recommended for HF’ subgroup (53% vs. 40% respectively, P < 0.05).
Two hundred and twenty-three patients (12.9%) died within 90 days of hospital discharge (248/2257 hospitalizations, 11.0%). They were characterized by older age, higher levels of B-type natriuretic peptide (BNP), serum creatinine and pacemaker implantation rate, as well as lower LVEF and prescription rate of RAS inhibitors. Furthermore, these patients were more likely to have 0 or 1 medication recommended for HF (Table 1).
Only three variables had more than 14% missing data. Of these, aspartate aminotransferase (ASAT) and BNP were retained in the final model, despite missing values rate of respectively 24.15% and 14.18%. The other variables in the dataset had missing values of less than 10% (Table S2).
Characteristics of the evaluation cohorts
Evaluation Cohort A included 312 patients (348 hospitalizations) and evaluation Cohort B included 353 patients (388 hospitalizations). There were several statistically significant differences between Cohort A and Cohort B. Patients of Cohort B were characterized by younger age, higher levels of BNP, higher prescription rate of diuretics and MRAs, as well as lower prescription rate of beta-blocker and anticoagulant, and pacemaker implantation rate. In addition, these patients were less likely to have cancer, whereas ischaemic HF and the presence of an implantable cardiac defibrillator (ICD) were more common in cohort B (Table 1).
Performance of the prediction model
The 90 day mortality rates were similar in the three cohorts (11.0% of the patients discharged in the derivation cohort, 10.9% in the evaluation Cohort A, and 10.3% in the evaluation Cohort B) (Table 2). Across all cohorts, the mortality rate tended to be higher in the ‘LVEF <50%’ subgroup and in the ‘0 or 1 medication recommended for HF’ subgroup.
| Na | Mortality rate, % | Model performance, AUC (95% CI) | |
|---|---|---|---|
| Derivation cohort | |||
| Overall | 2257 | 11.0 | 0.817 (0.789–0.845) |
| LVEF subgroups | |||
| ≥50% | 1102 | 9.9 | 0.779 (0.689–0.817) |
| <50% | 1115 | 11.6 | 0.827 (0.764–0.859) |
| Number of HF therapies subgroups | |||
| 0 or 1 | 1483 | 12.4 | 0.807 (0.750–0.835) |
| ≥2 | 774 | 8.3 | 0.793 (0.686–0.841) |
| Sex subgroups | |||
| Female | 1006 | 10. | 0.810 (0.766–0.854) |
| Male | 1251 | 11.0 | 0.823 (0.787–0.860) |
| Age subgroups | |||
| >80 years | 1222 | 14.2 | 0.792 (0.754–0.829) |
| <80 years | 1035 | 7.1 | 0.843 (0.797–0.889) |
| Evaluation Cohort A | |||
| Overall | 348 | 10.9 | 0.750 (0.672–0.829) |
| LVEF subgroups | |||
| ≥50% | 138 | 10.1 | 0.673 (0.413–0.794) |
| <50% | 210 | 11.4 | 0.793 (0.618–0.860) |
| Number of HF therapies subgroups | |||
| 0 or 1 | 140 | 10.2 | 0.721 (0.509–0.802) |
| ≥2 | 150 | 8.3 | 0.791 (0.589–0.895) |
| Sex subgroups | |||
| Female | 159 | 8.8 | 0.742 (0.643–0.841) |
| Male | 189 | 12.7 | 0.753 (0.641–0.864) |
| Age subgroups | |||
| >80 years | 163 | 1 | 0.701 (0.586–0.817) |
| <80 years | 185 | x | 0.795 (0.689–0.902) |
| Evaluation Cohort B | |||
| Overall | 388 | 10.3 | 0.723 (0.644–0.803) |
| LVEF subgroups | |||
| ≥50% | 173 | 9.8 | 0.673 (0.453–0.776) |
| <50% | 215 | 10.7 | 0.763 (0.589–0.844) |
| Number of HF therapies subgroups | |||
| 0 or 1 | 128 | 11.7 | 0.691 (0.513–0.778) |
| ≥2 | 161 | 8.1 | 0.765 (0.540–0.853) |
| Sex subgroups | |||
| Female | 157 | 11.5 | 0.703 (0.577–0.829) |
| Male | 231 | 9.5 | 0.748 (0.643–0.853) |
| Age subgroups | |||
| >80 years | 89 | 18.0 | 0.741 (0.617–0.866) |
| <80 years | 299 | 8.0 | 0.697 (0.588–0.805) |
- Abbreviations: AUC, area under the receiver operating characteristic curve; CI, confidence interval; HF, heart failure; LVEF, left ventricular ejection fraction.
- a N indicates the number of hospitalizations included in each group (patients could contribute more than one hospitalization event to the dataset).
The prediction model had an AUC: 0.817; 95% CI (0.789–0.845), in the derivation cohort. Performance in predicting 90 day post-discharge mortality was consistent in evaluation Cohort A [AUC: 0.750; 95% CI (0.672–0.829)] and evaluation Cohort B [AUC: 0.723; 95% CI (0.644–0.803)] (Table 2 and Figure 1). Across the derivation and the two evaluation cohorts, model performance was better in the ‘LVEF < 50%’ and in the ‘0 or 1 medication recommended for HF’ subgroups (Table 2).
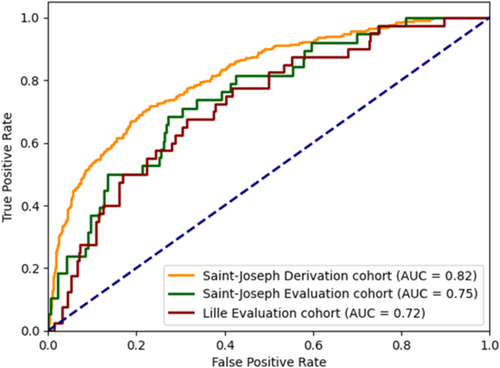
The calibration curves showed that there was a good consistency between predicted probability and actual probability (Figure S3). Moreover, the three risk subgroups (‘low-risk’, ‘intermediate-risk’ and ‘high-risk’) had different survival curves and 90 day mortality rate (3.8%, 8.4% and 19.4% respectively) (Figure 2).
The PROTECT model had an AUC: 0.710; 95% CI (0.676–0.744), in the derivation cohort, and an AUC: 0.687; 95% CI (0.591–0.783), and an AUC: 0.655; 95% CI (0.571–0.739) in the evaluation Cohorts A and B, respectively.
Discussion
To the best of our knowledge, this is the first model developed to predict 90 day post-discharge mortality for HF decompensation episodes using data from the EHR available up to 48 h after admission. The model is based on a limited number of routine care data (42 variables) extracted from the EHR data. The model performed well in the derivation cohort [AUC: 0.817; 95% CI (0.789–0.845)], indicating good discriminative ability. Evaluation in two independent cohorts from different hospitals [Cohort A AUC: 0.750; 95% CI (0.672–0.829), and Cohort B AUC: 0.723; 95% CI (0.644–0.803)] indicated that there was no overfitting of the model. Furthermore, despite some differences in clinical profiles or management modalities between the two evaluation cohorts, the overall model performance remained consistent. This indicates a good level of robustness and provides further support for the reliability and generalizability of the model.
However, subgroup analyses suggest that model performance may differ according to patient profile in the evaluation cohorts: model performance in patients with LVEF < 50% was higher than in patients with preserved ejection fraction (LVEF ≥ 50%). This could be explained by the difference in pathophysiology and treatment modalities according to ejection fraction. A higher model performance was also observed in the ‘≥2 medications recommended for HF’ subgroup. This may be related to lower proportion of patients with preserved LVEF in the ‘≥2 medications recommended for HF’ subgroup, as there is less evidence of benefit from currently available pharmacological therapies in HF with preserved ejection fraction.
It is difficult to compare our model with other predictive models because the study populations are different, as are the primary outcomes (mortality at 30 days, 180 days or 1 year). Previous scores have predicted mortality at 90 days, the Organized Program To Initiate lifesaving treatMent In hospitaliZEd patients (OPTIMIZE-HF),23 the Acute Physiology And Chronic Health Evaluation in Heart Failure (APACHE-HF)24 and the PROTECT scores. However, the methodology was different, with a pre-specified selection of variables, no evaluation cohort and standard statistical analyses. We compared our score with the PROTECT score and obtained a better performance [Cohort A AUC: 0.750; 95% CI (0.672–0.829), and Cohort B AUC: 0.723; 95% CI (0.644–0.803), vs. Cohort A AUC: 0.687; 95% CI (0.591–0.783), and Cohort B AUC: 0.655; 95% CI (0.571–0.739), respectively]. However, we calculated the PROTECT score without the uric acid variable, which was not available in our data. However, the effect of the uric acid may be small, as its hazard ratio (95% CI) was low [1.01 (1.00–1.02)] in the PROTECT model. The performance of the current model is also better than that of the ADHERE19 score used to predict in-hospital mortality in acutely decompensated HF, which had an AUC of 0.63. In addition, although the SHFM model applied on discharge after a decompensated HF hospitalization had good risk discrimination for predicting 1 year mortality in patients aged <65 years (AUC 0.704), it showed poor performance in older individuals,25 which is an important limitation given the age of HF patients referred to hospitals. Other scores have been developed to predict in-hospital mortality,26-29 but this is out of the scope of our work as we were interested in studying the post-discharge mortality during the vulnerable phase.
Other researchers have applied machine learning approaches to determine risk in patients with HF. The AUC in our model was slightly lower than that of a machine learning model developed to predict 1 year mortality risk in HF patients [Machine learning Assessment of RisK and EaRly mortality in Heart Failure (MARKER-HF)], which had an AUC 0.81–0.88.30 However, the training population of the MARKER-HF study included both hospitalized and stable ambulatory patients and focussed on 1 year mortality. Moreover, the mean age of the derivation cohort was 59 years, which is not representative of the older HF population usually seen in clinical practice. Another machine learning model with a LASSO algorithm used data on 27 continuous and 44 categorical variables from patients hospitalized for acute HF syndrome.31 The model's AUC for 30 day mortality in this study was 0.78 in the derivation set and 0.76 in the evaluation set. However, this study included only data from East-Asian patients, and therefore, its usefulness in individuals from other ethnic groups is not known.
In contrast to previous machine-learning models that looked at mortality and/or readmission risk over longer time periods, the model developed in this study was designed to specifically predict mortality risk in the vulnerable 90 day period after discharge from a HF-related hospitalization. This is particularly important as it has recently been suggested that early uptitration of guideline-directed medical therapies in decompensated HF is associated with significant clinical benefit.32
It has been suggested that the ideal risk score for HF would have the highest level of ‘goodness of fit’ and the lowest level of complexity.33 This means that the variables included in the model must be easy to obtain, routinely available in clinical practice, and as objective as possible.33 Therefore, our risk prediction model was designed to be easily integrated into the hospital information system using data already available in the first hours after admission. It can then be used to provide an automated calculation of the risk before a patient is discharged. This automation is a major advantage, as it does not add to the workload of physicians or other healthcare professionals involved in patient management.
Study strengths
This study has several strengths. The model was developed using data from all hospitalized HF patients, regardless of LVEF, and validated in two independent cohorts from different hospitals, indicating the model's robustness for different HF populations. As 90 day mortality was obtained by matching with the French national mortality database, very few patients were lost to follow-up. The calibration of the model was assessed with concordance between predicted and actual 90 day mortality to ensure accuracy. NLP was used to complement ICD-10 codes and improve the accuracy of diagnostic and comorbidity information, as previously demonstrated in HF.34
Study limitations
Several limitations need to be acknowledged when interpreting our findings. The model was developed using data from a single centre in France, and although it was subsequently validated on two independent and clinically different populations, the possibility of location and selection bias cannot be completely excluded. Our model requires therefore further external validation and will be tested in multicentre studies to confirm its robustness and applicability across different healthcare facilities and hospitals.
Although the model gives promising results, the lower limit of the CI, falling below 0.7, could indicate a limitation in the model's discriminative power. A larger sample size of the evaluation cohorts could contribute to strengthening the model's robustness as well as including other populations from different cities and countries.
SGLT2 inhibitors have been shown to significantly improve mortality in HF in several large outcome clinical trials. The timeframe of development of our study did not allow to enrol many patients treated by SGLT2 inhibitors, which were not yet widely used. They were approved for the management of HF in our country in August 2021. The relevance of our model will therefore need to be reassessed in populations treated by this new class of HF. Nevertheless, practices for HF management in the current datasets are more contemporary than those used in older HF risk models.
Our population was made of elderly patients. However, subgroup analyses suggested that the algorithm performed well for the age group <80 years [Cohort A AUC 0.795 (0.689–0.902) and Cohort B AUC 0.697 (0.588–0.805)]. Whether our model is relevant for a younger HF population needs therefore further studies.
The current model is specific to the setting in which the data informing the model were obtained, that is, the prediction of 90 day mortality after hospitalization for decompensated HF, and it does not address prediction of rehospitalization or longer-term mortality. However, the 6 month survival curves show consistency in risk prediction.
Conclusions
The model developed in this study provides a prediction of early post-discharge mortality in patients with decompensated HF. This could help to identify patients at very high risk of mortality after a HF decompensation episode and allow appropriate intensification of their medical management before and after discharge.
To minimize performance gaps between different populations and improve replicability, further model training could be undertaken on cohorts from different hospitals. This could be achieved using innovative machine learning approaches such as federated learning. In addition, this algorithm must be prospectively tested in clinical practice to measure its real impact on the 90 day survival rates.
Acknowledgements
Medical writing assistance was provided by Nicola Ryan, independent medical writer, funded by Hôpital Paris Saint-Joseph, Paris, France. We thank Xavier Maynadier for his help with data verification and Olivier Billuart for his help with data management.
Conflict of interest statement
None declared.
Funding
This work was supported by the French National Research Agency (ANR) under the PREDHIC project (ANR-21-CE23-0039-03).




