Prevalence and prognostic value of different iron deficiency definitions in light chain cardiac amyloidosis patients
Abstract
Aims
Research on iron deficiency (ID) in patients with light chain cardiac amyloidosis (AL-CM) has been limited in previous studies. The purpose of this study was to investigate the prevalence and its association with prognosis of ID based on different definitions in patients with AL-CM.
Methods and results
Three different ID definitions were applied: (1) serum ferritin concentration of <100 ng/mL, or 100–299 ng/mL with transferrin saturation (TSAT) < 20% (according to guidelines on heart failure), (2) TSAT < 20% and (3) serum iron <13 μmol/L. The primary outcome measure was all-cause mortality. Prevalence and outcomes of various ID definitions were evaluated among patients diagnosed with AL-CM at Heart Failure Center, Fuwai Hospital between September 2017 and October 2023. Overall, 149 patients were included with a mean age of 60.71 ± 10.11 years, of whom 60 (40.3%) patients were female, 127 (85.2%) patients were in New York Heart Association (NYHA) class III–IV and 136 (91.3%) patients were in the revised Mayo 2012 stage III–IV. Assessments of iron biomarkers revealed the following results: median ferritin levels were 183.68 ng/mL (interquartile range [IQR] 95.48, 339.01), median TSAT was 21.80% (IQR 16.34, 29.48), and median serum iron was 10.87 μmol/L (IQR 7.38, 13.71). Serum iron were highly correlated with TSAT (r = 0.85, P < 0.0001). Depending on the definition used, 63 (42.3%) patients, 61 (40.9%) patients and 105 (70.5%) patients were defined as ID, respectively (P < 0.0001). ID defined by TSAT and serum iron was associated with the primary outcome [hazard ratio (HR) 2.14, 95% confidence interval (CI) 1.36–3.37, P < 0.001, and HR 2.16, 95% CI (1.23–3.80), P < 0.01], but the same association was not seen with the guideline definition of ID [HR 1.39, 95% CI (0.88–2.18), P = 0.158]. In the multivariable model adjusting for age, gender, haemoglobin, and revised 2012 Mayo staging, the predictive value of TSAT < 20% [adjusted HR 2.49, 95% CI (1.54–4.05), P < 0.001] and serum iron < 13 μmol/L [adjusted HR 2.24, 95% CI (1.23–4.09), P < 0.01] remained.
Conclusions
Different definitions of ID yield inconsistent results in terms of prevalence and prognosis. ID, as defined by TSAT < 20% or serum iron < 13 μmol/L, rather than the guideline definition, emerged as an independent predictor of all-cause mortality in patients with AL-CM.
Introduction
Iron deficiency (ID) is a prevalent co-morbidity in patients with heart failure (HF), impacting approximately 50%–80% of patients.1 It has been shown to be correlated with diminished quality of life, reduced exercise capacity and adverse outcomes.2, 3 However, diagnosing ID solely based on circulating iron biomarkers can be challenging considering their susceptibility to the influence of inflammation and hypoxia.4 The current ESC and AHA/ACC/HFSA guidelines of heart failure defined ID as a serum ferritin concentration of <100 ng/mL or a serum ferritin of concentration of 100–299 ng/mL with a transferrin saturation (TSAT) < 20%, mainly based on the selection criteria of large clinical trials of intravenous iron in HF.1, 5 However, recent research has questioned the guideline definition of ID, as it appears to lack prognostic relevance among heart failure patients,6-8 which contradicts common expectations. Alternative definitions of ID (TSAT < 20% or a serum iron ≤ 13 μmol/L) have been proposed and confirmed to offer valuable prognostic insight.7-9
Systemic light chain amyloidosis (AL) is characterized by the presence of monoclonal plasma cells and the deposition of amyloid fibrils, which are composed of misfolded immunoglobulin light chains, in various organs. Cardiac involvement is seen in over 70% of patients, leading to a condition known as light chain cardiac amyloidosis (AL-CM). AL-CM represents a frequently overlooked aetiology of heart failure, and recent advances in cardiac diagnostic strategies have significantly enhanced the recognition of AL-CM, revealing a higher prevalence than previously presumed.10-12 AL-CM is characterized by progressive cardiac dysfunction with a dismal prognosis. The widely recognized revised Mayo 2012 staging system classifies patients into four stages (I–IV) based on three biomarkers: cardiac troponin T < 0.025 ng/mL [or high-sensitivity troponin T (hs-cTnT) < 40 ng/L], N terminal pro brain natriuretic peptide (NT-proBNP) < 1800 pg/mL and the difference between involved and uninvolved serum free light chains (dFLC) < 180 mg/L. The median overall survival from diagnosis was 94.1, 40.3, 14 and 5.8 months, respectively.13, 14
As a systemic disease, AL amyloidosis can affect not only the heart but also the soft tissue, the gastrointestinal tract and other tissue and organs.15 Several unique disease characteristics may predispose patients to ID. First, intestinal congestion caused by cardiac dysfunction and intestinal infiltration of amyloid fibrils may lead to decreased iron intake and absorption. Second, blood vessel infiltration, coagulopathy from co-morbid acquired X factor deficiency and the recurrent administration of novel oral anticoagulants could cause inconspicuous bleeding and therefore cause increased iron loss. Moreover, chronic inflammatory activation could lead to disordered iron metabolism.
Studies pertaining to ID in AL-CM patients are limited and the iron metabolism in AL-CM patients remains poorly characterized. The only previous study concerning ID in cardiac amyloidosis patients found that ID defined by guideline definition affected nearly half of the AL-CM patients, yet there was no difference in all-cause mortality considering ID status.16 Given the previously mentioned concerns regarding the guideline definition, it is plausible to hypothesize that this definition may not accurately capture patients with true ID. Using the appropriate criteria based on serum iron biomarkers for assessing ID in AL-CM patients is of great significance for identifying patients with this co-morbidity and initiating interventions.
The aim of this study is to delineate iron metabolism in AL-CM patients, evaluate how different definitions of ID affect the prevalence of ID and prognosis in this population and explore the prognostic stratification value of iron metabolism markers in AL-CM patients.
Methods
Study population
Consecutive hospitalized patients diagnosed with AL-CM between September 2017 and October 2023 in Heart Failure Center, Fuwai Hospital (Beijing, China) were included. AL-CM is diagnosed based on the algorithms proposed by the position statement of the ESC Working Group on Myocardial and Pericardial Diseases on Diagnosis and Treatment of Cardiac Amyloidosis11 and 2023 ACC Expert Consensus Decision Pathway on Comprehensive Multidisciplinary Care for the Patient with Cardiac Amyloidosis.17 It was confirmed by endomyocardial biopsy proof of amyloid deposition based on the presence of apple-green appearance viewed under cross-polarized light with Congo red staining and tissue typing by immunohistochemistry or immunoelectron microscopy or proof of extra-cardiac amyloid deposition accompanied by characteristic features of cardiac amyloidosis by echocardiography or cardiac magnetic resonance (CMR). All patients underwent a comprehensive evaluation, including a clinical evaluation and laboratory measurements completed within 24 h after being admitted. The investigation conforms with the principles outlined in the Declaration of Helsinki (Br Med J 1964; ii: 177). Ethical approval for this study was obtained from the Ethics Committee of Fuwai Hospital, Chinese Academy of Medical Sciences, and written informed consent was obtained from all patients.
Definitions
Anaemia was defined according to World Health Organization (WHO) criteria as haemoglobin < 120 g/L in women and <130 g/L in men. Serum ferritin, TSAT, and serum iron were used as biomarkers of ID. Serum concentrations of ferritin and iron were measured, and TSAT (%) were calculated using the formula: [iron (μmol/L)/(transferrin(g/L) × 25.2) × 100]. Three definitions of ID in HF patients were used: (1) guideline definition of ID: serum ferritin concentration of <100 ng/mL, or 100–299 ng/mL with TSAT < 20%, (2) TSAT < 20% and (3) serum iron concentration ≤13 μmol/L. Only patients with available iron biomarkers and haemoglobin values were included in this analysis.
To investigate the differences between subgroups included in the guideline-defined ID and TSAT-defined ID, patients were further categorized into six groups based on their TSAT levels (<20% or ≥20%) and ferritin levels (<100 ng/mL, 100–299 ng/mL and ≥300 ng/mL). Patients with ferritin between 100 and 299 ng/mL and TSAT > 20% are designated as the reference group.
Follow-up and study endpoint
The follow-up protocol adhered to standard clinical practices. This encompassed monthly medical evaluations following the initial discharge from the hospital and hospital visits tailored to meet the needs of each patient. Study endpoint was all-cause death. Patients were censored if they were still alive at that time or were lost to follow-up, on which occasion their last clinic visit or correspondence time was used.
Statistical analysis
Continuous variables were tested for normal distribution using Shapiro–Wilk test, presented as median with 25th and 75th percentiles or mean with standard deviation, and compared using Student's t-test or Mann–Whitney U test. Categorical variables are presented as numbers and percentages and compared through the use of Fisher's exact tests or chi-square tests.
Kaplan–Meier cumulative endpoint curves were used to compare groups. The log-rank test was used to evaluate statistical significance. Cox proportional hazard models were used to identify variables associated with all-cause mortality. Multivariable model was adjusted for age, gender, haemoglobin, and revised Mayo 2012 staging. Hazard ratio (HR) with 95% confidence interval (CI) are reported. Restricted cubic splines were constructed for each continuous iron biomarker to examine its association with all-cause mortality.
Additional interaction analyses for the TSAT definition in subgroups [age, gender, anaemia, high-sensitivity C reactive protein (hsCRP), NT-proBNP, left ventricular ejection fraction (LVEF), ferritin and admission time categorized as before or after 2021] were performed. All analysis were performed with R version 4.1.3 (R Foundation for Statistical Computing, Vienna, Austria). The two-tailed levels of statistical significance were set at P < 0.05.
Results
Overall, 149 patients diagnosed with AL-CM between September 2017 and October 2023 with available full iron indices were included. In the overall population, the mean age was 60.71 ± 10.11 years, with 60 (40.3%) patients being female, 127 (85.2%) were in New York Heart Association (NYHA) class III–IV, and 136 (91.3%) were in the revised Mayo 2012 stage III–IV. And a total of 63 (42.3%) patients presenting with concomitant anaemia. Assessments of iron biomarkers revealed the following results: Ferritin levels were 183.68 ng/mL (interquartile range [IQR] 95.48, 339.01), TSAT was 21.80% (IQR 16.34, 29.48), and serum iron was 10.87 μmol/L (IQR 7.38, 13.71). The distribution of iron biomarkers among our study population is shown in Figure S1. Serum iron were highly correlated with TSAT (r = 0.85, P < 0.0001).
The prevalence of ID in this AL-CM population was 42.3% and 40.9%, respectively, based on the guideline definition and TSAT definition, while reached up to 70.5% based on the serum iron definition. Each definition delineated a unique patient population, although there was a significant overlap between them. Out of the 149 AL-CM patients, 45 patients (30.2%) met the criteria for all three definitions, and only 32 (21.5%) did not meet any of the diagnostic criteria for ID (Graphic Abstract). ID patients defined by the TSAT definition is fully encompassed within the patient populations defined by the guideline and serum iron definition.
Baseline characteristics according to three different ID definitions were described in Table 1.
| Overall (n = 149) | Guideline definition | TSAT definition | Serum iron definition | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Non-ID (n = 86) | ID (n = 63) | P | Non-ID (n = 88) | ID (n = 61) | P | Non-ID (n = 44) | ID (n = 105) | P | ||
| Demographics and co-morbidities | ||||||||||
| Age, years | 60.71 (10.11) | 61.93 (9.80) | 59.05 (10.36) | 0.085 | 60.95 (10.24) | 60.36 (9.98) | 0.726 | 59.48 (9.85) | 61.23 (10.21) | 0.336 |
| Female sex | 60 (40.3) | 25 (29.1) | 35 (55.6) | 0.002 | 26 (29.5) | 34 (55.7) | 0.002 | 11 (25.0) | 49 (46.7) | 0.023 |
| BMI | 22.59 (2.98) | 22.45 (2.87) | 22.77 (3.12) | 0.524 | 22.64 (2.73) | 22.51 (3.32) | 0.793 | 22.61 (2.51) | 22.58 (3.16) | 0.961 |
| Diabetes | 21 (14.1) | 15 (17.4) | 6 (9.5) | 0.257 | 13 (14.8) | 8 (13.1) | 0.963 | 3 (6.8) | 18 (17.1) | 0.163 |
| Hypertension | 51 (34.2) | 27 (31.4) | 24 (38.1) | 0.499 | 31 (35.2) | 20 (32.8) | 0.894 | 15 (34.1) | 36 (34.3) | 1 |
| Anaemia | 63 (42.3) | 32 (37.2) | 31 (49.2) | 0.195 | 32 (36.4) | 31 (50.8) | 0.112 | 13 (29.5) | 50 (47.6) | 0.064 |
| Current smoker | 15 (10.1) | 10 (11.6) | 5 (7.9) | 0.642 | 11 (12.5) | 4 (6.6) | 0.364 | 6 (13.6) | 9 (8.6) | 0.523 |
| SBP, mmHg | 103.00 [95.00, 114.00] | 103.00 [94.25, 113.75] | 104.00 [95.00, 113.00] | 0.858 | 106.00 [95.75, 115.00] | 102.00 [92.00, 111.00] | 0.187 | 106.00 [97.00, 114.25] | 102.00 [93.00, 113.00] | 0.145 |
| DBP, mmHg | 75.91 (48.41) | 77.77 (62.38) | 73.37 (15.71) | 0.585 | 78.44 (61.63) | 72.25 (15.77) | 0.444 | 86.52 (86.24) | 71.46 (13.85) | 0.083 |
| HR, b.p.m. | 81.20 (14.98) | 81.07 (15.62) | 81.38 (14.19) | 0.901 | 80.69 (14.89) | 81.93 (15.21) | 0.621 | 79.68 (13.00) | 81.84 (15.76) | 0.425 |
| NYHA class III–IV | 127 (85.2) | 74 (86.0) | 53 (84.1) | 0.926 | 73 (83.0) | 54 (88.5) | 0.479 | 33 (75.0) | 94 (89.5) | 0.043 |
| Mayo 2012 stage | 0.516 | 0.379 | 0.01 | |||||||
| II | 13 (8.7) | 7 (8.1) | 6 (9.5) | 10 (11.4) | 3 (4.9) | 8 (18.2) | 5 (4.8) | |||
| III | 43 (28.9) | 22 (25.6) | 21 (33.3) | 24 (27.3) | 19 (31.1) | 15 (34.1) | 28 (26.7) | |||
| IV | 93 (62.4) | 57 (66.3) | 36 (57.1) | 54 (61.4) | 39 (63.9) | 21 (47.7) | 72 (68.6) | |||
| Laboratory | ||||||||||
| RBC, ×1012/L | 4.30 (0.68) | 4.34 (0.63) | 4.25 (0.75) | 0.41 | 4.35 (0.63) | 4.24 (0.76) | 0.358 | 4.50 (0.55) | 4.23 (0.72) | 0.027 |
| Haemoglobin, g/L | 130.44 (19.10) | 134.58 (16.57) | 124.78 (20.93) | 0.002 | 134.12 (17.25) | 125.11 (20.49) | 0.004 | 137.27 (15.14) | 127.57 (19.91) | 0.004 |
| PLT, ×109/L | 188.00 [157.00, 250.00] | 188.00 [153.50, 249.75] | 187.00 [160.50, 251.50] | 0.706 | 191.00 [155.75, 243.25] | 188.00 [159.00, 260.00] | 0.668 | 187.50 [145.00, 235.00] | 188.00 [159.00, 257.00] | 0.253 |
| MCV, fL | 91.70 [88.90, 95.10] | 93.05 [90.30, 95.92] | 89.60 [86.80, 93.30] | 0.001 | 92.75 [90.07, 95.50] | 90.30 [87.50, 93.80] | 0.015 | 92.20 [89.83, 94.60] | 91.60 [88.80, 95.10] | 0.641 |
| MCH, pg | 30.30 [29.30, 31.90] | 31.30 [30.02, 32.20] | 29.50 [28.55, 30.70] | <0.001 | 31.15 [29.98, 32.02] | 29.70 [28.80, 30.70] | <0.001 | 30.70 [29.87, 31.92] | 30.20 [29.20, 31.90] | 0.309 |
| MCHC, g/L | 332.00 [326.00, 339.00] | 335.50 [328.25, 340.75] | 329.00 [320.00, 334.00] | <0.001 | 335.00 [328.75, 341.25] | 329.00 [321.00, 334.00] | <0.001 | 333.00 [328.00, 339.00] | 332.00 [323.00, 339.00] | 0.242 |
| RDW, % | 14.30 [13.20, 15.20] | 14.20 [13.12, 14.90] | 14.30 [13.55, 15.90] | 0.057 | 14.20 [13.17, 14.93] | 14.30 [13.40, 15.90] | 0.136 | 14.20 [13.07, 14.62] | 14.30 [13.30, 15.50] | 0.177 |
| CRP, mg/L | 4.42 [2.19, 9.27] | 4.43 [2.23, 9.24] | 4.28 [2.17, 9.54] | 0.796 | 3.37 [2.10, 7.06] | 6.74 [3.38, 17.80] | <0.001 | 2.24 [1.67, 4.25] | 6.09 [3.19, 12.15] | <0.001 |
| hs-CRP, mg/L | 2.87 [0.83, 8.30] | 3.14 [0.86, 8.21] | 2.84 [0.86, 8.50] | 0.77 | 1.81 [0.66, 6.18] | 6.22 [1.89, 10.05] | <0.001 | 1.11 [0.40, 2.56] | 5.02 [1.64, 9.22] | <0.001 |
| NT-proBNP, pg/mL | 8851.00 [4614.00, 16 590.60] | 8880.50 [4561.50, 20 612.75] | 8342.00 [4763.50, 14 875.00] | 0.577 | 7857.50 [3864.25, 14 307.28] | 11 732.00 [6423.00, 20 099.00] | 0.008 | 4381.00 [3216.00, 8004.50] | 11 251.00 [7278.90, 20 655.00] | <0.001 |
| dFLC, mg/L | 408.68 [182.49, 771.70] | 355.30 [182.78, 769.20] | 433.00 [184.05, 768.54] | 0.481 | 352.30 [162.17, 632.85] | 466.20 [234.40, 879.20] | 0.066 | 295.58 [130.94, 439.73] | 509.60 [217.22, 879.20] | 0.004 |
| hs-cTnT, ng/L | 92.00 [49.20, 162.30] | 92.00 [51.23, 177.50] | 94.00 [45.10, 143.00] | 0.577 | 82.40 [46.00, 150.50] | 107.00 [67.90, 162.60] | 0.125 | 64.50 [37.50, 100.50] | 109.00 [67.90, 165.00] | 0.003 |
| Ferritin, ng/mL | 183.68 [95.48, 339.01] | 316.99 [203.28, 456.45] | 81.33 [46.58, 129.27] | <0.001 | 231.64 [120.61, 373.79] | 134.72 [56.83, 258.21] | 0.001 | 203.36 [111.59, 339.83] | 173.49 [92.73, 332.66] | 0.75 |
| TSAT, % | 21.80 [16.34, 29.48] | 25.60 [21.48, 34.94] | 16.34 [10.98, 20.41] | <0.001 | 26.73 [23.29, 35.06] | 14.76 [10.29, 17.47] | <0.001 | 34.42 [26.95, 40.43] | 19.12 [13.89, 22.76] | <0.001 |
| Serum iron, μmol/L | 10.87 [7.38, 13.71] | 12.09 [9.36, 15.25] | 8.09 [6.16, 11.04] | <0.001 | 12.80 [11.14, 15.78] | 6.86 [5.31, 8.97] | <0.001 | 15.90 [14.04, 19.94] | 8.50 [6.58, 10.99] | <0.001 |
| Echocardiographic measurements | ||||||||||
| LVEF, % | 52.00 [40.00, 58.00] | 53.00 [40.50, 57.00] | 50.00 [40.00, 58.00] | 0.524 | 53.00 [41.50, 58.00] | 50.00 [40.00, 57.00] | 0.179 | 50.00 [40.00, 57.00] | 52.00 [40.00, 59.00] | 0.695 |
| E/e′ | 21.84 [18.20, 27.92] | 21.92 [17.20, 29.49] | 21.43 [19.06, 26.77] | 0.91 | 22.82 [17.17, 28.33] | 20.96 [19.37, 26.47] | 0.711 | 22.40 [17.03, 29.92] | 21.43 [19.32, 26.99] | 0.956 |
| TAPSE ≥ 16 mm, n (%) | 75 (54.7) | 45 (59.2) | 30 (49.2) | 0.318 | 49 (62.0) | 26 (44.8) | 0.068 | 25 (65.8) | 50 (50.5) | 0.156 |
| FAC, % | 30.91 (9.48) | 32.39 (9.27) | 29.16 (9.56) | 0.123 | 31.52 (9.36) | 30.08 (9.71) | 0.5 | 33.26 (10.27) | 29.71 (8.92) | 0.107 |
| IVS, mm | 15.00 [13.00, 17.00] | 15.00 [13.00, 17.00] | 15.00 [13.00, 16.00] | 0.256 | 15.00 [13.00, 17.00] | 15.00 [14.00, 17.00] | 0.792 | 15.00 [13.00, 16.25] | 15.00 [13.00, 17.00] | 0.361 |
| LVPW, mm | 14.00 [12.00, 16.00] | 14.00 [12.25, 16.00] | 14.00 [12.00, 15.00] | 0.632 | 13.00 [12.00, 15.00] | 14.00 [13.00, 16.00] | 0.375 | 13.00 [12.00, 15.00] | 14.00 [13.00, 16.00] | 0.24 |
| Medications | ||||||||||
| Beta-blocker | 76 (51.0) | 44 (51.2) | 32 (50.8) | 1 | 46 (52.3) | 30 (49.2) | 0.838 | 24 (54.5) | 52 (49.5) | 0.704 |
| ACEi, ARB or ARNI | 3 (2.0) | 0 (0.0) | 3 (4.8) | 0.146 | 1 (1.1) | 2 (3.3) | 0.747 | 1 (2.3) | 2 (1.9) | 1 |
| MRA | 86 (57.7) | 49 (57.0) | 37 (58.7) | 0.963 | 53 (60.2) | 33 (54.1) | 0.565 | 30 (68.2) | 56 (53.3) | 0.136 |
| Loop diuretic | 130 (87.2) | 75 (87.2) | 55 (87.3) | 1 | 80 (90.9) | 50 (82.0) | 0.174 | 41 (93.2) | 89 (84.8) | 0.256 |
| SGLT2i | 26 (17.4) | 17 (19.8) | 9 (14.3) | 0.514 | 16 (18.2) | 10 (16.4) | 0.949 | 6 (13.6) | 20 (19.0) | 0.577 |
| Aspirin | 14 (9.4) | 10 (11.6) | 4 (6.3) | 0.42 | 12 (13.6) | 2 (3.3) | 0.065 | 5 (11.4) | 9 (8.6) | 0.822 |
| Clopidogrel | 8 (5.4) | 3 (3.5) | 5 (7.9) | 0.411 | 3 (3.4) | 5 (8.2) | 0.365 | 1 (2.3) | 7 (6.7) | 0.492 |
| Ticagrelor | 1 (0.7) | 1 (1.2) | 0 (0.0) | 1 | 1 (1.1) | 0 (0.0) | 1 | 0 (0.0) | 1 (1.0) | 1 |
| NOACS | 38 (25.5) | 27 (31.4) | 11 (17.5) | 0.082 | 21 (23.9) | 17 (27.9) | 0.719 | 7 (15.9) | 31 (29.5) | 0.125 |
| Chemotherapy regimens, n (%) | 86 (57.7) | 50 (58.1) | 36 (57.1) | 1 | 55 (62.5) | 31 (50.8) | 0.211 | 27 (61.4) | 59 (56.2) | 0.688 |
| Melphalan, n (%) | 1 (0.7) | 1 (1.2) | 0 (0.0) | 1 | 1 (1.1) | 0 (0.0) | 1 | 0 (0.0) | 1 (1.0) | 1 |
| Dexamethasone, n (%) | 86 (57.7) | 50 (58.1) | 36 (57.1) | 1 | 55 (62.5) | 31 (50.8) | 0.211 | 27 (61.4) | 59 (56.2) | 0.688 |
| Bortezomib, n (%) | 84 (56.4) | 50 (58.1) | 34 (54.0) | 0.734 | 55 (62.5) | 29 (47.5) | 0.1 | 27 (61.4) | 57 (54.3) | 0.539 |
| Daratumumab, n (%) | 68 (45.6) | 39 (45.3) | 29 (46.0) | 1 | 42 (47.7) | 26 (42.6) | 0.654 | 21 (47.7) | 47 (44.8) | 0.88 |
| Immunomodulatory drug, n (%) | 4 (2.7) | 2 (2.3) | 2 (3.2) | 1 | 4 (4.5) | 0 (0.0) | 0.241 | 3 (6.8) | 1 (1.0) | 0.143 |
| ASCT, n (%) | 1 (0.7) | 1 (1.2) | 0 (0.0) | 1 | 1 (1.1) | 0 (0.0) | 1 | 0 (0.0) | 1 (1.0) | 1 |
| Palliative care, n (%) | 63 (42.3) | 36 (41.9) | 27 (42.9) | 1 | 33 (37.5) | 30 (49.2) | 0.211 | 17 (38.6) | 46 (43.8) | 0.688 |
- Note: Values are n (%), median (IQR) or mean (SD).
- Abbreviations: ACEi, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker; ARNI, angiotensin receptor-neprilysin inhibitor; BMI, body mass index; DBP, diastolic blood pressure; dFLC, difference between involved and uninvolved serum free light chains; FAC, fractional area change; HR, heart rate; hs-cTnT, high-sensitivity troponin T; IVS, interventricular septum; LVEF, left ventricular ejection fraction; LVPW, left ventricular posterior wall; MCH, mean corpuscular haemoglobin; MCHC, mean corpuscular haemoglobin concentration; MCV, mean corpuscular volume; MRA, mineralocorticoid receptor antagonist; NOACS, novel oral anticoagulants; NT-proBNP, N-terminal pro-B-type natriuretic peptide; NYHA, New York Heart Association; PLT, platelets; RBC, red blood cell; RDW, red cell distribution width; SBP, systolic blood pressure; SGLT2i, sodium-glucose cotransporter 2 inhibitor; TAPSE, tricuspid annular plane systolic excursion; TSAT, transferrin saturation.
Regardless of the definition used, patients with ID were more likely to be women and tended to exhibit lower levels of haemoglobin. When using the guideline and TSAT definitions, patients with ID had lower MCH, MCHC and MCV compared with non-ID patients. However, when using the serum iron definition, no significant differences were observed between ID and non-ID patients. There was no statistical significance in NT-proBNP levels between patients with or without ID when using the guideline definition, whereas differences were evident under the other two definitions.
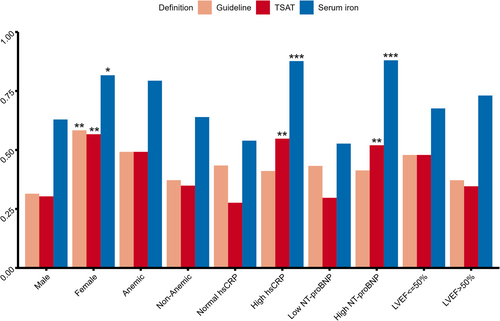
The prevalence of ID according to the three definitions in various patient subgroups is shown in Figure 1. ID was more common in women across all definitions. While the prevalence of ID is slightly higher in anaemic patients compared with non-anaemic patients, it is not statistically significant regardless of the definition used. Compared with patients with lower NT-proBNP and hsCRP levels, those with higher levels were more likely to have a low serum iron or TSAT but not guideline criteria defined ID. There is no significant difference in the prevalence of ID between patients with different LVEF levels.
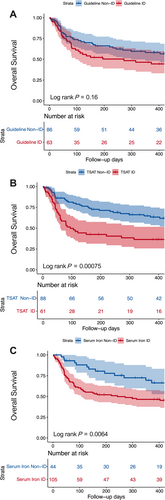
The average follow-up duration for all patients in this study was 359 days, during which 76 (51.0%) patients died. Mortality at 6 months was 39.6% (95% CI, 31%–47.2%) and at 12 months was 46.8% (95% CI, 37.7–54.5%). The Kaplan–Meier curves for the primary outcome according to the three different ID definitions are shown in Figure 2. While the guideline ID definition did not prove to be predictive of the primary outcome, TSAT < 20% (log rank P < 0.001) and serum iron < 13 μmol/L (log rank P < 0.01) were identified as robust predictors.
In univariable analysis, the guideline ID criteria were not associated with higher risk of all-cause mortality [HR 1.39, 95% CI (0.88–2.18), P = 0.158] (Table 2). Conversely, there was a notable difference in the risk of all-cause mortality between patients with or without ID according to the TSAT [HR 2.14, 95% CI (1.36–3.37), P < 0.001] and serum iron [HR 2.16, 95% CI (1.22–3.80), P < 0.01] definitions. Using patients with ferritin levels of 100–299 ng/mL and TSAT > 20% as the reference group, patients with ferritin > 300 ng/mL and TSAT < 20% are associated with the most unfavourable prognosis [HR 2.77, CI (1.28–6.01), P = 0.01], while those with ferritin < 100 ng/mL and TSAT < 20% exhibit a prognosis ranking slightly better [HR 2.26, CI (1.10–4.65), P = 0.027] (Table S2).
| Univariable model | Multivariable modela | |||
|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | |
| Iron biomarkers | ||||
| Ln (ferritin), ng/mL | 0.98 (0.77–1.25) | 0.87 | 0.93 (0.71–1.22) | 0.599 |
| Ln (TSAT), % | 0.60 (0.39–0.91) | 0.0156 | 0.49 (0.29–0.81) | 0.00544 |
| Ln (serum iron), μmol/L | 0.57 (0.37–0.86) | 0.00995 | 0.48 (0.29–0.79) | 0.00371 |
| ID definitions | ||||
| Guideline definition | 1.39 (0.88–2.18) | 0.158 | 1.56 (0.97–2.52) | 0.0658 |
| TSAT definition | 2.14 (1.36–3.37) | 0.00093 | 2.49 (1.54–4.05) | 0.000217 |
| Serum iron definition | 2.16 (1.22–3.80) | 0.0078 | 2.24 (1.23–4.09) | 0.00825 |
- Abbreviations: CI, confidence interval; cTnI, cardiac troponin I; dFLC, difference immunoglobulin free light chains; HR, hazard ratio; NT-proBNP, N-terminal pro-B-type natriuretic peptide; TSAT, transferrin saturation.
- a Multivariable model adjusted for age, gender, haemoglobin and revised Mayo 2012 staging.
Multivariable Cox regression analyses revealed that the ID definition based on TSAT [adjusted HR 2.49, 95% CI (1.54–4.05), P < 0.001] and serum iron [adjusted HR 2.24, 95% CI (1.23–4.09), P < 0.01] was independently associated with higher risk of mortality even after adjustment for age, gender, haemoglobin and revised Mayo 2012 staging. Additionally, patients in the ferritin > 300 ng/mL and TSAT < 20% category were consistently confirmed to have the highest HR value after adjustment for the aforementioned variables [adjusted HR 2.55, CI (1.15–5.65), P = 0.021].
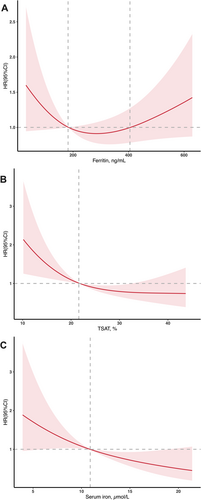
In Figure 3, we used restricted cubic splines to flexibly model and visualize the relation of iron biomarkers with all-cause mortality in AL-CM patients. The association between ferritin levels and all-cause mortality was J-shaped (P for non-linearity < 0.05). The plot showed a substantial reduction of risk within the lower range, which reached the lowest risk around 300 ng/mL and then increased thereafter. Patients with ferritin level between 180 and 400 ng/mL showed relatively lower risks. In contrast, the relationships between TSAT, serum iron and all-cause mortality were linear, with HRs decreasing as their levels increased.
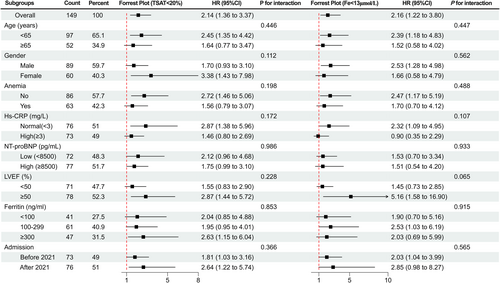
Subgroup analysis showed that there were no significant interactions for the prognostic value of both the TSAT definition and the serum iron definition in all subgroups (based on age, gender, anaemia, hsCRP, NT-proBNP, LVEF, ferritin and admission time categorized as before or after 2021), validating the robustness of TSAT < 20% and serum iron < 13 μmol/L as robust predictor of prognosis (Figure 4).
Discussion
Iron plays a pivotal role in both haematopoietic processes, such as erythropoiesis, oxygen transport and storage, as well as non-haematopoietic processes, including substrate utilization and mitochondrial energy production.18 Hence, ID can result in decreased oxygen delivery and impaired oxygen utilization. Moreover, cells with high energy demand such as cardiac myocytes are particularly vulnerable to the effects of ID on metabolism and cellular energetics. ID may aggravate AL-CM patients' symptoms, reduce their quality of life and worsen their prognosis. It is crucial to accurately and comprehensively study iron metabolism in patients with AL-CM.
To our knowledge, this is the first study to assess systematically the prevalence and prognostic utility of different definitions of ID in a population of advanced AL-CM patients, a significant proportion of whom were classified as NYHA III–IV. Our findings confirm that ID is common in patients with advanced AL-CM, but the prognostic implications differ according to different definitions. ID defined by the current guideline was not associated with all-cause mortality; instead, the TSAT and serum iron definitions were independently associated with worse prognosis even after adjustment for age, gender, haemoglobin, and revised Mayo 2012 staging. Patients with ferritin > 300 ng/mL and TSAT < 20% exhibited the worst prognosis. This subset of patients was not included in the guideline-defined criteria for ID, which could be one of the reasons why guideline-defined ID is not associated with prognosis.
Prevalence of ID in AL-CM
The prevalence of ID based on the guideline and TSAT definition in our AL-CM patients cohort was around 40%, which aligns with the reported prevalence of 45% among AL-CM patients in a prior research.16 But the prevalence of ID reached up to 70%, using the serum iron definition. Nevertheless, these results indicate that the concomitant burden of ID in advanced AL-CM patients is significant and merits heightened clinical awareness. It is suggested that female patients, or patients with elevated hsCRP, NT-proBNP levels may face heightened risk of experiencing concomitant ID.
Prognostic value of different ID definitions in AL-CM
There is already an extensive body of research pertaining to the different definition and prognostic value of ID in patients with heart failure. Among the key studies, Grote Beverborg et al. utilized bone marrow iron staining to propose a biomarker-based definition of ID in heart failure patients. They demonstrated that a TSAT < 19.8% or a serum iron concentration of 13 μmol/L or less offered optimal performance in identifying patients with ID, thereby casting doubt on the diagnostic utility of ferritin. Similarly, Jeness Campodonico et al. found that patients with a TSAT below 20% exhibited a worse prognosis compared with those with a TSAT of 20% or higher. Echoing these findings, Charikleia Papadopoulou et al. reported that ID, whether defined by serum iron concentration or TSAT, was linked to an increased frequency of adverse clinical outcomes in patients with advanced heart failure.
Given that prior studies have shown that guideline-defined ID does not provide prognostic value in patients with CA,16 we seek to investigate whether alternative definitions of ID might offer prognostic insights, thus enabling the more effective detection of high-risk individuals. Our research findings validate that, compared with ID as defined by guidelines, the TSAT or the serum iron defined ID is an independent risk factor for the prognosis of patients with advanced AL-CM and offers superior prognostic predictive value, which is congruent with outcomes from multiple prior studies among the heart failure population.
This might stem from that the TSAT and serum iron definition more precisely captures true ID patients. It is indicated by our study that the current guideline definition of ID may incorporate subsets of patients with low HRs for adverse outcomes while dismiss patient subgroups with high risks. Our study results show that patients in the ferritin > 300 ng/mL and TSAT < 20% category were confirmed to have the highest HR value [adjusted HR 2.55, 95% CI (1.15–5.65)] (Table S2), which was not contained in the guideline criteria. This subset of patients exhibited higher CRP and hsCRP levels, suggesting that they have elevated levels of inflammation. On the contrary, patients with low ferritin (<100 ng/mL) but high TSAT (>20%) tend to exhibit a relatively favourable prognosis [adjusted HR 1.12 95% CI (0.47–2.66)], despite being encompassed within the guideline's definition. These findings are consistent with the results of a previous study conducted by Gabriele Masini et al. in patients with heart failure.19
Circulating iron biomarkers in AL-CM
Moreover, our study further investigated the relationship between circulating iron biomarkers and adverse outcomes. Firstly, ferritin serves as an iron-storage protein and also acts as a positive acute-phase protein.4 Although ferritin is a reliable indicator of total body iron store, it may offer limited diagnostic and prognostic utility in patients with concurrent inflammatory conditions. Our study results indicate that elevated ferritin levels may result from a complex interaction between ID and inflammation, without excluding the possibility of ID. The association between ferritin levels and all-cause mortality was J-shaped (Figure 3), and both low and high levels of ferritin are indicative of poor prognosis. Secondly, TSAT represents the proportion of iron bound to transferrin, calculated as the serum iron divided by TIBC and multiplied by 100. This value reflects the amount of iron accessible for cellular processes. A TSAT value lower than 20% is therefore interpreted as a sign of inadequate iron for metabolic needs. Thirdly, serum iron is almost entirely bound to transferrin, which is consistent with our observation of a strong correlation between serum iron and TSAT. Therefore, it is not surprising that TSAT and serum iron can both function as key variables for prognostic prediction in AL-CM patients. However, given the excessive high prevalence under the serum iron-based definition, combined with the restricted cubic splines (RCS) curves for iron biomarkers, a TSAT of 20% and a serum iron level of 13 μmol/L may not be the corresponding cut-off values in our study population.
Some novel iron biomarkers, such as hepcidin and soluble transferrin receptor (sTfR), have been proposed and demonstrated to have promising diagnostic and prognostic value.20, 21 However, their potential application in AL-CM patients requires further investigation and exploration.
Iron therapy in AL-CM
Randomized controlled trials (RCTs) have demonstrated the safety and efficacy of intravenous iron supplementation in enhancing exercise capacity, and quality of life, as well as reducing the risk of heart failure (HF) hospitalization and cardiovascular/all-cause death in patients with HF with reduced ejection fraction (HFrEF) or HF with mid-range ejection fraction (HFmrEF) and concomitant ID.22-25 However, the impact of intravenous iron supplementation in patients with HF with preserved ejection fraction (HFpEF) and cardiac amyloidosis remains uncertain. Many clinical trials have excluded patients with AL-CM, the AFFIRM-AHF excluded patients with restricted amyloid cardiomyopathy,26 and the IRONMAN study excluded patients with short life expectancy.27 Consequently, the management of CA patients with concurrent ID presents numerous unresolved issues that urgently require answers.
Moreover, the response to intravenous iron therapy differs among patients with ID defined by different criteria in HF. Intravenous iron is likely to benefits for those with anaemia and TSAT < 20%, especially if serum ferritin exceeds 100 μg/L.28 A meta-analysis investigating intravenous iron in patients with heart failure and ID found that patients with TSAT < 20% may have benefited more than those with values ≥ 20%.29 Accurately defining ID is of paramount significance for conducting clinical trials and identifying patients most likely to benefit from IV iron. Our research not only indicates the prognostic significance of TSAT < 20% in risk stratification for patients with advanced AL-CM but also furnishes valuable evidence for defining potential inclusion and exclusion criteria in future randomized controlled trials (RCTs) targeting the AL-CM patients with co-morbid ID.
Limitations
Our study is not without limitation. First, this is a single-centre cohort study that presents data measured at a single time point and does not offer insight into temporal trends in iron status. Second, other biomarkers of iron metabolism such as hepcidin and soluble transferrin receptor were not included in this study. These novel biomarkers may be promising yet are not routinely tested in clinical practice. Last, our study lacks data pertaining to quality of life and exercise tolerance, including the 6-min walk test and cardiopulmonary exercise testing. Future research incorporating these tests within the AL-CM population will be crucial for enhancing patient quality of life and exercise capacity.
Conclusions
In conclusion, different definitions of ID yield inconsistent results in terms of prevalence and prognosis. ID is a prevalent co-morbidity in patients with AL-CM, regardless of the definition used. ID, as defined by TSAT < 20% or serum iron < 13 μmol/L, rather than the guideline definition, emerged as an independent predictor of all-cause mortality in patients with AL-CM.
Acknowledgements
We are grateful to all members who contributed to the study.
Conflict of interest statement
No conflicts of interest to declare.
Funding
This work was supported by National High Level Hospital Clinical Research Funding at Fuwai Hospital, Chinese Academy of Medical Sciences (Grant Number 2023-GSP-GG-26).




