Prognostic impact of gait speed, muscle strength and muscle mass in chronic heart failure—A prospective cohort study
Klemens Ablasser and Nicolas Verheyen contributed equally as last authors.
Abstract
Aims
Heart failure (HF) impairs skeletal muscle mass and function, which contributes to reduced physical performance. We investigated the prognostic impact of gait speed (GS), handgrip strength (HG) and appendicular skeletal muscle index (ASMI) on cardiovascular outcomes in a prospective HF cohort.
Methods
This single-centre prospective cohort study included adults with stable chronic HF with a previous diagnosis of overtly reduced left ventricular ejection fraction (LVEF) <40% and LVEF < 50% at enrolment. GS was measured by the 4 m GS test, maximal HG was measured with a hydraulic dynamometer, and ASMI was measured by dual-energy X-ray absorptiometry. The primary combined outcome was cardiovascular death or worsening HF. Fine and Gray regression models were calculated, treating non-cardiovascular death as the competing event.
Results
Two hundred five patients (78% male) were analysed. The median age was 66 (quartiles: 58–74) years, 31% had diabetes mellitus, and the median LVEF was 37 (30–43) %. Median GS was 1.0 (0.8–1.0) m/s, median HG was 32 (24–40) kg, and median ASMI was 8.0 (7.2–8.9) kg/m2. During a median follow-up of 4.7 (4.0–5.3) years, the primary outcome was observed in 52 patients. In models adjusted for key clinical covariates, lower GS predicted a higher risk of cardiovascular death or worsening HF [subdistribution hazard ratio (SHR) per 0.1 m/s increase = 0.81, 95% confidence interval (CI) 0.68–0.95], whereas HG (SHR per 5 kg increase = 0.97, 95% CI 0.84–1.10) and ASMI (SHR per 1 kg/m2 increase = 1.17, 95% CI 0.94–1.44) did not. In the analysis of effect modification, these associations were consistent across key clinical subgroups.
Conclusions
Higher GS was independently associated with a lower risk of cardiovascular death or worsening HF, whereas HG and ASMI were not. We prospectively confirm GS as a physical performance measure with clear prognostic significance for patients with HF.
Introduction
Heart failure (HF) is a severe debilitating disease with high mortality, primarily driven by cardiovascular (CV) death.1-3 The prevalence in the general population is estimated at 1%–2%,4 reaching up to 9% in those aged 60–79 years.5 With the ageing population, a 46% increase in prevalence by 2030 is estimated,6 further aggravating the already substantial financial burden on healthcare systems, chiefly propelled by frequent hospitalizations.4, 5 HF causes reduced physical performance, which is defined as a whole-body function related to locomotion.7 Although reduced cardiac reserve plays a pivotal role, the reduction of physical performance in HF is not attributable only to impaired cardiac function. Alterations of skeletal muscle functioning and mass are important contributors.8-10
Up to 20% of HF patients suffer from sarcopenia, which is defined as a generalized skeletal muscle disorder and determined by three components: low physical performance, low muscle strength and low muscle mass.7, 11 The diagnosis of sarcopenia is confirmed by evidence of low muscle mass. When all three components are present, sarcopenia is declared as severe.7 Gait speed (GS), handgrip strength (HG) and appendicular skeletal muscle index (ASMI), the sum of the muscle masses of all extremities indexed to height squared, are measures of physical performance, muscle strength and muscle mass, respectively, and can be utilized to assess sarcopenia.7 We refer to GS, HG and ASMI as muscle parameters in further text. Previous prospective studies of these muscle parameters in HF predominantly investigated their impact on all-cause mortality and focused on analysing the effect of a single muscle parameter per cohort.12-17 Less is known about the differential prognostic impact of muscle parameters that reflect all three sarcopenia components within a particular HF cohort. Moreover, the prognostic impact of muscle parameters on adverse CV outcomes in HF is underinvestigated. From a practical perspective, performing multiple muscle tests in clinical practice requires time and financial resources. Therefore, clinicians might prefer to assess only parameters with relevant prognostic information. A differentiated analysis of multiple muscle parameters within a single cohort can provide useful evidence to identify which are clinically most important.
In this study, we aimed to investigate the prognostic impact of GS, HG and ASMI on CV death and worsening HF (WHF) in a prospectively followed cohort of patients with stable chronic HF.
Methods
Study design
This is an analysis of the Role of Comorbidities in Chronic Heart Failure (RoC-HF) study, a prospective, single-centre cohort study enrolling consecutive HF patients of the Outpatient Clinic of the Department of Cardiology of the Medical University of Graz, Austria (ClinicalTrials.gov ID NCT02922478). The study was approved by the institutional review board of the Medical University of Graz (EC Number 28-467 ex 15/16), and written informed consent was obtained from every participant. The RoC-HF study was conducted in compliance with the Good Clinical Practice, the Declaration of Helsinki and Austrian laws. The study included 205 adult patients with stable chronic HF with a previous diagnosis of overtly reduced left ventricular ejection fraction (LVEF) <40% and LVEF < 50% at enrolment. Patients were enrolled between September 2016 and December 2018, as reported previously.18, 19
Study procedures were performed at inclusion following standardized operating procedures predefined per study protocol (ClinicalTrials.gov ID NCT02922478). Blood samples for instant laboratory assessment and storage of blood samples in a biobanking facility (Biobank Graz, Medical University of Graz, Graz, Austria) were drawn in the morning (7:00 am to 11:00 am) after an overnight fast. Estimated glomerular filtration rate (eGFR) was calculated based on serum creatinine using the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) formula.20 N-terminal pro-brain natriuretic peptide (NT-proBNP) was quantified from one-time frozen plasma using an electrochemiluminescence immunoassay based on a polyclonal antibody-based sandwich chemiluminescence assay (Roche Diagnostics) and an autoanalyser (Elecsys 2010). Concomitant medications were determined from patient interviews and matched with medical records. Transthoracic echocardiography was performed with a Vivid 7 or Vivid 9 (GE Healthcare, Chalfont St Giles, UK). LVEF was measured using the Simpson's biplane method.21 Diabetes mellitus was defined as concurrent antidiabetic therapy for the treatment of diabetes or fasting plasma glucose ≥126 mg/dL or glycated haemoglobin (HbA1c) ≥48 mmol/mol.22
Muscle parameters
Skeletal muscle mass was determined using dual-energy X-ray absorptiometry (DXA) with the Lunar iDXA system (GE Healthcare GmbH, Vienna, Austria), as reported previously.23 ASMI was defined as the sum of the muscle mass of all extremities in kg divided by height in m2. Total skeletal muscle mass index (TSMI) was defined as the total muscle mass in kg divided by height in m2. Muscle wasting, that is, low muscle mass, was defined as ASMI < 7.0 kg/m2 in men and <5.5 kg/m2 in women. HG was measured with a JAMAR Hydraulic Hand Dynamometer (Model J00105, Lafayette Instrument Company, USA) in kg, with three consecutive measurements performed with each hand. Maximum HG was defined as the peak strength out of all six measurements. Low muscle strength was defined as <27 kg in men and <16 kg in women. Physical performance was operationalized as GS measured by a 4 m walk test. Low physical performance was defined as GS ≤ 0.8 m/s. These cut-offs are based on the revised European consensus on definition and diagnosis of sarcopenia.7
Outcomes
The primary combined endpoint was defined as CV death or WHF. Patient outcomes were systematically assessed using medical and health insurance records and accessing the Federal Statistical Office of Austria (Statistics Austria) as reported previously.18 Cause of death was adjudicated based on available data by an experienced cardiologist who was blinded to individual patient data (D. v. L.).
Statistical methods
All statistical analyses were performed with Stata (Windows Version 18.0, Stata Corp., Houston, TX, USA). Continuous variables were summarized as medians (25th–75th percentile) and count data as absolute frequencies (column %). The distribution of variables between two groups was compared with rank-sum tests and χ2 tests and Fisher's exact tests, as appropriate. Median follow-up was estimated with a reverse Kaplan–Meier estimator.24 Overall survival (OS) was computed with a Kaplan–Meier estimator, whereas all other time-to-event outcomes were computed with competing risk cumulative incidence estimators. Cumulative incidences were compared with Gray's test, and subdistribution hazards were modelled with univariable and multivariable Fine and Gray models. Co-variables for multivariable adjustment were pre-selected by clinical subject matter knowledge. Discrimination was assessed with the concordance coefficient (C index) from Cox proportional hazard models and model fit with Akaike information criterion (AIC).25 Multivariable models and curves in Figure 2 were fitted on multiple-imputed data (chained equations algorithm, n = 10 imputation datasets). Missing data are reported in Table 1. Gender was accounted for by using gender-specific cut-offs for HG and ASMI7 and performing subgroup analyses. Subgroup analyses were performed by fitting an interaction term between the respective muscle parameter and the selected subgroup variable (Figure 4). Interaction analyses in Figure 4 considered a two-sided ‘relaxed’ alpha of ≤0.1 to indicate statistical significance, whereas all other analyses considered the standard two-sided alpha of ≤0.05.
| Variable | n (% miss.) | Overall (n = 205) | No WHF or CV death (n = 153) | WHF or CV death (n = 52) | Pa |
|---|---|---|---|---|---|
| Demographic variables | |||||
| Age (years) | 205 (0%) | 66 [58–74] | 65 [57–73] | 69 [62–74] | 0.064 |
| Female sex | 205 (0%) | 45 (22%) | 37 (24%) | 8 (15%) | 0.185 |
| Caucasian ethnicity | 205 (0%) | 205 (100%) | 153 (100%) | 52 (100%) | n/a |
| Height (m) | 205 (0%) | 1.73 [1.67–1.79] | 1.73 [1.67–1.78] | 1.74 [1.69–1.81] | 0.341 |
| Weight (kg) | 205 (0%) | 86 [76–95] | 82 [75–95] | 90 [81–96] | 0.061 |
| Body mass index (kg/m2) | 205 (0%) | 28 [25–32] | 28 [25–31] | 30 [27–32] | 0.087 |
| HF aetiology | 205 (0%) | - | - | - | 0.113 |
| Ischaemic | - | 86 (42%) | 58 (38%) | 28 (54%) | - |
| Idiopathic dilated cardiomyopathy | - | 103 (50%) | 83 (54%) | 20 (38%) | - |
| Other aetiologiesb | - | 16 (8%) | 12 (8%) | 4 (8%) | - |
| Comorbidities | |||||
| Diabetes mellitus (all types) | 205 (0%) | 63 (31%) | 41 (27%) | 22 (42%) | 0.036 |
| Arterial hypertension | 195 (5%) | 143 (73%) | 107 (74%) | 36 (72%) | 0.805 |
| Atrial fibrillation | 205 (0%) | 85 (41%) | 55 (36%) | 30 (58%) | 0.006 |
| Coronary artery disease | 205 (0%) | 143 (70%) | 104 (68%) | 39 (75%) | 0.341 |
| Echocardiographic parameters | |||||
| LVEF (%) | 205 (0%) | 37 [30–43] | 39 [31–45] | 32 [26–39] | 0.002 |
| E/e′ | 196 (4%) | 13.6 [10.3–19.0] | 13.3 [10.0–17.5] | 15.2 [10.7–21.0] | 0.052 |
| Laboratory parameters | |||||
| NT-proBNP (pg/mL) | 205 (0%) | 964 [363–2151] | 635 [249–1516] | 2173 [1129–3910] | <0.0001 |
| eGFR (mL/min/1.73 m2) | 199 (3%) | 64 [48–80] | 68 [51–83] | 49 [38–68] | <0.0001 |
| Albumin (g/L) | 197 (4%) | 4.5 [4.3–4.7] | 4.5 [4.3–4.7] | 4.4 [4.1–4.5] | 0.024 |
| Co-medication | |||||
| Beta-blocker | 205 (0%) | 196 (96%) | 146 (95%) | 50 (96%) | 0.825 |
| ACEi/ARB/ARNi | 205 (0%) | 189 (92%) | 142 (93%) | 47 (90%) | 0.573 |
| MRA | 205 (0%) | 159 (78%) | 120 (78%) | 39 (75%) | 0.608 |
| SGLT2i | 205 (0%) | 9 (4%) | 7 (5%) | 2 (4%) | 0.999 |
| Loop diuretic | 205 (0%) | 120 (59%) | 81 (53%) | 39 (75%) | 0.005 |
| VKA | 205 (0%) | 51 (25%) | 35 (23%) | 16 (31%) | 0.255 |
| NOAC | 205 (0%) | 52 (25%) | 33 (22%) | 19 (37%) | 0.032 |
| Muscle parameters | |||||
| 4 m gait speed (m/s) | 185 (10%) | 1.0 [0.8–1.0] | 1.0 [0.8–1.0] | 0.8 [0.7–1.0] | <0.0001 |
| Low 4 m gait speedc | 185 (10%) | 78 (42%) | 45 (32%) | 33 (73%) | <0.0001 |
| Maximum handgrip strength (kg) | 201 (2%) | 32 [24–40] | 32 [24–42] | 30 [23–36] | 0.100 |
| Low handgrip strengthc | 201 (2%) | 37 (18%) | 24 (16%) | 13 (25%) | 0.131 |
| ASMI (kg/m2) | 176 (14%) | 8.0 [7.2–8.9] | 8.0 [7.2–8.9] | 8.0 [7.6–9.0] | 0.493 |
| Low ASMIc | 176 (14%) | 16 (9%) | 11 (8%) | 5 (12%) | 0.430 |
- Note: Reported data are medians [25th–75th percentile] for continuous variables and absolute frequencies (column %) for count data. ‘n (% miss.)’ indicates the number of patients with the observed variable (% missing).
- Abbreviations: ACEi, angiotensin-converting enzyme inhibitor; ARB, angiotensin II receptor blocker; ARNi, angiotensin receptor neprilysin inhibitor; ASMI, appendicular skeletal muscle index; CV, cardiovascular; eGFR, estimated glomerular filtration rate; HF, heart failure; HG, handgrip strength; LVEF, left ventricular ejection fraction; MRA, mineralocorticoid receptor antagonist; NOAC, non-vitamin K-dependent oral anticoagulant; NT-proBNP, N-terminal pro-brain natriuretic peptide; SGLT2i, sodium–glucose cotransporter 2 inhibitor; VKA, vitamin K antagonist; WHF, worsening heart failure.
- a P values are from rank-sum tests or χ2 tests or Fisher's exact tests, as appropriate.
- b Other aetiologies include inflammatory CMP (n = 13), hypertrophic CMP (n = 2) and cardiac sarcoidosis (n = 1).
- c Low 4 m gait speed was defined as a 4 m gait speed ≤0.8 m/s, low maximum HG was defined as <27 kg in males and <16 kg in females, and low ASMI was defined as <7 kg/m2 in males and <5.5 kg/m2 in females.
Results
Descriptive statistics of the cohort and outcomes
Two hundred five Caucasian patients with a median age of 66 years (25th–75th percentile: 58–74), 45 (22%) females, were included in the study (Table 1). These patients were followed up for a median interval of 4.7 years, with 75% and 25% of the cohort being followed up for at least 4.0 and 5.3 years, respectively. During this period, we observed 46 patients (22%) to have at least one WHF event, and the number of events per patient with at least one event ranged from 1 to 10. Among the 58 patients (28%) who died, 18 deaths (31%) were adjudicated to CV causes and 40 deaths (69%) to other causes. The corresponding 5 year estimates [95% confidence interval (CI)] for OS, all-cause mortality, WHF, CV death and death from other causes were 69% (61–75), 31% (25–39), 23% (17–29), 9% (5–14) and 22% (16–29), respectively (Figure S1). The primary outcome (WHF or CV death) and secondary outcome (WHF or all-cause death) occurred in 52 (25%) and 79 (39%) patients, corresponding to 5 year cumulative incidences (95% CI) of 26% (20–32) and 41% (34–49), respectively (Figure S2).
Descriptive statistics of muscle parameters and their association with covariates
Median estimates of GS, maximum HG and ASMI were 1.0 (quartiles: 0.8–1.0) m/s, 32 (quartiles: 24–40) kg and 8.0 (quartiles: 7.2–8.9) kg/m2; 78 patients (42%), 37 patients (18%) and 16 patients (9%) were classified to have low GS, low maximum HG and low ASMI, respectively (Table 1). Having low measurements of these parameters was not consistently associated with baseline covariates. The strongest associations were observed between GS and higher age, atrial fibrillation, higher NT-proBNP, lower eGFR and treatment with loop diuretics (Table S1).
Muscle parameters and outcome—Univariable analysis
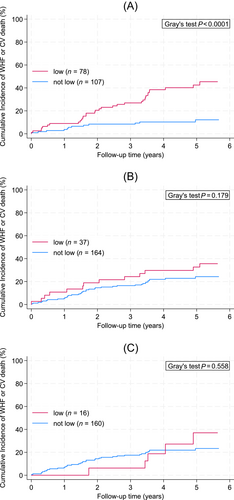
The primary outcome of this study, a composite of WHF and CV death, occurred more frequently in patients with low GS, whereas results for low maximum HG and low ASMI were less pronounced. In detail, 5 year cumulative incidences of WHF and CV death were 43%, 33% and 37% in patients with GS, HG and ASMI measurements below the respective cut-offs, and 12%, 24% and 23% in patients with measurements greater than or equal to these cut-offs (Figure 1A–C). In univariable competing risk regression, the risks of developing WHF or CV death were 4.4-fold, 1.5-fold and 1.29-fold higher in patients with low GS, HG and ASMI measurements, respectively (Table 2). Treating the three muscle parameters as continuous variables in these models increased the strength of association with the outcome for GS and HG, but not for ASMI (Table 2). In the analysis of model fit and discriminatory performance, only GS (both continuous and cut-off specifications) appeared to be a meaningful predictor of the primary outcome (Table S2).
| Variable | Univariable subdistribution hazard ratio (SHR) | 95% CI | P |
|---|---|---|---|
| Demographic variables | |||
| Age (per 5 year increase) | 1.17 | 1.03–1.34 | 0.019 |
| Female sex | 0.64 | 0.30–1.36 | 0.245 |
| Body mass index (per 5 kg/m2 increase) | 1.13 | 0.92–1.40 | 0.257 |
| Comorbidities | |||
| Diabetes mellitus (all types) | 1.76 | 1.02–3.04 | 0.043 |
| Atrial fibrillation | 2.12 | 1.22–3.67 | 0.008 |
| Coronary artery disease | 1.36 | 0.73–2.54 | 0.339 |
| Echocardiographic parameters | |||
| LVEF (per 5% increase) | 0.79 | 0.69–0.90 | <0.0001 |
| E/e′ | 1.04 | 1.01–1.07 | 0.007 |
| Laboratory parameters | |||
| NT-proBNP (per 1000 pg/mL increase) | 1.22 | 1.16–1.28 | <0.0001 |
| eGFR (per 10 mL/min/1.73 m2 increase) | 0.75 | 0.66–0.86 | <0.0001 |
| Albumin (per 1 g/L increase) | 0.51 | 0.25–1.03 | 0.061 |
| Co-medication | |||
| Beta-blocker | 1.17 | 0.26–5.26 | 0.839 |
| ACEi/ARB/ARNi | 0.83 | 0.34–2.02 | 0.681 |
| MRA | 0.89 | 0.48–1.65 | 0.705 |
| SGLT2i | 0.87 | 0.21–3.50 | 0.841 |
| Loop diuretic | 2.34 | 1.25–4.39 | 0.008 |
| VKA | 1.40 | 0.78–2.52 | 0.265 |
| NOAC | 1.89 | 1.08–3.31 | 0.027 |
| Muscle parameters | |||
| 4 m gait speed (per 0.1 m/s increase) | 0.71 | 0.63–0.81 | <0.0001 |
| Low 4 m gait speeda | 4.40 | 2.24–8.61 | <0.0001 |
| Maximum handgrip strength (per 5 kg increase) | 0.87 | 0.78–0.97 | 0.009 |
| Low handgrip strengtha | 1.54 | 0.82–2.88 | 0.179 |
| ASMI (per 1 kg/m2 increase) | 1.08 | 0.87–1.35 | 0.477 |
| Low ASMIa | 1.29 | 0.56–2.97 | 0.558 |
- Note: Regression results were obtained from univariable Fine and Gray models, treating death from other causes as the competing event of interest.
- Abbreviations: ACEi, angiotensin-converting enzyme inhibitor; ARB, angiotensin II receptor blocker; ARNi, angiotensin receptor neprilysin inhibitor; ASMI, appendicular skeletal muscle index; CI, confidence interval; eGFR, estimated glomerular filtration rate; HG, handgrip strength; LVEF, left ventricular ejection fraction; MRA, mineralocorticoid receptor antagonist; NOAC, non-vitamin K-dependent oral anticoagulant; NT-proBNP, N-terminal pro-brain natriuretic peptide; P, Wald test P value; SGLT2i, sodium–glucose cotransporter 2 inhibitor; SHR, subdistribution hazard ratio; VKA, vitamin K antagonist.
- a Low 4 m gait speed was defined as a 4 m gait speed ≤0.8 m/s, low maximum HG was defined as <27 kg in males and <16 kg in females, and low ASMI was defined as <7 kg/m2 in males and <5.5 kg/m2 in females.
Muscle parameters and outcome—Multivariable analysis
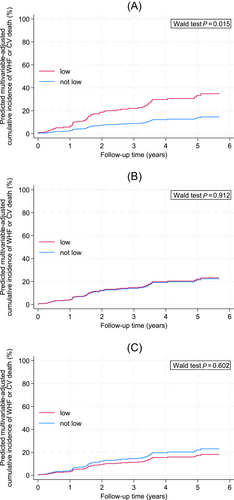
Other predictors of an increased risk of WHF or CV death were, among others, higher age, diabetes, atrial fibrillation, lower LVEF, higher E/e′, higher NT-proBNP, lower eGFR and treatment with loop diuretics (Table 2). In multivariable analysis adjusting for the clinically most important co-variables, namely, age, diabetes mellitus, atrial fibrillation, LVEF, eGFR and NT-proBNP, only low GS remained independently associated with an increased risk of the primary outcome (Table 3 and Figure 2A–C).
| Variable | Multivariable subdistribution hazard ratio (SHR) | 95% CI | P |
|---|---|---|---|
| Multivariable Model 1 | |||
| 4 m gait speed (per 0.1 m/s increase) | 0.81 | 0.68–0.95 | 0.017 |
| Age (per 5 year increase) | 0.95 | 0.78–1.11 | 0.537 |
| Diabetes | 1.14 | 0.63–2.09 | 0.660 |
| Atrial fibrillation | 1.19 | 0.65–2.17 | 0.583 |
| LVEF (per 5% increase) | 0.84 | 0.68–0.99 | 0.062 |
| eGFR (per 10 mL/min/1.73 m2 increase) | 0.89 | 0.74–1.05 | 0.200 |
| NT-proBNP (per 1000 pg/mL increase) | 1.15 | 1.06–1.23 | <0.0001 |
| Multivariable Model 2 | |||
| Low 4 m gait speeda | 2.71 | 1.21–6.06 | 0.015 |
| Age (per 5 year increase) | 0.96 | 0.80–1.13 | 0.670 |
| Diabetes | 1.17 | 0.65–2.10 | 0.600 |
| Atrial fibrillation | 1.14 | 0.63–2.06 | 0.674 |
| LVEF (per 5% increase) | 0.82 | 0.68–0.96 | 0.025 |
| eGFR (per 10 mL/min/1.73 m2 increase) | 0.87 | 0.73–1.01 | 0.097 |
| NT-proBNP (per 1000 pg/mL increase) | 1.15 | 1.08–1.22 | <0.0001 |
| Multivariable Model 3 | |||
| Maximum handgrip strength (per 5 kg increase) | 0.97 | 0.84–1.10 | 0.619 |
| Age (per 5 year increase) | 1.02 | 0.85–1.18 | 0.849 |
| Diabetes | 1.20 | 0.67–2.16 | 0.538 |
| Atrial fibrillation | 1.32 | 0.72–2.43 | 0.366 |
| LVEF (per 5% increase) | 0.79 | 0.65–0.93 | 0.010 |
| eGFR (per 10 mL/min/1.73 m2 increase) | 0.84 | 0.70–0.98 | 0.045 |
| NT-proBNP (per 1000 pg/mL increase) | 1.13 | 1.06–1.20 | <0.0001 |
| Multivariable Model 4 | |||
| Low handgrip strengtha | 1.04 | 0.50–2.17 | 0.912 |
| Age (per 5 year increase) | 1.02 | 0.86–1.19 | 0.774 |
| Diabetes | 1.22 | 0.68–2.17 | 0.502 |
| Atrial fibrillation | 1.32 | 0.70–2.49 | 0.382 |
| LVEF (per 5% increase) | 0.80 | 0.66–0.94 | 0.011 |
| eGFR (per 10 mL/min/1.73 m2 increase) | 0.84 | 0.70–0.98 | 0.034 |
| NT-proBNP (per 1000 pg/mL increase) | 1.14 | 1.07–1.21 | <0.0001 |
| Multivariable Model 5 | |||
| ASMI (per 1 kg/m2 increase) | 1.17 | 0.94–1.44 | 0.159 |
| Age (per 5 year increase) | 1.07 | 0.88–1.25 | 0.467 |
| Diabetes | 1.22 | 0.68–2.18 | 0.505 |
| Atrial fibrillation | 1.26 | 0.69–2.32 | 0.456 |
| LVEF (per 5% increase) | 0.80 | 0.66–0.94 | 0.012 |
| eGFR (per 10 mL/min/1.73 m2 increase) | 0.83 | 0.70–0.97 | 0.029 |
| NT-proBNP (per 1000 pg/mL increase) | 1.13 | 1.06–1.21 | <0.0001 |
| Multivariable Model 6 | |||
| Low ASMIa | 0.76 | 0.26–2.17 | 0.602 |
| Age (per 5 year increase) | 1.03 | 0.86–1.20 | 0.754 |
| Diabetes | 1.25 | 0.70–2.24 | 0.455 |
| Atrial fibrillation | 1.34 | 0.73–2.44 | 0.344 |
| LVEF (per 5% increase) | 0.80 | 0.66–0.94 | 0.013 |
| eGFR (per 10 mL/min/1.73 m2 increase) | 0.83 | 0.69–0.97 | 0.027 |
| NT-proBNP (per 1000 pg/mL increase) | 1.14 | 1.07–1.21 | <0.0001 |
- Note: The table reports six models, one for each of the three muscle parameters as a continuous variable (Models 1, 3 and 5) and one for each of the parameters as a binary variable. Regression results were obtained from univariable Fine and Gray models, treating death from other causes as the competing event of interest.
- Abbreviations: ASMI, appendicular skeletal muscle index; CI, confidence interval; eGFR, estimated glomerular filtration rate; HG, handgrip strength; LVEF, left ventricular ejection fraction; NT-proBNP, N-terminal pro-brain natriuretic peptide; P, Wald test P value; SHR, subdistribution hazard ratio.
- a Low 4 m gait speed was defined as a 4 m gait speed ≤0.8 m/s, low maximum HG was defined as <27 kg in males and <16 kg in females, and low ASMI was defined as <7 kg/m2 in males and <5.5 kg/m2 in females.
Muscle parameters and outcome—Subgroup analyses and possible effect modifiers
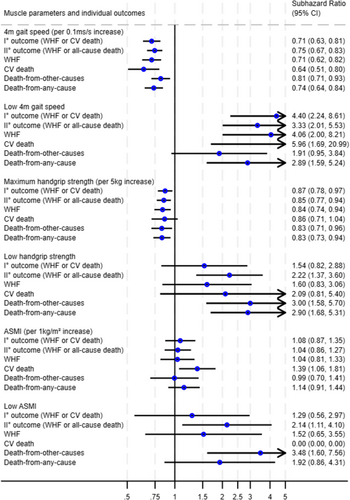
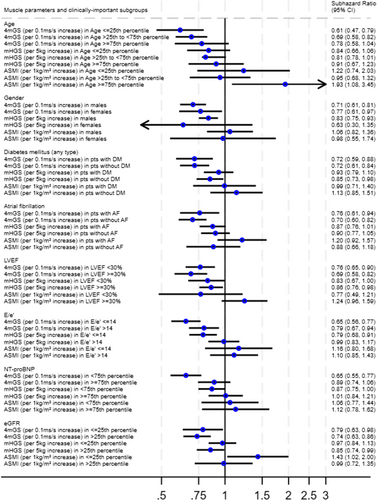
The above-described associations between low measurements of muscle parameters and worse outcome were broadly consistent across the secondary and exploratory outcomes WHF, CV death, all-cause death and death from other causes (Figure 3). In an exploratory analysis of potential interactions across clinically meaningful patient and biomarker subgroups (age, gender, diabetes, atrial fibrillation, systolic function as represented by LVEF, left ventricular filling pressures as represented by E/e′, NT-proBNP and eGFR), the associations between muscle parameters and the primary outcome were also broadly consistent across these groups, such as women and men, patients with and without evidence of elevated filling pressures, and patients with and without diabetes (Figure 4).
Discussion
This study investigated the prognostic impact of multiple muscle parameters, that is, GS (a measure of physical performance), HG (muscle strength) and ASMI (muscle mass), on CV outcomes in a prospectively followed HF cohort. Our work extends previous research by analysing the combined outcome of CV death or WHF while simultaneously taking death from non-CV causes into account by applying the Fine and Gray regression method.26, 27 Although CV death is the leading mode of death in HF,1, 2 previous prospective studies of these muscle parameters mainly focused on the outcome of all-cause mortality.13, 16, 17, 28 In our analysis, higher GS was significantly associated with a decreased rate of the primary combined outcome of CV death or WHF in multivariable-adjusted analysis. Although higher HG was significantly associated with a decreased rate of the primary outcome in univariable analysis, the significant effect vanished after adjustment for confounders. ASMI turned out to have the weakest association with the primary outcome, because its effect did not reach statistical significance either in univariable or in multivariable models. The results were consistent when analysing muscle parameters as both continuous and cut-off-based (binary) variables. GS showed the highest univariable discrimination (C index of 0.70) of the primary outcome, followed by HG (C index of 0.57) and, lastly, ASMI (C index of 0.54).
The results of our study are in line with previous findings of the prognostic impact of GS in HF. Chaudhry et al. found a slow GS, defined as <0.8 m/s, to be an independent predictor of hospitalizations in 758 prospectively followed patients with HF, hazard ratio (HR) 1.28, 95% CI 1.06–1.55.29 Pulignano et al. have found higher GS to be independently associated with all-cause death and providing incremental value in risk stratification. The study prospectively followed 331 patients with HF for 1 year. GS was stratified into tertiles: ≤0.65, 0.66–0.99 and ≥1.0 m/s. In multivariable-adjusted analysis, the risk of all-cause death significantly decreased with higher GS, HR 0.62 per tertile, 95% CI 0.43–0.88. When GS was added to the 3C-HF score, risk stratification improved as shown by a net reclassification improvement of 0.49, 95% CI 0.26–0.73.13 Our study expands the existing literature by demonstrating a significant association of GS with adverse CV outcomes, defined by CV death or WHF, in patients with stable chronic HF. A possible advantage of GS over other muscle parameters, especially muscle mass, might be its sensitivity to change with regard to the instant health status of the individual. In fact, an increase in GS during hospitalization for acute decompensated HF was demonstrated to be associated with improved outcome.28 Many cut-offs for GS have been proposed, depending on the condition studied.7, 30, 31 In our study, GS ≤ 0.8 m/s, which is recommended as a cut-off to declare reduced physical performance by the European consensus on definition and diagnosis of sarcopenia,7 was associated with an increased rate of the primary outcome, subdistribution HR (SHR) 2.71, 95% CI 1.21–6.06. It is, however, debatable whether the dichotomization of prognostic factors is meaningful in contrast to the use of continuous values. From a methodological perspective, dichotomization is associated with loss of information and power in prognostic regression modelling and should rather be avoided.32, 33 Our analysis supports these recommendations. We assessed discrimination in univariable Cox proportional hazard regression of the primary outcome using GS, HG and ASMI as both dichotomized binary and continuous variables. GS displayed the best discrimination and had as a continuous variable a larger C index (0.70) than as a dichotomous variable (0.67) (see Table S2 for more information).
Results from the SICA-HF study have shown that patients with HF and low skeletal muscle mass have lower HG and peak oxygen consumption in cardiopulmonary exercise testing and walk at slower pace.11 In our study, HG did not show any significant association with the primary outcome, SHR 0.97, 95% CI 0.84–1.1, in the multivariable-adjusted Fine and Gray regression model. This is in contrast to previous findings. In an individual participant data meta-analysis of 23 480 patients, HG was associated with CV death [odds ratio (OR) 0.84, 95% CI 0.79–0.89], all-cause death (OR 0.87, 95% CI 0.85–0.89) and hospital admission for HF (OR 0.88, 95% CI 0.84–0.92). However, the definition of HF varied across studies, and studies that defined HF according to echocardiography and Framingham criteria did not yield consistently significant associations.34 In another study, Castillo-Martínez et al. prospectively followed 546 stable outpatients with HF for 36 months. Low HG in combination with abnormal fluid distribution was associated with an increased risk of death (HR 2.8, 95% CI 1.25–6.4). However, this association was observed only in men. Furthermore, multivariable models that included low HG without abnormal fluid distribution were coherent with findings of our present study, as the effect of HG on survival did not reach statistical significance (P = 0.8).14 While the evidence on the prognostic impact of HG in patients with HF is currently inconclusive, HG could represent a simple tool for the risk assessment of developing HF. In a prospective cohort study from Laukkanen et al., HG in the elderly was inversely associated with incident HF during a median follow-up of 17.1 years.35 A similar association has been reported also for GS.36 Low muscle strength and physical performance might well reflect an unfavourable metabolic state leading to insulin resistance, diabetes mellitus and eventually HF. In fact, there is evidence that low HG is also associated with incident diabetes mellitus,37, 38 which makes patients particularly prone to HF. In the Framingham Heart Study, diabetes mellitus was associated with as much as a four-fold risk increase of incident HF.39
We found no significant effect of muscle mass, operationalized as ASMI, on the primary outcome studied, which is divergent from previous research.12, 15 Von Haehling et al. investigated the prognostic effect of muscle wasting in 268 patients of the SICA-HF cohort, including 17.5% with low muscle mass, and found an 80% relative risk increase of all-cause death in patients with an ASMI < 7.26 or <5.45 kg/m2 in men and women, respectively.12 In a Japanese study, Konishi et al. performed DXA after decongestion therapy in 418 patients admitted for WHF and found a 17% relative risk increase per 1 kg decrement in appendicular skeletal muscle mass. The cohort included as many as 44% of participants with reduced muscle mass, according to the definition of the Asian Working Group for Sarcopenia.15, 31 In comparison with the above-mentioned studies, the proportion of patients with muscle wasting was much lower in our cohort. Only 16 of our patients (9%) fulfilled the criterion of low muscle mass, ASMI < 7.0 kg/m2 in men and <5.5 kg/m2 in women, according to the European Working Group on Sarcopenia in Older People.7 The fact that most participants in our cohort did not present with sarcopenia, as defined by low ASMI, suggests that the systemic catabolic processes associated with HF were not as pronounced in our cohort, and therefore, we did not find a significant association between ASMI and the primary outcome.
The strength of our study is the comprehensive assessment of muscular function and quantity by validated diagnostic tests and the simultaneous consideration of GS, HG and ASMI, reflecting all three components instrumental in the diagnosis of sarcopenia, in our analyses. We studied whether key subgroups defined by such variables as gender, comorbidities and organ function biomarkers may modify the association between muscle parameters and the primary outcome. While some of these analyses may have limited power due to smaller subgroup sizes [e.g., only 22% (n = 45) of our cohort were female], we nonetheless did not observe an inconsistent effect of muscle parameters across these key subgroups.
An important limitation of our study is the fact that physical performance, muscle strength and muscle quantity can be assessed by alternative tests as well, for example, physical performance by the 6 min walk distance, muscle strength by the chair stand test, and muscle mass by computational tomography or magnetic resonance imaging.7 Our results are thus confined to the muscle parameters investigated in this work. Further limitations include a solely Caucasian population with a predominantly reduced LVEF. Whether our results apply to other ethnic groups and whether our findings extrapolate to HF with preserved ejection fraction remain unclear. Future research should elucidate whether GS is a suitable prognostic factor across the spectrum of left ventricular function and ethnic diversity. Dedicated studies on the clinical significance of diverse muscle parameters in females with HF will be necessary as well.
Conclusions
To conclude, in patients with stable HF with reduced or improved LVEF, out of GS, HG and ASMI, only GS had a significant prognostic impact on adverse CV outcomes in our study. Evaluating muscular function with respect to locomotion for the risk assessment of adverse CV outcomes seems more important than the evaluation of muscle strength and mass.
Acknowledgements
We thank all our patients who participated in the RoC-HF study. Samples used for the present analyses were partly provided by the Biobank Graz. We thank Karin Brander and Sandra Sailer for their assistance in conducting the study.
Conflict of interest statement
None declared.




