Heart failure decompensation with cardiogenic shock exhibits distinct sequential inflammatory profiles
Darshan H. Brahmbhatt and Fernando Luis Scolari contributed equally to this work.
Abstract
Aims
The inflammatory profile of cardiogenic shock (CS) after myocardial infarction affects outcomes; however, little is known about the impact of inflammatory changes in CS caused by acute decompensated heart failure (ADHF-CS). We measured levels of inflammatory cytokines in patients with ADHF-CS admitted to a cardiac intensive care unit (CICU).
Methods
We identified patients admitted to our CICU with ADHF-CS who had consented to having biospecimens stored. We identified two comparator groups of patients with HF seen as outpatients with stored biospecimens: firstly, those who had no history of decompensation and did not develop CS during follow-up after sample acquisition (stable HF), and secondly, a group of patients who developed CS during follow-up (pre-CS). All samples underwent 48-plex cytokine and white blood cell differential testing with the differences between groups analysed by comparing means.
Results
Eighty-four ADHF-CS patients were identified who had samples obtained at a median of 2 [inter-quartile range (IQR) 0–7] days after CICU admission. Thirty-six pre-CS outpatients had samples taken 137 (IQR 41–258) days before admission with CS, and 338 stable HF control patients were included. Cytokine profiles differed between ADHF-CS and stable HF. Patients with CS had higher pro-inflammatory cytokine levels [including interleukin-1 (IL-1), interleukin-6 (IL-6) and interleukin-8 (IL-8)] and total white cell counts than stable HF patients. Analysis of the pre-CS outpatient group suggested an intermediate stage in subacute transition to CS.
Conclusions
ADHF-CS is characterized by high levels of pro-inflammatory cytokines and total white count, compared with ambulatory HF. Decompensation from HF has two distinct inflammatory phases that may help identify outpatients at risk of CS.
Introduction
Cardiogenic shock (CS) is the most severe form of acute heart failure (HF) characterized by severely reduced cardiac output and inadequate end-organ perfusion leading to tissue hypoxia. Patients with CS suffer significant morbidity and mortality1 with in-hospital mortality reaching up to 50%.2 The pathophysiological underpinnings of CS remain to be fully elucidated, but inflammation is thought to play a key role.3 One of the several potential mechanisms for worsening of the shock state has been proposed to occur through the release of inducible nitric oxide and its mediators, resulting in inappropriate vasodilatation.4 Additionally, the development of systemic inflammatory response syndrome (SIRS) is seen in up to a third of patients at presentation and portends a more severe clinical syndrome associated with worse outcomes.5
The inflammatory profile associated with CS is now being studied in greater detail. However, the majority of studies have focused on CS following acute myocardial infarction (AMI-CS). In AMI-CS, an acute ischaemic insult leads to an immediate drop in cardiac output, which may then be compounded by the ischaemia–reperfusion injury associated with acute revascularization.6 In contrast to this, CS caused by acute decompensated HF (ADHF-CS) often has a heterogeneous trajectory, even before presentation. This realization is important in that these patients may have a different clinical profile and outcomes after discharge.7 The HF state has its own heightened inflammatory state, which is also known to affect outcomes.8 Thus far, the inflammatory profile of patients with ADHF-CS has not been fully characterized nor has the transition from a clinically stable, outpatient-managed HF state to ADHF-CS, and it is unclear if there are changes in the inflammatory profile that occur during this clinical deterioration.
This retrospective, cross-sectional study was designed to investigate the profile and prognostic significance of circulating cytokines and cellular inflammatory profiles in patients with ADHF-CS admitted to a cardiac intensive care unit (CICU) and to define the association between circulating cytokines in outpatients with HF and those admitted with CS.
Methods
Study location
The Peter Munk Cardiac Centre (PMCC) is a large, quaternary, cardiovascular referral centre located at the University Health Network (UHN) in Ontario, Canada. It offers a full range of advanced HF assessment and treatment modalities, including temporary and durable mechanical circulatory support (MCS), alongside orthotopic heart transplant (OHT). The PMCC Cardiovascular Biobank has collected biospecimens from consented patients treated at the centre for future studies. This study was approved by the UHN Research Ethics Board (#18-6188) and complied with the Declaration of Helsinki.
Patient selection
A prospective database of all CICU admissions at our institution was used to identify patients admitted with CS. Patients with a diagnosis of AMI-CS, a previous admission with CS, a history of OHT or a durable ventricular assist device (VAD) were excluded. Patients with ADHF-CS were composed of either those patients with chronic HF or those patients presenting for the first time with HF decompensation. All patients who had biospecimens collected while in the CICU were included in this study.
Two cohorts of HF outpatients with biospecimens were identified. The first comparison cohort included patients with no history of HF decompensation in the prior 12 months to assessment and biospecimen collection and were denoted as the ‘stable HF’ cohort. Any of these patients with an admission for CS during follow-up were also excluded from the analysis. The second comparator cohort were HF patients who had been assessed while outpatients, who deteriorated during follow-up after biospecimen collection, requiring admission to an intensive care unit (ICU) with CS, and were denoted as the ‘pre-CS’ cohort. This was a cross-sectional study of patients with no repeated measures in the same patients at different time points.
Data collection
Clinical and laboratory data for CS patients were collected within the first 24 h of admission to the CICU. The Society for Cardiovascular Angiography and Interventions (SCAI) CS stage was determined at 24 h after admission.9 Interventions including renal replacement therapy, mechanical ventilation, and institution of MCS during CICU admission and the outcomes of in-hospital mortality, durable VAD and OHT were also recorded. Data for HF patients were collected at the time of the clinic visit closest to biospecimen collection, and follow-up continued until the last clinic visit at our institution.
Biospecimen analysis
In total, 84 patients had biospecimens collected at a median of 2 [inter-quartile range (IQR) 0–7] days after CICU admission. The stable HF cohort included 338 patients, the majority of whom had samples collected on the day of the clinic visit where baseline clinical data were collected. The pre-CS comparator group, comprising 36 ambulatory patients who went on to deteriorate and be admitted with CS, had biospecimens collected at a median of 137 (41–258) days before admission with CS. All biospecimens were stored at −80°C. Aliquots of plasma were used in this study and analysed with the Luminex™ 200 System (Luminex, Austin, TX, USA) by Eve Technologies Corp. (Alberta, Canada) using the Human Cytokine 48-Plex Discovery Assay® (MilliporeSigma, MA, USA) according to the manufacturer's protocol. The 48-plex consisted of sCD40L, EGF, CCL11, FGF-2, FLT3 ligand, CX3CL1, G-CSF, GM-CSF, CXCL1, IFNα2, IFNγ, interleukin (IL)-1α, IL-1β, IL-1 receptor antagonist (IL-1RA), IL-2, IL-3, IL-4, IL-5, IL-6, IL-7, IL-8, IL-9, IL-10, IL-12p40, IL-12p70, IL-13, IL-15, IL-17A, IL-17E/IL-25, IL-17F, IL-18, IL-22, IL-27, CXCL10, CCL2, CCL7, M-CSF, MDC, CXCL9, CCL3, CCL4, PDGF-AA, PDGF-AB/BB, CCL5, TGF-α, tumour necrosis factor-α (TNF-α), TNF-β and VEGF-A. White blood cell counts were obtained through in-hospital testing using a Sysmex XN-10 Hematology Analyzer (Sysmex Corporation, Kobe, Japan).
Data analysis
All continuous data are reported as mean ± standard deviation (SD) or median (IQR) as appropriate. Categorical data are reported with absolute and relative frequencies. Cytokine levels were log-transformed to normalize data distribution. Mean values were compared between groups using independent-samples Mann–Whitney U test. Clinical variables such as mean arterial pressure (MAP) and number of vasopressors/inotropes and laboratory values including B-type natriuretic peptide (BNP) and admission serum lactate levels were correlated with log-transformed cytokine levels with statistical significance set at 0.05 for these analyses using a two-sided P value. Finally, an exploratory analysis of correlation between cytokines was undertaken using Pearson's test. Given the large number of comparisons, the statistical significance was set at 0.001 for these analyses. All analyses were performed with SPSS, Version 25.0 (SPSS Inc., DE, USA).
Results
Numbers of patients
Samples were analysed from 84 patients admitted with CS to the CICU with ADHF-CS (CS patients); 338 ambulatory patients with a diagnosis of HF, with no history of OHT, VAD and CS, or subsequent episodes of CS during a median follow-up of 1266 (796–1680) days (stable HF patients); and 36 ambulatory HF patients who went on to be admitted with CS, with a median of 137 (41–258) days after outpatient biospecimen collection (pre-CS patients). Baseline characteristics of these patients are summarized in Table 1.
| Stable HF (N = 338) | Pre-CS (N = 36) | Cardiogenic shock (n = 84) | P value | |
|---|---|---|---|---|
| Age (years) | 57 ± 15 | 57 ± 15 | 56 ± 15 | 0.882 |
| Male sex | 241 (71%) | 27 (75%) | 61 (73%) | 0.882 |
| Heart failure aetiology | 0.898 | |||
| Ischaemic heart disease | 105 (31%) | 11 (31%) | 30 (36%) | |
| Non-ischaemic cardiomyopathy | 220 (65%) | 24 (67%) | 52 (62%) | |
| Congenital disease | 13 (4%) | 1 (3%) | 2 (2%) | |
| Ejection fraction (%) | 27 ± 9 | 26 ± 10 | 24 ± 12 | 0.140 |
| Other baseline characteristics | ||||
| Body mass index (kg/m2) | 29 ± 6 | 27 ± 7 | 25 ± 6 | <0.0001 |
| Hypertension | 108 (32%) | 14 (39%) | 31 (37%) | 0.530 |
| Dyslipidaemia | 121 (36%) | 15 (42%) | 32 (38%) | 0.752 |
| Diabetes | 77 (23%) | 8 (22%) | 29 (35%) | 0.078 |
| Prior/current smoker | 81 (24%) | 10 (28%) | 25 (30%) | 0.517 |
| Prior history of cancer | 42 (12%) | 3 (8%) | 9 (11%) | 0.727 |
| Prior coronary revascularization | 83 (25%) | 8 (23%) | 18 (21%) | 0.826 |
| Previous cerebrovascular disease/transient ischaemic attack | 32 (10%) | 4 (11%) | 13 (16%) | 0.280 |
| Atrial fibrillation/flutter | 99 (29%) | 16 (44%) | 30 (36%) | 0.120 |
| Chronic heart failure | 338 (100%) | 35 (97%) | 51 (61%) | <0.0001 |
| Implantable cardioverter-defibrillator | 104 (31%) | 13 (36%) | 20 (24%) | 0.321 |
| Cardiac resynchronization therapy | 67 (20%) | 11 (31%) | 15 (18%) | 0.220 |
| White blood count (× 109/L) | 7.3 ± 2.4 | 9.8 ± 5.5 | 10.7 ± 5.0 | <0.0001 |
| Creatinine (μmol/L) | 110 ± 73 | 236 ± 207 | 191 ± 155 | <0.0001 |
| Sodium (mmol/L) | 138 ± 3 | 134 ± 8 | 134 ± 6 | <0.0001 |
| B-type natriuretic peptide (pg/mL) | 166 (325) | 1309 (1932) | 1534 (1877) | <0.0001 |
- Abbreviations: HF, heart failure; pre-CS, patients who were seen in the outpatient heart failure clinic and went on to develop cardiogenic shock.
Clinical profile of CS patients
The majority of CS patients were SCAI stage D (69 patients, 82.1%) at presentation with 50 (59.5%) patients having a history of chronic HF and 33 (39.3%) presenting with de novo HF (Table S1). Forty-five (53.6%) CS patients had a pulmonary artery catheter used to guide management, with the mean number of vasoactive medications being 1.2 ± 0.4 per patient. Nineteen patients (22.6%) required temporary MCS, 15 (17.9%) were mechanically ventilated and 10 (11.9%) required renal replacement therapy. Overall, 20 (23.8%) patients died as an inpatient, 21 (25.0%) required durable VAD implantation and 18 (21.4%) had OHT during this index admission (Table S2).
Inflammatory profiles of CS and HF patients
Cytokines
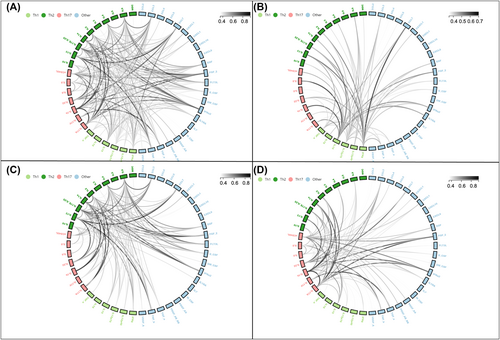
The cytokine levels for all three groups are summarized in Table S3. The first comparison undertaken was between stable HF patients and those with CS (Table S4). Patients with CS had significantly higher levels of IL-1α, IL-1β, IL-1RA, IL-6, IL-8, IL-10, IL-15, IL-17F, IL-18, IL-27, CXCL1, CXCL9, CXCL10, M-CSF, CCL2, CCL3, CCL4, CCL5, TGF-α and TNF-α than stable HF patients. Levels of IFNγ, IL-13, CCL7 and MDC were decreased in CS patients compared with stable HF patients (Figure 1).
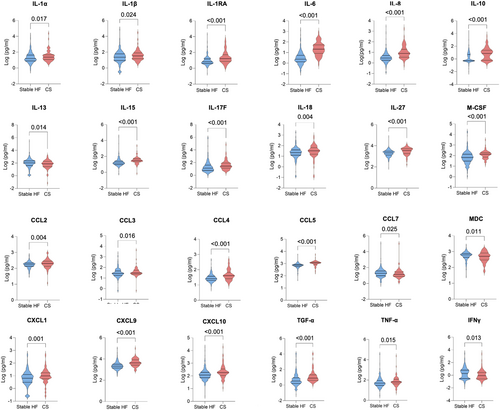
Comparisons were subsequently undertaken between the stable HF group and the pre-CS group, who included ambulatory HF patients who would subsequently be admitted to the CICU with CS. Pre-CS patients had elevated levels of IL-10, IL-27, CXCL9, CXCL10, CCL4 and CCL5 compared with stable HF patients. Levels of IL-5 were decreased in the pre-CS group, compared with stable HF patients (Table S5 and Figure 2). When comparing CS patients with the pre-CS group, CS patients had higher levels of IL-1α, IL-1RA, IL-6, IL-8, IL-15, IL-17F, CXCL1, CXCL9, CCL3, CCL5 and TGF-α and lower MDC levels than pre-CS patients (Table S6 and Figure 3).

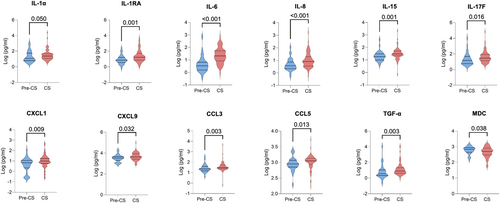
Cellular inflammation
The total white cell count was higher in patients with CS than those in the stable HF group (9.10 vs. 7.34 × 109/L; P < 0.001), with an increased number of neutrophils and monocytes and a reduction in the number of lymphocytes, eosinophils and basophils (Table S7). When comparing pre-CS patients with stable HF patients, there was a modest increase in total white cell count (8.51 vs. 7.34 × 109/L; P < 0.001). This was largely accounted for by an increase in neutrophils and monocytes, with a reduction in eosinophils (Table S8). Total white cell count was similar between pre-CS and CS (8.51 vs. 9.10 × 109/L; P = 0.390), with a similar distribution of cellular types too (Table S9).
Association between inflammatory profile and clinical parameters
Correlation of clinical measures with cytokine levels in CS patients
Admission MAP of CS patients weakly correlated with CXCL9 (r = 0.318, P = 0.004) and TNF-α (r = 0.228, P = 0.044) levels, as did admission BNP and IL-13 (r = 0.234, P = 0.038). There was a weak negative correlation between admission lactate levels and IL-12p40 (r = −0.297, P = 0.006) and CCL4 (r = −0.301, P = 0.006). There was a negative correlation between the number of vasopressors/inotropes and the following cytokines: sCD40L (r = −0.226, 0.039), CX3CL1 (r = −0.274, P = 0.012), G-CSF (r = −0.282, P = 0.009), IL-3 (r = −0.241, P = 0.027), IL-4 (r = −0.267, P = 0.014), IL-5 (r = −0.268, P = 0.014), IL-17E/IL-25 (r = −0.297, P = 0.006), IL-22 (r = −0.309, P = 0.004), CCL7 (r = −0.282, P = 0.009) and TGF-α (r = −0.278, P = 0.010). These correlations and correlations between individual cytokines are summarized in Table S10.
Survival
We compared circulating cytokine profiles and cellular counts between patients who went on to survive CS compared with those who died before hospital discharge. Among the 48 cytokines tested, there was a significant difference between IL-27 levels between the two groups: Survivors from the CS group had lower levels of IL-27 compared with those that died during admission for CS (Table S11 and Figure 4). The total white cell count was similar between survivors and non-survivors of CS (9.20 vs. 8.73 × 109/L; P = 0.620) with a similar differential cell count (Table S12).
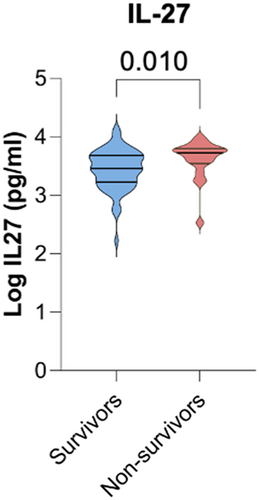
Discussion
Inflammation has been previously demonstrated to be strongly associated with outcomes in AMI-CS.10 IL-6 levels have been shown to be higher in those who died at 30 days after presentation with AMI-CS, compared with survivors.11 Further studies have demonstrated that alongside IL-6, TNF-α and IL-1RA are elevated in patients who develop AMI-CS compared with patients who recover from acute myocardial infarction (AMI) without developing CS.12
Inflammation is also believed to play a pivotal role in the progression of chronic HF,8 as seen in the RELAX trial, where over half of the patients had an elevated high-sensitivity C-reactive protein (CRP), which has been further corroborated in subsequent studies.13, 14
In terms of circulating cytokines, IL-1 has been shown to depress myocardial function by reducing the function of beta-adrenergic receptors in humans. In animal models, elevated levels of IL-1 are associated with reduced exercise performance in systolic HF with both IL-1β and TNF-α inducing left ventricular dilation and reduced contractile function, which was resolved after removal of the stimulus.15-17 TNF-α has also been implicated in the induction of hypertrophy and myocardial fibrosis formation in laboratory experiments.18, 19 Therefore, it is not surprising that CS patients presenting with the most severe form of HF have further dysregulation of their inflammatory cytokine profile.
In our study, we present the cellular inflammatory profile of 84 patients with ADHF-CS who had peripheral blood samples obtained while admitted in the CICU. We compared these patients with two groups of ambulatory patients: firstly, 338 outpatients who were clinically stable with HF, who did not have a history of hospitalization in the preceding year, did not go on to develop CS and did not require advanced HF therapies such as OHT or durable VAD during follow-up (stable HF group); and secondly, a group of 36 patients who were seen in the outpatient HF clinic but went on to develop CS, with a median of 137 (41–258) days later (pre-CS group). We then compared differences between the two ambulatory groups to identify any early changes in inflammatory profile. Finally, we compared the inflammatory profile of CS patient survivors and non-survivors admitted to our ICU.
Patterns of inflammation from HF to CS
There is a pro-inflammatory change in both cytokines and chemokines alongside an increase in circulating white blood cells observed in patients that exhibited a progression of their HF syndrome to ADHF-CS (Figure 5), suggesting that inflammation is a key mediator in the pathogenesis of CS from HF. IL-1, IL-6, IL-8, IL-18, CXCL1, CXCL9, CXCL10, CCL3, CCL4 and CCL5 are all pro-inflammatory and were significantly higher in CS patients than in stable HF comparators. At the same time, immunoregulators that minimize inflammation, such as IL-13, were reduced. The cellular component of the immune system was also activated, with an increase in circulating white cells, in particular a neutrophilia.
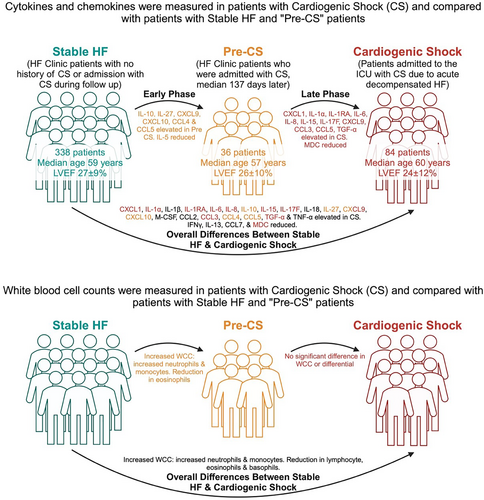
IL-1 and IL-8 are known to stimulate the production of neutrophils,20 and IL-6 is recognized to facilitate the adaptive immune response, responsible for the conversion of mature B lymphocytes into plasma cells.21, 22 The CXCL1 chemokine mediates its activity through the CXCR2 receptor, acting similar to IL-8, attracting and activating neutrophils and basophils,23 while CXCL9 and CXCL10 both activate the CXCR3 receptor, and these chemokines have been shown to increase remodelling of the heart and are elevated 1 week after myocardial infarction in a rat model of HF.24 The family of chemokines CCL3, CCL4 and CCL5 are all potent chemoattractants for eosinophils, basophils23 and monocytes.25 At the same time, IL-13, an immunoregulatory cytokine, responsible for inhibiting many pro-inflammatory cytokines (such as IL-1α, IL-1β, IL-6, IL-8, G-CSF and IFN-α), was decreased in patients with CS compared with patients with stable HF.
Early and late phase changes
The design of this study allowed an assessment of early changes from HF to those beginning the process of decompensation and then comparing this latter group with patients admitted with ADHF-CS. Overall, as seen in Figure 5, there are some cytokine and chemokine changes that predominate early in the process from HF to pre-CS, such as IL-10, IL-27, CXCL9, CXCL10, CCL4 and CCL5. These are largely mediators of the Th1 immune response. In comparison, the response seen later in the process of developing CS, from decompensation to fulminant CS, features a number of cytokines associated with a Th17 immune response, such as IL-1α, IL-6 and IL-17F. The overall drop in IFNγ, which is key in promoting the Th1 response, also supports this explanation. These classes of CD4+ T cells are implicated in autoimmune diseases, with a differential effect on tissues, but their significance in cardiac disease is not well-studied26 and could be a target for future research. The correlation between different cytokine levels also favours this two-phase model of deterioration from stable HF to CS (Figure 6).
Inflammation as a diagnostic and therapeutic target
The changes in inflammatory profile of HF patients who develop CS provide a useful means to assess and risk-stratify patients. There is evidence that pro-inflammatory proteins are linked with the development of HF,27 and markers such as clonal haematopoiesis28 and CRP confer an increased risk in acute HF.29 Given the changes early in the process being different to later on during admission with CS, this could provide a way to test ambulatory HF patients and determine their risk of deteriorating into CS. Whether this could provide additional information beyond assessment by clinical examination and traditional biomarkers alone is untested, but nonetheless is a potential use of precision medicine to better phenotype patients seen in the outpatient clinic.
Beyond simply identifying patients at higher risk of deterioration, it is possible that the changes in inflammatory response seen over the spectrum of ADHF-CS may provide a potential therapeutic target in themselves. Previous studies in both human and animal models of HF have seen a reduction in inflammation with the use of beta-adrenoceptor and renin–angiotensin inhibitors.30, 31 However, studies of immunomodulators in CS have not shown any morbidity or mortality benefit, with negative results in clinical trials of both TNF-α32 and IL-1 blockade.33 Steroid therapy may provide a possible therapeutic option to augment inflammation. A small, open-label study of burst steroid therapy in patients with acute HF reduced inflammatory markers and the incidence of worsening HF during follow-up.34 Further randomized trials of immunomodulation therapy are warranted in ADHF-CS.
Study limitations
This investigation into inflammatory cytokine profiles of ADHF-CS patients compared with ambulatory HF patients is novel, and few previous reports have undertaken such a study with as many participants. However, there are a number of limitations. This is a retrospective study possible only due to the comprehensive biospecimen collection at the institutional biobank; therefore, selection into the study was only possible when patients consented to participate. There is no doubt that there are a number of CS patients who chose not to consent and a number who died soon after presentation to the CICU who would not have been included. Secondly, the CS patients had their samples collected at various time points after CICU admission. While this was a median of 2 (0–7) days after admission, there was no fixed protocol for sample collection. Additionally, the comparisons are made between different groups of patients at different points in the HF trajectory, rather than repeated measures in the same patients. This could be overcome by undertaking a prospective study with regular sample collection. While this is feasible for HF outpatients and during CICU admission, it would be a challenge to prospectively obtain samples from patients on a sufficiently regular basis to be able to capture the pre-CS group, where samples were acquired shortly before admission for CS.
Conclusions
In conclusion, this study identifies the differences in inflammatory profiles of patients in HF and patients in CS due to acute decompensated HF (ADHF). There appears to be a distinct pro-inflammatory state associated with CS in these patients that develops in an early and late phase, corresponding with Th1 and Th17 immune profiles, respectively. The detailed description of these inflammatory profiles may allow earlier recognition of HF patients at risk of developing CS and could provide a potential novel therapeutic target.
Conflict of interest statement
FB has received research support from Abbott outside of the submitted work. All other authors declare no conflicts of interest.
Funding
DHB was supported by a post-doctoral fellowship award from TRANSFORM HF (Ontario, Canada). FLS is supported by a post-doctoral fellowship award from the Ted Rogers Centre for Heart Research (Ontario, Canada). ACL is supported by the Heart and Stroke Foundation/University of Toronto Polo Chair in Cardiology Young Investigator Award. PRL is currently supported by the Fonds de Recherche du Quebec (Sante). FB is supported by the Peter Munk Cardiac Centre Innovation Fund, the Canadian Institutes of Health Research (Project Grant No. 400345) and the Department of Medicine, University of Toronto.




