Risk classification for long-term mortality among patients with acute heart failure: China PEACE 4YMortality
Wei Wang and Lihua Zhang are joint co-first authors.
Abstract
Aims
There are limited tools to predict long-term mortality among patients hospitalized with acute heart failure (AHF) in China. This study aimed to develop and validate a model to predict long-term mortality risk among patients who were hospitalized with AHF and discharged alive.
Methods
We used data from China Patient-Centred Evaluative Assessment of Cardiac Events Prospective Heart Failure Study. Multivariate Cox proportional hazard model was used to develop and internal validate a model to predict 4 year mortality risk.
Results
The study included 4875 patients hospitalized for AHF, of whom 2066 (42.38%) died within 4 years following admission, with a median survival time of 3.91 (interquartile range: 1.67, 4.00) years. We selected 13 predictors to establish the model, including age, medical history of hypertension, chronic obstructive pulmonary disease and HF, systolic blood pressure, blood urea nitrogen, albumin, high-sensitivity troponin T, N-terminal pro-brain natriuretic peptide, serum creatine, Kansas City Cardiomyopathy Questionnaire-12 score and left ventricular ejection fraction. The model showed a reasonable performance with the discrimination [C-index was 0.726 (95% confidence interval, CI: 0.714, 0.739) in the development cohort and 0.727 (95% CI: 0.708, 0.747) in the validation cohort]. We then built a point-based risk score algorithm and the patients were stratified to low-risk (0–14), intermediate-risk (15–19) and high-risk (≥20) groups.
Conclusions
By using readily accessible predictors, we developed and validated a risk prediction model to predict 4 year mortality risk among patients who were hospitalized with AHF and discharged alive. This model proved beneficial for individual risk stratification and facilitating ongoing enhancements in patient outcomes.
Introduction
Heart failure (HF) is a life-threatening syndrome with substantial morbidity and mortality, affecting an estimated 56 million patients worldwide, and this number is expected to increase continuously during the next few decades.1, 2 It was reported that China accounted for about 20% of total patients with HF worldwide, where population ageing, urbanized industrialization and high-income-country lifestyle changes lead to more risk factors for HF development.3 Among whom, over 50% patients with acute HF (AHF) admitted in emergency departments died within 5 years after the onset of AHF in Beijing, China.4 Although advances in medical therapy and device assistance have significantly improved the outcomes of HF, implications of HF were still dramatic.5 Therefore, quantifying survival prospects based on individual risk profile will aid to perform risk stratification and facilitate clinical decisions for regular management in the long term.6-8
Previously, several risk prediction models aimed at predict in-hospital or short-term risk after discharge have been generally introduced to identify patients with AHF at high risk of mortality.9-19 Nevertheless, they might not be able to predict long-term mortality risk among patients with AHF, such as Get With The Guideline-Heart Failure (GWTG-HF),11 Emergency Heart Failure Mortality Risk Grade (EHMRG),19 Acute Decompensated Heart Failure National Registry (ADHERE)15 and Organized Program to Initiate Lifesaving Treatment in Hospitalized Patients with Heart Failure (OPTIMIZE-HF).20 Apart from this, some of these models were derived by use of data from randomized controlled trials; thus, the patients were highly selected, and the results were limited in generalizability, such as Acute Study of Clinical Effectiveness of Nesiritide in Decompensated Heart Failure (ASCEND-HF)9 and Enhanced Feedback for Effective Cardiac Treatment (EFFECT).13 In the consideration of predictor diversities, most of models were developed by clinical routinely collected materials, thus insufficient to fully demonstrate individual perceived status in response to clinical care, such as patient-reported outcomes (PROs).21 While in China, little risk prediction tools were observed for long-term risk stratifications in patients with AHF in Chinese population.22 Because it was reported that significant disparities exited between western and Chinese patients, such as population features, clinical pathways and healthcare system, current models derived from western participants were limited in their feasibility to perform mortality risk stratification in China.23-26 Therefore, it was an imperative necessity to establish a practical tool to predict long-term mortality risk among patients with AHF in China.
In this study, we used data from a nationwide, multi-centre, prospective cohort, the China Patient-Centred Evaluative Assessment of Cardiac Events Prospective Heart Failure (China PEACE 5p-HF) study, and primarily purposed to: (i) identify significant predictors that associated with 4 year mortality risk among patients who were hospitalized with AHF and discharged alive; (ii) develop, validate and evaluate a risk prediction model in predicting 4 year mortality risk, namely, the China PEACE-HF 4YMortality; (iii) establish a risk score algorithm based on China PEACE-HF 4YMortality for clinical use.
Methods
Study design and participants
The China PEACE 5p-HF Study is a nationwide, prospective, multi-centre cohort study with an established protocol. This study screened patients hospitalized for HF within 48 h of admission at 52 hospitals across 20 provinces in China from August 2016 to May 2018, considering factors like geographic distribution and study capacity (including facilities, staff and patient volume). We invited patients aged 18 years and older who were hospitalized due to newly diagnosed or acutely decompensated chronic HF and provided written informed consent.27 Specifically, to align with aims of the study, which was mainly focused on clinical outcomes after discharge, patients who died in-hospital were excluded. Local residents with no communication barriers were invited to participate, with HF diagnoses made by local physicians following the Chinese Guidelines for Heart Failure,28 which is in accordance with guidelines from the European Society of Cardiology, the American College of Cardiology and the American Heart Association.29, 30 The study was approved by ethics committees of Fuwai Hospital and collaborating sites and was registered at www.clinicaltrials.gov (NCT 02878811).
Data collection
We gathered data through patient interview, local examination, laboratory tests, central laboratory analysis and central medical chart abstraction. Patient interviews were conducted at the time of admission for AHF, and subsequently at 1, 6 and 12 months after hospital discharge. The trained staff at the national coordinating centre would conduct interviews via telephone for patients who did not attend the scheduled in-person interview. We collected information of demographics, socioeconomic status, cardiovascular risk factors, healthcare services, medications and PROs (including generic and HF-specific health status, health-related quality of life, depression, stress, anxiety, social support and cognitive function) at admission. We conducted local examination and laboratory tests to acquire physical examination [blood pressure (BP), weight and waist circumference and height and neck circumstance], electrocardiograph (12-lead ECG), transthoracic echocardiogram, chest X-ray (or chest CT), blood cell count, and so forth. We performed central laboratory analysis by using bio-samples, including blood and urine samples, which were obtained at the earliest convenient time after enrolment, typically within 48 h of hospital admission. During the index hospitalization, we carried out central medical chart abstraction, requiring detailed clinical data from an electronic version of the complete medical record. This included demographics, medical history, risk factors, clinical characteristics, medications at discharge, diagnostic tests, treatments, procedures, in-hospital complications, outcomes and discharge diagnoses.27
Outcome
The outcome was all-cause mortality within 4 years after discharge from an index hospitalization. We verified death events in accordance with protocols used in international multi-centre clinical trials. Additionally, vital status and cause of death were centrally adjudicated by linking to the National Mortality Surveillance System.27 Detailed description of central adjudication criteria for death events was reported in the Supporting information Part 2.
Candidate predictors
In our study, we evaluated all available variables using existing literature and professional experiences, and a comprehensive review of data quality, along with considerations of accessibility, feasibility and interpretability in clinical practice. As a result, we identified 44 candidate predictors associated with 4 year all-cause mortality. These predictors were selected as follows: demographics such as sex and age; medical history including hypertension, stroke, coronary heart disease (CHD), atrial fibrillation (AF), valvular heart disease (VHD), HF, cancer, diabetes mellitus (DM), renal dysfunction, chronic obstructive pulmonary disease (COPD); clinical characteristics at admission and/or at discharge including systolic BP (SBP, mmHg), diastolic BP (DBP, mmHg), heart rate (bpm), dyspnoea at rest, New York Heart Association Functional Classification of HF (NYHA); laboratory tests at admission and/or at discharge including N-terminal pro-brain natriuretic peptide (NT-proBNP, ng/L), high-sensitivity troponin T (hsTNT, ng/L), creatinine (CRE, μmol/L), highly sensitive C-reactive protein (hsCRP, mg/L), low-density lipoprotein (LDL-C, mg/dL), glycated haemoglobin A1c (HbA1c, %), blood urea nitrogen (BUN, mmol/L), haemoglobin (Hgb, g/L), albumin (Alb, g/L), serum sodium (Na, mg/L) and serum potassium (K, mg/L); medications at discharge [angiotensin-converting enzyme inhibitor or angiotensin receptor blocker (ACEI or ARB), aldosterone receptor blockers (AldA), β-receptor blockers (BB), calcium channel blockers (CCB), diuretic, digoxin and nitrate]; echocardiogram during hospitalization which measured by left ventricular ejection fraction (LVEF, %); health status at admission and at 1 month follow-up, which was measured by Kansas City Cardiomyopathy Questionnaire-12 score (KCCQ-12); depression at admission and at 1 month follow-up, which was measured by Patient Health Questionnaire 2 item (PHQ-2) depression scale; cognitive function at admission which measured by Mini-Cog score; hospitalization services, which was measured by length of hospital stay (LOS, day) and discharge symptom. Meanwhile, we made adjustment to the data by recoding one categorical variable concerning near-zero variance, discharge symptom and performing log-transformation on skewed distributed continuous variables like NT-proBNP, hsTNT, CRE and hsCRP. Detailed description of full list of candidate predictors and operational definitions was reported in Table S1.
Sample size
In this study, we hypothesized that 4 year mortality rate would be 40%, with no more than 15 predictors included in the model, achieving an adjusted R2 of 0.05. We considered a sample size ratio of 7:3 between the development and validation cohorts. Utilizing methods for calculating sample sizes for survival outcomes in clinical prediction model development,31 it was estimated that a maximum of 1260 patients would be needed for the development cohort and 540 for the validation cohort. These requirements were met by the nearly 5000 participants enrolled in the China PEACE 5p-HF study.
Missing data
We excluded participants who had a missing data rate exceeding 10% for clinically important candidate predictors. We analysed the pattern of missingness and the missingness at random was confirmed. For those predictors with a missing rate below 10%, we employed multivariate imputation by chained equations, using a fully conditional specification across five imputed datasets to derive final estimates. Specifically, we applied predictive mean matching to impute 17 continuous variables, which included clinical characteristics at admission and/or at discharge (SBP, DBP and heart rate), laboratory tests at admission and/or at discharge (NT-proBNP, hsTNT, CRE, hsCRP, LDL-C, HbA1c, BUN, Hgb, Alb, Na, K), echocardiogram during hospitalization (LVEF), health status at admission and at 1 month follow-up (KCCQ-12 score) and hospitalization services (LOS). We used the imputed values to select predictors and develop the model. Detailed information of multiple imputation process for handling missing data was reported in Table S2.
Statistical analysis
Development and validation cohort sampling
Patients who died in-hospital were excluded, and the remaining were randomly allocated into a development cohort (70% of total population) and a validation cohort (30% of total population). We reported the distribution of candidate predictors by comparing the development and validation cohort, as well as by 4 year mortality status (yes vs. no). This involved using frequencies (percentage, %) for categorical variables, and either the mean (standard deviation, SD) or median (interquartile range, IQR) for continuous variables. Differences were assessed using Wilcoxon test for continuous variables and χ2 test for categorical variables. We used log-rank test to compare the survival probability between development and validation cohort and presented with Kaplan–Meier curve.
Predictors selection
In the development cohort, we undertook a structured approach for predictor selection. Initially, we applied Pearson correlation analysis by use of Pearson correlation coefficient and excluded one out of two highly correlated candidate predictors (|Pearson correlation coefficient| > 0.75) based on its associations with mortality. Next, we performed collinearity diagnostics using the variance inflation factor (VIF), removing predictors that showed linear combinations (VIF > 10) to preserve domain diversity. Subsequently, we used a univariate Cox proportional hazard model to identify significant predictors with a P < 0.05. We then employed the least absolute shrinkage and selection operator (LASSO) and random survival forest (RSF) for feature selection among significant variables. To determine the optimal form for inclusion in the model, we conducted a multivariate Cox model with restricted cubic splines (RCS) of four knots, assessing the nonlinear association between each significant continuous variables and 4 year mortality, while adjusting for all other significant variables. Finally, we performed a stepwise multivariable Cox model to finalize predictors selection, by using minimum Akaike information criterion (AIC) with a P value threshold of 0.2 for adding variables and 0.1 for removing them. As a result, the set of candidate predictors retained were those commonly selected through the LASSO, RSF and stepwise Cox model approaches. Detailed description of predictor selection was reported in Figures S1–S6 and Tables S3 and S4.
Model development and validation
In the development cohort, we employed a multivariate Cox model to construct the China PEACE-HF 4YMortality in development cohort. Internal validation was carried out to assess the model's performance in the validation cohort by use of discrimination, accuracy and calibration. We presented model discrimination by determining the likelihood of 4 year mortality through using Harrell's C-index and area under the receiver operating characteristic (ROC) curve.32 Furthermore, we delineated the probability of 4 year mortality across deciles 1 to 10 of predicted risk, thereby illustrating the model's discrimination capability. We quantified model accuracy and interpreted it by mean absolute difference (MAD), which reflected the average discrepancy between the observed and predicted event times across the entire study population.33 We assessed model calibration graphically by comparing predicted vs. observed probability of 4 year mortality.32 Additionally, we performed a sensitivity analysis by constructing a model using raw data without imputation for missing values.
Additionally, by employing the C-index and ROC, we assessed the predictive ability of China PEACE-HF 4YMortality relative to several established prognostic scores recommended by the Guideline for the Management of Heart Failure34 to enhance clinical application among Chinese patients who were hospitalized with AHF and discharged alive. These existing scores included the following: (1) GWTG-HF, which predicted in-hospital mortality among patients hospitalized for HF and incorporated seven variables: age, race, SBP, BUN, Na, heart rate and medical history of COPD11; (2) EFFECT risk score, which designed to predict 1 year all-cause mortality among patients with HF and incorporated 11 variables: age, respiratory rate, SBP, BUN, Na, Hgb and medical history of cerebrovascular disease, dementia, COPD, hepatic cirrhosis and cancer13; (3) ASCEND-HF, which was utilized to predict 30 day mortality among patients with AHF and incorporated five variables: age, SBP, Na, BUN and dyspnoea at rest.9
Risk score and risk stratification
To elucidate the clinical implications of the model, we developed a point-based risk score algorithm based on the estimated coefficients of each selected predictors. To mitigate the issues of excessively high or low scores for individual predictors, thereby ensuring a more uniform and continuous range for clinical application, we considered their relative weights against a reference group. Specifically, for each predictor, we first established the theoretical minimum risk level to determine its reference value, then assessed the variance between this reference group and others to gauge shifts across the entire spectrum. Afterwards, we assigned the theoretical minimum risk group a baseline risk score of zero, with other groups receiving scores proportionate to their relative distance from this baseline. Consequently, we computed an individual risk score by summing the scores for each predictor and utilized the multivariate Cox model formula to estimate the 4 year mortality risk for each integral score. An optimal cutoff point derived from the development cohort, specifically, the 25% and 75% quantiles of the total risk score, was used to categorize patients in the validation cohort into low-, intermediate- and high-risk groups. Finally, we employed the log-rank test to assess the differences in overall survival across these risk groups.35
Clinical utility
We conducted decision curve analysis (DCA) to provide a comprehensive view of the net benefit across various harm–benefit thresholds concerning 4 year mortality probabilities among patients who were hospitalized with AHF and discharged alive. This analysis evaluated clinical utility and potential population impact of incorporating a risk prediction instrument into practice.36-39 Briefly, the premise of the decision curves was to assess the mortality risk within 4 year and recommend further clinical actions, such as interventions or treatments, for patients identified as high risk. The underlying assumption was that the intervention or treatment would offer expected benefits to those who might otherwise die within 4 years without it while also incurring certain costs or harms to those who would survive without intervention. We presented net benefit by comparing the expected benefits and associated costs or harms to two scenarios: one where no patients would die within 4 years without needing intervention (net benefit is 0, labelled ‘None’) and another where every patient would die within 4 years and receive intervention (net benefit equals to the negative value of individual slope, labelled ‘All’). Specifically, the net benefit was calculated as the number of true-positive classifications (the benefit of correctly predicting 4 year mortality risk for a patient who would have died) minus the number of false-positive classifications (the harm of an unnecessary prediction in a patient who would not die), where false positives were weighted by a factor that considered the relative harm of missing a death case versus an unnecessary prediction. This weighting was derived from the threshold probabilities at a defined time point for 4 year mortality. Additionally, we presented a clinical impact curve (CIC) to further identify true high-risk patients hospitalized for AHF who will die within 4 years from all patients classified as high risk.36, 40
This study was conducted in accordance with the Transparent Reporting of a Multivariable Prediction Model for Individual Prognosis of Diagnosis (TRIPOD) statement for reporting.41 Detailed description of TRIPOD checklist for Prediction Model Development and Validation was reported in Supporting information Part 1. All confidence intervals (CIs) presented were derived from standard bootstrapping with 500 samples using the standard unconditional method. Specifically, we first obtained regression coefficients and corresponding standard errors (SEs) by using Cox regressions; afterwards, we took a random sample for 500 times from Kaplan–Meier estimator by using established model and acquired corresponding SEs for each predictor; we thus calculated CIs based on those known SEs.42, 43 A P value <0.05 was considered statistically significant and all tests were two sided. We performed the main analysis in R version 4.3.1 (the R foundation for Statistical Computing, Vienna, Australia).
Results
Study cohort
Of the 4907 patients enrolled in the China PEACE 5p-HF study, 32 individuals who died during their index hospitalization were excluded, leaving 4875 participants for the current analysis (Figure S7). Among these, 2066 patients (42.38%) experienced mortality within 4 years following admission to the index hospitalization, with a median survival time of 3.91 years (IQR: 1.67, 4). Table 1 presented the characteristics of patients admitted for AHF, categorized by their 4 year mortality status, in both the development/validation cohort. Overall, 1830 patients (37.54%) were female, with a median age of 67 years (IQR: 57, 75). Compared with survivors, deceased patients tended to be older and more likely to have a medical history of hypertension, stroke, CHD, HF, cancer, renal dysfunction and COPD. They also exhibited lower levels of BP, LDL-C, Hgb, Alb, Na and LVEF, as well as higher levels of NT-proBNP, hsTNT, CRE, hsCRP, HbA1c and BUN. Additionally, they presented with more severe HF conditions, poorer health status, depression, cognitive function and worsened discharge symptoms compared with admission. All patients were randomly assigned to either the development cohort (n = 3412) or the validation cohort (n = 1463), with all candidate predictors equally distributed between the two cohorts. Moreover, the overall survival probability was comparable between the cohorts (Figure S8).
| Total | Survivors | Deaths | P | Development cohort | Validation cohort | P | |
|---|---|---|---|---|---|---|---|
| Total | 4875 (100) | 2809 (57.62) | 2066 (42.38) | 3412 (70.00) | 1463 (30.00) | ||
| 4 year mortality, n (%) | 0.902 | ||||||
| Yes | - | - | - | 1445 (42.35) | 621 (42.45) | ||
| No | - | - | - | 1967 (57.65) | 842 (57.55) | ||
| Demographics | |||||||
| Sex, n (%) | 0.021 | 0.360 | |||||
| Male | 3045 (62.46) | 1716 (61.09) | 1329 (64.33) | 2117 (62.05) | 928 (63.43) | ||
| Female | 1830 (37.54) | 1093 (38.91) | 737 (35.67) | 1295 (37.95) | 535 (36.57) | ||
| Age, years, median (IQR) | 67.00 (57.00, 75.00) | 65.00 (54.00, 73.00) | 70.00 (61.00, 78.00) | <0.001 | 67.00 (57.00, 75.00) | 67.00 (57.00, 75.00) | 0.570 |
| Age groups, years, n (%) | <0.001 | 0.169 | |||||
| <60 | 1443 (29.60) | 997 (35.49) | 446 (21.59) | 1008 (29.54) | 435 (29.73) | ||
| 60–70 | 1415 (29.03) | 838 (29.83) | 577 (27.93) | 1016 (29.78) | 399 (27.27) | ||
| ≥70 | 2017 (41.37) | 974 (34.67) | 1043 (50.48) | 1388 (40.68) | 629 (42.99) | ||
| Medical history | |||||||
| Hypertension, n (%) | 2845 (58.36) | 1686 (60.02) | 1159 (56.10) | 0.006 | 1978 (57.97) | 867 (59.26) | 0.402 |
| Stroke, n (%) | 999 (20.49) | 527 (18.76) | 472 (22.85) | <0.001 | 710 (20.81) | 289 (19.75) | 0.403 |
| CHD, n (%) | 2818 (57.81) | 1575 (56.07) | 1243 (60.16) | 0.004 | 1969 (57.71) | 849 (58.03) | 0.834 |
| AF, n (%) | 1777 (36.45) | 1003 (35.71) | 774 (37.46) | 0.208 | 1231 (36.08) | 546 (37.32) | 0.409 |
| VHD, n (%) | 794 (16.29) | 442 (15.74) | 352 (17.04) | 0.224 | 565 (16.56) | 229 (15.65) | 0.432 |
| HF, n (%) | 3415 (70.05) | 1812 (64.51) | 1603 (77.59) | <0.001 | 2395 (70.19) | 1020 (69.72) | 0.741 |
| Cancer, n (%) | 203 (4.16) | 97 (3.45) | 106 (5.13) | 0.004 | 136 (3.99) | 67 (4.58) | 0.342 |
| DM, n (%) | 881 (18.07) | 499 (17.76) | 382 (18.49) | 0.515 | 612 (17.94) | 269 (18.39) | 0.708 |
| Renal dysfunction, n (%) | 1398 (28.68) | 623 (22.18) | 775 (37.51) | <0.001 | 998 (29.25) | 400 (27.34) | 0.177 |
| COPD, n (%) | 950 (19.49) | 461 (16.41) | 489 (23.67) | <0.001 | 646 (18.93) | 304 (20.78) | 0.136 |
| Clinical characteristics | |||||||
| SBP at admission, mmHg, mean (SD) | 133.16 (24.52) | 135.00 (24.08) | 130.67 (24.89) | <0.001 | 133.18 (24.68) | 133.14 (24.14) | 0.959 |
| SBP at discharge, mmHg, mean (SD) | 121.05 (16.06) | 122.09 (15.21) | 119.64 (17.06) | <0.001 | 120.87 (16.23) | 121.48 (15.65) | 0.221 |
| DBP at admission, mmHg, mean (SD) | 81.04 (15.98) | 82.64 (16.33) | 78.85 (15.23) | <0.001 | 81.00 (16.27) | 81.12 (15.29) | 0.802 |
| DBP at discharge, mmHg, mean (SD) | 72.05 (10.30) | 72.92 (10.22) | 70.88 (10.28) | <0.001 | 71.95 (10.43) | 72.30 (9.98) | 0.269 |
| Heart rate at admission, bpm, mean (SD) | 89.19 (21.95) | 89.55 (22.51) | 88.69 (21.16) | 0.174 | 89.08 (22.07) | 89.44 (21.66) | 0.602 |
| Heart rate at discharge, bpm, mean (SD) | 74.02 (11.11) | 73.92 (10.77) | 74.15 (11.55) | 0.481 | 73.88 (11.04) | 74.32 (11.25) | 0.204 |
| Dyspnoea at rest at admission, n (%) | 2676 (54.89) | 1423 (50.66) | 1253 (60.65) | <0.001 | 1896 (55.57) | 780 (53.32) | 0.147 |
| NYHA at admission | <0.001 | 0.152 | |||||
| II | 701 (14.38) | 540 (19.22) | 161 (7.79) | 493 (14.45) | 208 (14.22) | ||
| III | 2160 (44.31) | 1268 (45.14) | 892 (43.18) | 1482 (43.43) | 678 (46.34) | ||
| IV | 2014 (41.31) | 1001 (35.64) | 1013 (49.03) | 1437 (42.12) | 577 (39.44) | ||
| NYHA at discharge | <0.001 | 0.124 | |||||
| II | 805 (16.52) | 596 (21.22) | 209 (10.12) | 564 (16.53) | 241 (16.47) | ||
| III | 2125 (43.59) | 1245 (44.32) | 880 (42.59) | 1460 (42.79) | 665 (45.45) | ||
| IV | 1945 (39.90) | 968 (34.46) | 977 (47.29) | 1388 (40.68) | 557 (38.07) | ||
| Laboratory tests | |||||||
| NT-proBNP at admission, ng/L, median (IQR) | 1450.00 (593.95, 3210.50) | 1017.00 (423.90, 2128.00) | 2418.50 (1059.00, 5118.00) | <0.001 | 1476.50 (610.30, 3246.00) | 1377.00 (553.30, 3141.00) | 0.269 |
| hsTNT at admission, ng/L, median (IQR) | 21.54 (12.82, 40.06) | 17.10 (10.59, 29.96) | 29.94 (17.79, 54.42) | <0.001 | 21.36 (12.84, 39.37) | 22.05 (12.71, 42.50) | 0.748 |
| CRE at admission, μmol/L, median (IQR) | 91.97 (77.04, 111.06) | 88.58 (75.32, 104.82) | 97.54 (80.28, 123.12) | <0.001 | 91.96 (76.87, 111.31) | 92.12 (77.60, 110.62) | 0.967 |
| hsCRP at admission, mg/L, median (IQR) | 4.02 (1.61, 11.91) | 3.27 (1.31, 9.08) | 5.49 (2.17, 15.89) | <0.001 | 3.99 (1.65, 12.10) | 4.11 (1.48, 11.55) | 0.536 |
| LDL-C at admission, mg/dL, mean (SD) | 2.51 (0.83) | 2.56 (0.82) | 2.44 (0.84) | <0.001 | 2.51 (0.83) | 2.50 (0.85) | 0.732 |
| HbA1c at admission, %, mean (SD) | 5.97 (1.34) | 5.91 (1.28) | 6.05 (1.42) | <0.001 | 5.96 (1.35) | 5.99 (1.33) | 0.413 |
| BUN at admission, mmol/L, mean (SD) | 7.85 (3.84) | 7.05 (3.06) | 8.93 (4.48) | <0.001 | 7.85 (3.89) | 7.83 (3.73) | 0.847 |
| BUN at discharge, mmol/L, mean (SD) | 7.70 (3.75) | 7.02 (3.06) | 8.63 (4.35) | <0.001 | 7.69 (3.79) | 7.72 (3.64) | 0.808 |
| Hgb at admission, g/L, mean (SD) | 133.08 (22.61) | 135.68 (21.10) | 129.54 (24.07) | <0.001 | 132.85 (22.48) | 133.60 (22.90) | 0.285 |
| Hgb at discharge, g/L, mean (SD) | 132.64 (23.16) | 135.36 (21.74) | 128.93 (24.50) | <0.001 | 132.44 (23.12) | 133.09 (23.27) | 0.374 |
| Alb at admission, g/L, mean (SD) | 38.56 (4.83) | 39.37 (4.63) | 37.44 (4.88) | <0.001 | 38.53 (4.84) | 38.62 (4.81) | 0.557 |
| Na at admission, mg/L, mean (SD) | 139.39 (4.44) | 139.97 (4.02) | 138.59 (4.84) | <0.001 | 139.35 (4.50) | 139.47 (4.29) | 0.371 |
| Na at discharge, mg/L, mean (SD) | 139.48 (3.91) | 139.88 (3.57) | 138.93 (4.28) | <0.001 | 139.51 (3.93) | 139.41 (3.87) | 0.431 |
| K at admission, mg/L, mean (SD) | 4.08 (0.58) | 4.04 (0.52) | 4.13 (0.64) | <0.001 | 4.08 (0.58) | 4.07 (0.57) | 0.495 |
| K at discharge, mg/L, mean (SD) | 4.19 (0.48) | 4.17 (0.46) | 4.21 (0.51) | 0.022 | 4.19 (0.48) | 4.19 (0.48) | 0.707 |
| Medications at discharge | |||||||
| ACEI or ARB, n (%) | 2541 (52.12) | 1559 (55.50) | 982 (47.53) | <0.001 | 1785 (52.32) | 756 (51.67) | 0.681 |
| AldA, n (%) | 3100 (63.59) | 1745 (62.12) | 1355 (65.59) | 0.013 | 2188 (64.13) | 912 (62.34) | 0.234 |
| BB, n (%) | 2879 (59.06) | 1749 (62.26) | 1130 (54.70) | <0.001 | 2009 (58.88) | 870 (59.47) | 0.703 |
| CCB, n (%) | 718 (14.73) | 436 (15.52) | 282 (13.65) | 0.068 | 496 (14.54) | 222 (15.17) | 0.565 |
| Diuretic, n (%) | 3364 (69.01) | 1877 (66.82) | 1487 (71.97) | <0.001 | 2368 (69.40) | 996 (68.08) | 0.360 |
| Digoxin, n (%) | 1171 (24.02) | 658 (23.42) | 513 (24.83) | 0.256 | 818 (23.97) | 353 (24.13) | 0.908 |
| Nitrate, n (%) | 1366 (28.02) | 747 (26.59) | 619 (29.96) | 0.01 | 961 (28.17) | 405 (27.68) | 0.731 |
| Echocardiograph | |||||||
| LVEF during hospitalization, %, mean (SD) | 44.32 (15.08) | 45.56 (14.78) | 42.64 (15.32) | <0.001 | 44.18 (15.08) | 44.66 (15.06) | 0.309 |
| HF phenotype, n (%) | <0.001 | 0.512 | |||||
| HFrEF | 2016 (41.35) | 1054 (37.52) | 962 (46.56) | 1423 (41.71) | 593 (40.53) | ||
| HFmrEF | 1036 (21.25) | 626 (22.29) | 410 (19.85) | 731 (21.42) | 305 (20.85) | ||
| HFpEF | 1823 (37.39) | 1129 (40.19) | 694 (33.59) | 1258 (36.87) | 565 (38.62) | ||
| Health status | |||||||
| KCCQ-12 score at admission, mean (SD) | 44.02 (22.71) | 48.68 (22.35) | 37.68 (21.65) | <0.001 | 43.70 (22.59) | 44.75 (23.00) | 0.140 |
| KCCQ-12 score at 1 month follow-up, mean (SD) | 61.61 (26.04) | 68.39 (22.18) | 52.38 (27.99) | <0.001 | 61.19 (26.19) | 62.57 (25.65) | 0.089 |
| Depression | |||||||
| Mini-Cog score at admission, median (IQR) | 4.00 (2.00, 5.00) | 4.00 (2.00, 5.00) | 3.00 (1.00, 5.00) | <0.001 | 4.00 (2.00, 5.00) | 4.00 (2.00, 5.00) | 0.958 |
| Cognitive function | |||||||
| PHQ-2 score at admission, median (IQR) | 2.00 (0.00, 3.00) | 2.00 (0.00, 2.00) | 2.00 (0.00, 4.00) | <0.001 | 2.00 (0.00, 3.00) | 2.00 (0.00, 3.00) | 0.807 |
| PHQ-2 score at 1 month follow-up, median (IQR) | 0.00 (0.00, 3.00) | 0.00 (0.00, 2.00) | 2.00 (0.00, 5.00) | <0.001 | 0.00 (0.00, 3.00) | 0.00 (0.00, 3.00) | 0.085 |
| Hospitalization services | |||||||
| LOS, day, mean (SD) | 9.00 (7.00, 13.00) | 9.00 (7.00, 12.00) | 10.00 (7.00, 13.00) | <0.001 | 9.00 (7.00, 13.00) | 9.00 (7.00, 13.00) | 0.746 |
| Discharge symptom, n (%) | <0.001 | 0.993 | |||||
| Improved, asymptomatic | 2962 (60.76) | 1855 (66.04) | 1107 (53.58) | 2075 (60.81) | 887 (60.63) | ||
| Improved, symptomatic | 1850 (37.95) | 948 (33.75) | 902 (43.66) | 1293 (37.90) | 557 (38.07) | ||
| Worsen/no changed | 63 (1.29) | 6 (0.21) | 57 (2.76) | 44 (1.29) | 19 (1.30) | ||
| Survival time, median (IQR) | 1427.00 (610.00, 1460.00) | 1460.00 (1460.00, 1460.00) | 472.50 (184.00, 893.25) | <0.001 | 1430.00 (606.00, 1460.00) | 1418.00 (632.00, 1460.00) | 0.635 |
- Abbreviations: ACEI or ARB, angiotensin-converting enzyme inhibitor or angiotensin receptor blocker; AF, atrial fibrillation; Alb, albumin; AldA, aldosterone receptor blockers; BB, β-receptor blockers; BUN, blood urea nitrogen; CCB, calcium channel blockers; CHD, Coronary heart disease; COPD, chronic obstructive pulmonary disease; CRE, serum creatinine; DBP, diastolic blood pressure; DM, diabetes mellitus; HbA1c, glycated haemoglobin A1c; HF, heart failure; HFmrEF, HF with mid-range ejection fraction; HFpEF, HF with preserved ejection fraction; HFrEF, HF with reduced ejection fraction; Hgb, haemoglobin; hsCRP, highly sensitive C-reactive protein; hsTNT, high-sensitivity troponin T; IQR, interquartile range; K, serum potassium; KCCQ-12, Kansas City Cardiomyopathy Questionnaire-12; LDL-C, low-density lipoprotein; LOS, length of stay; LVEF, left ventricular ejection fraction; Na, serum sodium; NT-proBNP, N-terminal pro-brain natriuretic peptide; NYHA, New York Heart Association Classification of HF; PHQ-2, Patient Health Questionnaire 2 item; SBP, systolic blood pressure; SD, standard deviation; VHD, valvular heart disease.
Predictors selection
A univariate Cox model identified 30 out of 44 significant predictors for subsequent feature selection using LASSO and RSF. Stepwise Cox modelling, along with professional expertise, retained 13 predictors, as presented in Figure S9. Among these predictors, cardio-specific biomarkers, particularly NT-proBNP and hsTNT levels at admission, contributed significantly to the overall predictive ability compared with other variables. These were followed by demographics (age), medical history (hypertension, COPD and HF), clinical characteristics (SBP at discharge), other laboratory tests (Alb and CRE at admission, BUN at discharge), echocardiographic findings (LVEF during hospitalization), health status (KCCQ-12 score at admission) and cognitive function (Mini-Cog score at admission).
Model development and validation
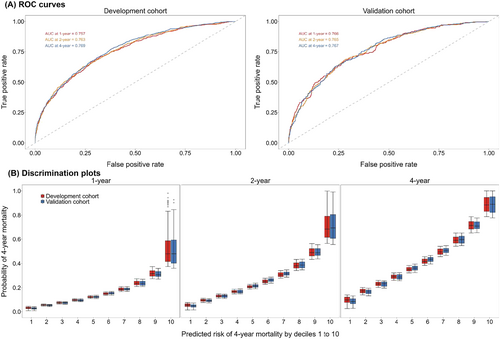
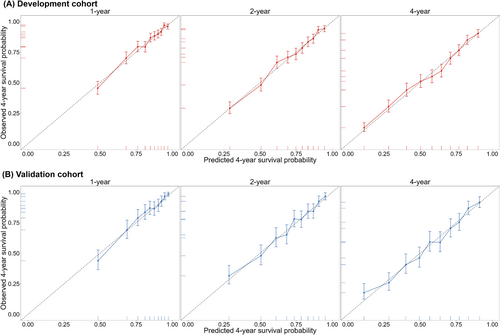
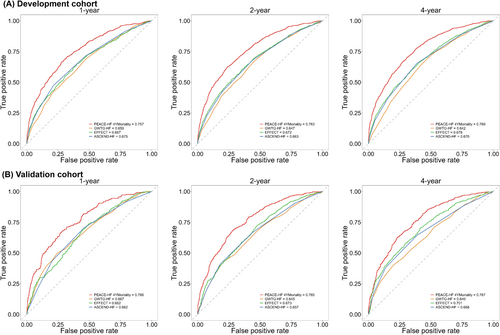
The developed China PEACE-HF 4YMortality demonstrated reasonable discrimination, accuracy and calibration. Regarding discrimination, the C-index was 0.726 (95% CI: 0.714, 0.739) in the development cohort and 0.727 (95% CI: 0.708, 0.747) in the validation cohort. Time-specific ROC curves at 1, 2 and 4 years after discharge from the index hospitalization yielded AUC of 0.757, 0.763 and 0.769 in the development cohort and 0.766, 0.765 and 0.767 in the validation cohort, respectively (Figure 1A). Time-dependent ROC curves exhibited a subtle downward trend after the first year of discharge across the entire study period (Figure S10). Additionally, discrimination plots depicted the distribution of predicted 4 year mortality risk by deciles 1 to 10 in both the development and validation cohorts. At 4 years, the median predicted event rate ranged from 9.67% to 88.54% in the development cohort and from 8.92% to 88.97% in the validation cohort (Figure 1B). Regarding accuracy, the mean absolute deviation (MAD) between observed and predicted survival time within 4 years following admission was estimated to be 57.83 (95% CI: 51.77, 64.12) days in the development cohort and 57.26 (95% CI: 49.12, 65.73) days in the validation cohort. For calibration, the predicted probabilities closely aligned with the observed ones but exhibited slight deviations at 4 years (Figure 2). Furthermore, the predictive ability of the China PEACE-HF 4YMortality was found to outperform GWTG-HF, EFFECT Risk Score and ASCEND-HF at 1, 2 and 4 years after discharge in predicting 4 year mortality risk among AHF patients in the Chinese population, regardless of the development or validation cohort (Figure 3).
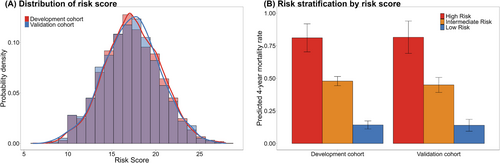
Risk score and risk stratification
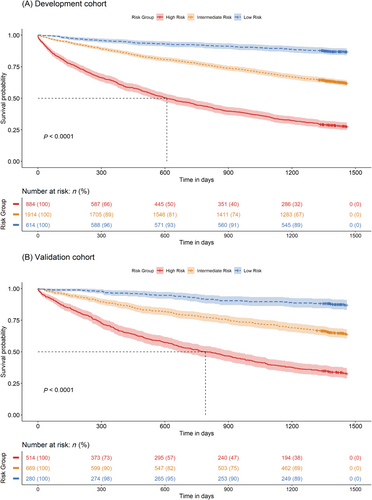
Individual risk scores of the China PEACE-HF 4YMortality ranged from 0 to 34 (Table 2), corresponding to predicted probabilities of 4 year mortality ranging from 0.079 to 1 (Table S5). Patients with HF were stratified into low-risk (score: 0–14), intermediate-risk (score: 15–19) and high-risk (score: ≥20) groups (Figure 4A). The corresponding predicted rates of 4 year mortality were 81.11% (95% CI: 70.36, 91.86%), 47.89% (95% CI: 44.36, 39.18%) and 14.22% (95% CI: 11.12, 17.32%) in the high-, intermediate- and low-risk groups, respectively, in the development cohort, and 81.57% (95% CI: 69.14%, 94.00%), 44.93% (95% CI: 39.18%, 50.69%) and 13.95% (95% CI: 9.38%, 18.52%) in the validation cohort, respectively (Figure 4B). The risk stratification in the validation cohort was similar to that of the development cohort, demonstrating substantial discrimination of survival probability (Figure 5).
| Items and categories | Risk score |
|---|---|
| Age, years | |
| <10 | 0 |
| [10, 30) | 1 |
| [30, 50) | 2 |
| [50, 70) | 3 |
| [70, 80) | 4 |
| ≥80 | 5 |
| Hypertension | |
| No | 1 |
| Yes | 0 |
| COPD | |
| No | 0 |
| Yes | 1 |
| HF | |
| No | 0 |
| Yes | 1 |
| SBP at discharge, mmHg | |
| <80 | 4 |
| [80, 110) | 3 |
| [110, 140) | 2 |
| [140, 180) | 1 |
| ≥180 | 0 |
| BUN at discharge, mmol/L | |
| <10 | 0 |
| [10, 20) | 1 |
| ≥20 | 2 |
| Alb at admission, g/L | |
| <30 | 3 |
| [30, 40) | 2 |
| [40, 50) | 1 |
| ≥50 | 0 |
| Log-transformed hsTNT at admission, ng/L | |
| <0.8 | 0 |
| [0.8, 1.4) | 1 |
| [1.4, 2.2) | 2 |
| ≥2.2 | 3 |
| Log-transformed NT-proBNP at admission, ng/L | |
| <1.5 | 0 |
| [1.5, 2) | 1 |
| [2, 2.5) | 2 |
| [2.5, 3) | 3 |
| [3, 3.5) | 4 |
| [3.5, 4) | 5 |
| ≥4 | 6 |
| Log-transformed CRE at admission, μmol/L | |
| <1.7 | 0 |
| [1.7, 2.1) | 1 |
| [2.1, 2.5) | 2 |
| ≥2.5 | 3 |
| KCCQ-12 score at admission | |
| <10 | 3 |
| [10, 50) | 2 |
| [50, 80) | 1 |
| ≥80 | 0 |
| LVEF during hospitalization, % | |
| <50 | 1 |
| ≥50 | 0 |
| Mini-Cog score at admission | |
| <2 | 1 |
| ≥2 | 0 |
- Abbreviations: Alb, albumin; BUN, blood urea nitrogen; COPD, chronic obstructive pulmonary disease; CRE, serum creatinine; HF, heart failure; hsTNT, high-sensitivity troponin T; KCCQ-12, Kansas City Cardiomyopathy Questionnaire-12; LVEF, left ventricular ejection fraction; NT-proBNP, N-terminal pro-brain natriuretic peptide; SBP, systolic blood pressure
Clinical utility
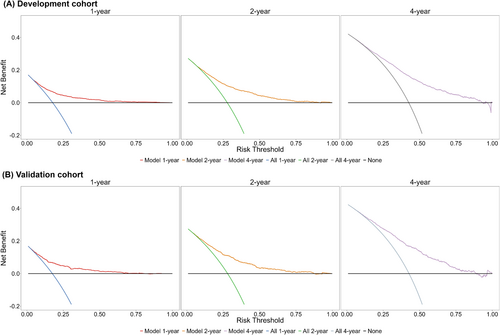
Figure 6 illustrated the net benefits of utilizing the China PEACE-HF 4YMortality for stratifying participants for intervention in comparison with other strategies, including the extremes of intervening on everyone (all) or no one (none). Across various 4 year absolute risk thresholds ranging from 0.029 to 0.913 at 1 year, 0.087 to 0.893 at 2 years and 0.103 to 0.947 at 4 years in the development cohort, China PEACE-HF 4YMortality consistently yielded the highest benefit scores compared with all other alternatives. Similar results were observed in the validation cohort (Figure 6). Within this range, the model demonstrated the highest net benefit compared with the none or all strategies, with the validation cohort showing identical outcomes. In addition to DCA, we conducted a cost–benefit analysis by providing the CIC for further evaluation (Figure S11). In the development cohort, overall, a high-risk threshold ranging from 0 to 0.50 for 1 year mortality yielded a net benefit from employing the China PEACE-HF 4YMortality in risk stratification. For instance, a 0.45 threshold for 4 year mortality risk could accurately identify 64.91% of high-risk patients who would experience mortality, with a cost–benefit ratio of 9:11. Similar findings were observed in the validation cohort.
Discussion
By utilizing data from a nationwide, multi-centre, prospective HF cohort study, we have developed a risk prediction model known as China PEACE-HF 4YMortality, which predicted 4 year all-cause mortality risk for patients who were hospitalized with AHF and discharged alive. This model demonstrated a relatively satisfied predictive capability as evidenced by internal validation, surpassing existing scores developed for western populations. The model incorporated easily accessible and collectible predictors for patients, including demographics, medical history, clinical characteristics, laboratory tests, echocardiogram, health status and cognitive function. Additionally, we have constructed a point-based risk score algorithm derived from this model and assessed its clinical utility. These risk stratification tools enabled physicians to enhance care triage, allocate resources more efficiently and implement personalized interventions, ultimately aiming to improve the quality of care provided.9
Our research contributed to the expanding corpus of literature on predicting mortality risk in patients with AHF and provided several additional insights. Actually, HF presented a strong heterogeneity in different countries and regions, which is mainly because of varying risk factors exposure. Also, there were disparities in aetiology spectrum across age groups and sex.5 Some surveys have reported that the incidence of HF have decreased in developed countries, and the epidemic of HF is likely to grow further in China in the coming decades, largely because of the millions of patients with risk factors, the improved outcomes of acute cardiac conditions and the subsequently developing HF as a result of increasing use of early interventional strategies. Given these differences, it is crucial that HF-related public health policies and strategies should be targeted to local conditions.3, 5 Given the complexity of AHF, previous efforts to develop risk models have faced challenges in adapting to clinical practice, generally achieving only moderate to acceptable levels of discrimination.9 Numerous systematic reviews have identified over 10 significant models for predicting mortality risk among patients with AHF, primarily developed in high-income regions such as the United States and European countries. However, the applicability of these models, often derived from clinical trials with strict inclusion and exclusion criteria, was limited in generalizing to broader population in Chinese patients. 8, 9, 12, 44 In contrast, the China PEACE 5p-HF study, supplementing prior literature, employed wide-ranging inclusion criteria and captured extensive clinical data from patients with AHF, enhancing its applicability to daily clinical settings. This approach could facilitate the identification of 4 year mortality risks among patients who were hospitalized with AHF and discharged alive, allowing for timely, targeted interventions and evidence-based, long-term care.
The China PEACE-HF 4YMortality demonstrated satisfactory performance in distinguishing high-risk patients from the general cohorts who were hospitalized with AHF and discharged alive. It exhibited good calibration in estimating the probabilities of 4 year mortality risk in the development cohort and maintained high consistency during internal validation. These findings were comparable with or superior to previous models. 8, 18, 45 Additionally, our study compared this model with three other risk assessment tools recommended by guidelines: GWTG-HF,11 EFFECT Risk Score13 and ASCEND-HF,9 which reported C-index values of 0.75, 0.77 and 0.74, respectively, for predicting in-hospital mortality, mortality within 1 after discharge and mortality within 30 days after discharge. When applied to our cohort, these tools yielded lower C-index values compared with the China PEACE-HF 4YMortality. This underscored the divergence in patient characteristics and prediction time between our cohort and those in existing risk assessment tools, making it challenging to achieve precise predictions in China using models developed in western populations. Notably, none of the existing risk scores were designed to forecast long-term outcomes, such as 4 year mortality after discharge. Furthermore, the patient populations enrolled in the EFFECT and ASCEND-HF trials were highly selective, contrasting with the heterogeneous characteristics of our patient cohort.13 Hence, the superior performance of the China PEACE-HF 4YMortality offered a valuable tool for efficiently stratifying risk among Chinese AHF patients during long-term care. In addition to model discrimination, we introduced MAD as a simple and clinically interpretable measurement of predictive accuracy, which indicated a slight difference in the current prediction model. Utilizing the algorithmic estimates from each predictor, a point-based risk score was generated, aiding physicians and patients in promptly identifying high-risk groups and advocating for evidence-based treatments for those at elevated risk who were hospitalized with AHF and discharged alive from the index hospitalization.
The China PEACE-HF 4YMortality identified 13 predictors that were easy to measure at low cost, widely accepted and readily available in clinical settings. Some of these predictors overlap with several existing risk scores (i.e.. GWTG-HF,11 EFFECT,13 ASCEND-HF,9 ADHERE15 and AHEAD10), including older age, elevated BUN levels at admission, decreased SBP at admission and the presence of COPD, which have been demonstrated as robust prognostic factors for predicting mortality risk in patients with AHF. Additionally, certain cardiac-specific biomarkers, such as elevated NT-proBNP and hsTNT levels at admission, contributed significantly to the overall risk score. NT-proBNP emerged as the most influential indicator in predicting AHF mortality risk, highlighting its indispensable predictive value for HF prognosis, despite some prior studies lacking BNP or NT-proBNP data. Meanwhile, recent experimental and clinical studies have clearly demonstrated the fundamental role of assay of cardio-specific biomarkers (especially hs-cTnI and hs-cTnT assay) for the cardiovascular risk assessment and for the early detection of asymptomatic individuals in the general population at high risk for a more rapid progression toward the symptomatic phase of HF (from B to C stage of HF), which were consistent with our findings that hsTNT at admission contributed largely in the prognosis prediction. In particular, the high-sensitivity cardiac troponin (hs-cTn) methods were characterized by a low intra-individual index of variation and reduced analytical imprecision at the clinical cutoff value.29, 46, 47 Second, KCCQ-12, an HF-specific health status assessment tool, quantified symptom frequency, physical limitations, social limitations and quality of life, serving as an initial assessment for physicians to rapidly gauge 4 year mortality risk. This holds particular importance in resource-limited settings like remote areas or clinics without biomarker analysis.48 Third, the presence of hypertension or elevated SBP predicted lower mortality risk among AHF patients, consistent with previous findings. Mortality risk decreased as SBP increases, up to an SBP of 160 mmHg, beyond which incremental benefits diminish.20 Patients hospitalized with AHF and elevated SBP may possess greater myocardial reserve and thus face lower short-term mortality risk, with potentially easier stabilization and compensation restoration compared with those with lower SBP. However, this should not be misconstrued to advocate for maintaining elevated BP long-term by avoiding beneficial drug therapies, alternatively, it should be interpreted at the situation that under certain range of SBP during which it could achieve in current study. 13, 20 Fourth, consistent with prior research, renal function emerged as a significant predictor of mortality risk, with both BUN and CRE serving as predictors of 4 year mortality in the China PEACE-HF 4YMortality. BUN was considered a more potent prognostic variable compared with CRE, owing to its dependence on a combination of protein metabolism, neurohormonal activation, and renal function. 9, 49, 50
The clinical relevance of understanding the risk of adverse outcomes in patients hospitalized with AHF had been highlighted in HF guidelines, which advocated the use of multivariate risk scores as a Class IIa recommendation. 34, 51 The China PEACE-HF 4YMortality had shown satisfactory clinical utility from an application standpoint. Prior researches on the clinical utility of HF mortality risk had been limited39 while its application had been more extensively utilized in disease screening studies, including various cancer subtypes.36, 40, 52 In assessments comparing the net benefit of using the China PEACE-HF 4YMortality to stratify patients against a baseline of intervening in all or none, a significant net benefit was noted at medium risk levels across a broad spectrum of threshold probabilities for 4 year mortality risk. Furthermore, by employing a high-risk threshold, the CIC provided a scalable metric to evaluate the cost–benefit ratio, determining which patient groups with AHF would benefit from the prediction model and which would not. This approach was particularly valuable in preventing the unnecessary use of the model, especially when the costs outweigh the benefits.
Implications
In order to develop evidence-based informed interventions to mitigate the burden imposed by HF, epidemiological differences across economic and demographic characteristics at regional and national levels should be taken into account. Adequate patient self-care is essential in the effective management of HF and allows patients to understand what is beneficial and to agree to self-monitoring and management plans. The establishment of the China PEACE-HF 4YMortality offered a framework for delivering personalized care and fostering shared decision making, thereby enhancing adherence to treatment and reducing adverse outcomes. This represented a significant advancement over previous models and risk scores used in the Chinese population. Clinically applied, this tool effectively identified patients at high risk who may benefit from intensive monitoring, early referral for advanced HF management, or if suitable, transition to hospice care or community-based settings in the long-term period.20, 53 Moreover, alerting physicians to these risks facilitates targeted interventions aimed at reducing mortality within this group.20 It also supported the design of preventive trials and the estimation of the absolute burden within specific populations. Nevertheless, the implementation of AHF mortality risk stratification and prevention strategies incorporating cardio-specific biomarkers, like hs-cTn, required further investigation to define the optimal target population, timing of measurement and preventive interventions.46, 47 At institutional level, the model was adaptable to various settings including metropolitan, regional, rural and remote areas, and an online calculator is in development to enhance its widespread use in mortality risk stratification among patients with AHF. At population level, the model served as an important tool for risk standardization during mortality rate assessments, facilitating the quantification and comparison of medical resource consumption across populations and regions. Given the constrained medical and economic resources in China, adopting such a risk identification tool is a cost-effective strategy for allocating preventive resources and supporting risk-based intervention strategies.53 Meanwhile, an essential future direction was to assess whether the prospective application of the risk prediction score positively impacts patient care and clinical outcomes, which should be prioritized in future research endeavours.
Strengths and limitations
A primary strength of our study was the utilization of data from a comprehensive, multi-centre, prospective cohort study, the China PEACE-HF 4YMortality, making it as the inaugural study to forecast the 4 year mortality risk after discharge across China. This study integrated a substantial number of participants from varied regions, embodying diverse sociodemographic profiles. Moreover, the collection of high-quality and multi-dimensional predictors through various methodologies strengthened the robustness of our model and enhanced its potential applicability to a wide spectrum of patients with AHF in China. In contrast to the increasing number of previously established and validated risk scores in western populations, the China PEACE-HF 4YMortality model includes predictors that are easy to measure, cost-effective and widely accepted in clinical practice. This model especially emphasizes the use of HF-specific health status assessment tools that can quantify patient-reported outcomes. Additionally, the assessment of net benefit derived from employing this prediction model underscored its utility in enhancing risk counselling and informing policy making.
Nevertheless, our study confronted several limitations that warrant attention. Primarily, the absence of other large-scale multi-dimensional domestic cohorts in China curtailed our capacity for effective external validation. However, with the recent surge in hospital-based national and regional HF cohorts, as well as the enhanced accessibility of claims data and EMR since the mid-2010s, there is a compelling need for further external validation and subsequent updates to our model. Furthermore, while the employment of multiple imputation for addressing missing data did not alter our principal findings, it introduced additional uncertainties and may related to an underestimation of the predictive capacities of the selected predictors. Third, we were unable to provide cause-specific predictions for AHF risk due to the limited number of events collected within the 4 years following discharge and the constraints on presenting AHF prediction models separately. However, based on the methods, results, interpretations and clinical applications from the current study, we will use it as a benchmark to develop cause-specific AHF predictions for more accurate risk stratifications in the future. Also, because we excluded patients who died in-hospital and only focused on patients who were hospitalized with AHF and discharged alive, generality should be interpreted with cautious. Further researches would be consistently expanded its coverage on patients and generality on clinical application.
Conclusions
In summary, we developed and validated a straightforward predictive model, China PEACE-HF 4YMortality, which effectively estimated 4 year mortality risk among patients who were hospitalized with AHF and discharged alive with notable model performance. This model proved beneficial in identifying high-risk patients, thereby facilitating ongoing enhancements in patient outcomes.
Acknowledgements
We appreciate the multiple contributions made by project teams at National Clinical Research Center for Cardiovascular Diseases in the realms of study operation and data collection. We are grateful for the support provided by the Chinese government.
Conflict of interest statement
There are no conflicts of interest to declare.
Funding
This work was supported by the Chinese Academy of Chinese Medical Sciences Innovation Fund for Medical Science (2021-I2M-1-009). The funders of the study have no role in study design, data collection, data analysis, data interpretation or writing of the report. The corresponding authors have full access to all the data in the study and have final responsibility for the decision to submit for publication.
Open Research
Data availability statement
The datasets generated and/or analysed during the current study are not publicly available due to the government policy stipulates; it is not permissible for the researchers to make the raw data publicly available at this time. And currently, it is not yet possible for other researchers to apply for the access.




