Relaxin mimetic in pulmonary hypertension associated with left heart disease: Design and rationale of Re-PHIRE
Registration URL: https://www.clinicaltrials.gov; Unique identifier: NCT05737940.
Abstract
Aims
Despite receiving guideline-directed medical heart failure (HF) therapy, patients with pulmonary hypertension associated with left heart disease (PH-LHD) experience higher mortality and hospitalization rates than the general HF population. AZD3427 is a functionally selective, long-acting mimetic of relaxin, a hormone that has the potential to induce vasodilation and prevent fibrosis. In a phase 1b study conducted in patients with HF, AZD3427 demonstrated a favourable safety and pharmacokinetic profile. To address the unmet medical need in patients with PH-LHD in the context of HF, AZD3427 is currently under development as a potential treatment option.
Methods and results
The Re-PHIRE study is a phase 2b, randomized, double-blind, placebo-controlled, multicentre, dose-ranging study to evaluate the effect of AZD3427 on a broad range of PH-LHD phenotypes. In total, 220 patients will be randomized to four treatment groups to receive a subcutaneous injection of AZD3427 or placebo every 2 weeks for 24 weeks. The primary endpoint of the study is the change in pulmonary vascular resistance in patients treated with AZD3427 versus placebo after 24 weeks of treatment. Key secondary endpoints include changes in mean pulmonary arterial pressure, pulmonary artery wedge pressure, systemic vascular resistance, 6-min walking distance, N-terminal pro B-type natriuretic peptide levels, echocardiographic parameters, and health-related quality of life (assessed by the Kansas City Cardiomyopathy Questionnaire).
Conclusions
Re-PHIRE is the first study of a relaxin mimetic in patients with PH-LHD. The insights gained from the Re-PHIRE study are expected to inform the further development of AZD3427 in the PH-LHD population, including identifying the most suitable pulmonary hypertension and HF phenotypes for treatment.
1 Introduction
Pulmonary hypertension (PH) associated with left heart disease (PH-LHD, group 2 PH) is characterized by elevated blood pressure within the pulmonary circulation. In the context of heart failure (HF), PH-LHD is primarily caused by systolic and/or diastolic dysfunction of the left ventricle (i.e., post-capillary PH). Current guidelines define PH as a mean pulmonary artery pressure (mPAP) of greater than 20 mmHg and a pulmonary artery wedge pressure (PAWP) of greater than 15 mmHg at rest.1 Right heart catheterization (RHC) is considered the gold standard for diagnosing PH-LHD.
PH-LHD often presents as a progressive continuum of disease severity and may differ among distinct HF and PH phenotypes. Initially, LHD-related elevated left-sided filling pressure leads to an increase in mPAP. This results in post-capillary PH with normal pulmonary vascular resistance (PVR), a state referred to as isolated post-capillary pulmonary hypertension (IpcPH). IpcPH may progress to combined post- and pre-capillary pulmonary hypertension (CpcPH) owing to vascular remodelling over time. These structural changes are associated with impaired pulmonary vascular compliance and subsequently contribute to increased PVR and further increases in mPAP (Figure 1). Furthermore, despite similar haemodynamic characteristics, there is growing evidence of differences in the mechanisms leading to PH-LHD associated with HF with reduced ejection fraction (HFrEF) and PH-LHD associated with HF with preserved ejection fraction (HFpEF). Notably, the latter is often related to a cardiometabolic phenotype.2
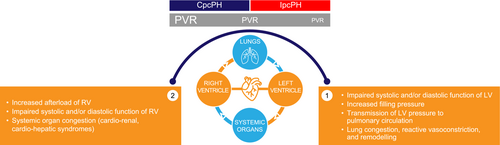
PH is observed in patients with HF regardless of left ventricular ejection fraction. Determining the exact prevalence of PH-LHD is challenging because of variations in diagnostic methods, such as echocardiography or invasive haemodynamics.1 However, studies have consistently shown that most patients with HF demonstrate increased mPAP.3-7 Elevated mPAP and PVR have been correlated with increased mortality. Conversely, lower mPAP and PVR levels have been linked to improved survival, enhanced exertional tolerance, and enhanced quality of life.5, 8-13
At present, there is no specific treatment available for patients with PH-LHD, who continue to experience high mortality and hospitalization rates despite receiving guideline-directed medical therapy for HF.1, 14-16
Relaxin is a naturally occurring hormone recognized for its vasodilatory, anti-inflammatory, and anti-fibrotic effects.17-22 Activation of the relaxin receptor RXFP1 signalling through AZD3427 holds the potential to induce favourable haemodynamic and structural changes. These include reductions in mPAP and systemic and pulmonary vascular resistance, and a halt or reversal of pathological cardiac and vascular remodelling. For the past two decades, there have been attempts to utilize the therapeutic effects of relaxin in patients with HF. However, the short-acting, intravenously administered compounds did not allow for chronic treatment effects to be utilized.23, 24
AZD3427 represents the first in its class of long-acting relaxin mimetics for chronic subcutaneous (SC) treatment, thus enabling sustained therapeutic actions. The Re-PHIRE study is the first study of a relaxin mimetic in the spectrum of HF and PH phenotypes.
2 Study design
Re-PHIRE (ClinicalTrials.gov identifier: NCT05737940) is a phase 2b randomized, double-blind, placebo-controlled, multicentre, dose-ranging trial aimed to evaluate the safety, pharmacokinetics, and efficacy of AZD3427. The study population comprises patients with HF and PH associated with left heart disease (PH-LHD, group 2 PH). The trial is not restricted by left ventricular ejection fraction or PVR at baseline, therefore it encompasses patients with HF across the spectrum of ejection fractions, and those with either IpcPH or CpcPH.
In total, 220 participants will be assigned at a 1:1:1:1 ratio to receive SC injections of AZD3427 at a dose of A, B, C, or placebo bi-weekly for 24 weeks. The study is projected to span 32–37 weeks, consisting of a screening period (up to 6 weeks), treatment period (24 weeks), and follow-up period (8 weeks post-final dose) (Figure 2).
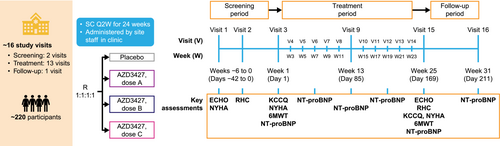
2.1 Objectives
The primary objective of the study is to investigate the effect of AZD3427 on haemodynamic measures that are relevant for survival, functional capacity, and extent of symptoms in patients with HF and PH. The primary endpoint is the change in PVR by AZD3427 versus placebo after 24 weeks of treatment. Among haemodynamic measures, PVR was shown to be the best prognostic indicator in patients with HF,13 and PVR has also been shown to correlate with mortality in patients with low and high PAWP.11
2.2 Study participants
Eligible patients are adults with New York Heart Association (NYHA) class II–IV HF, weighing more than 45 kg. Patients must be on a stable HF standard of care medication, including diuretics (if used), for at least 4 weeks before the initial screening visit (V1). Female participants must be of non-childbearing potential.
Patients enrolled in the study must have a pre-existing diagnosis of PH-LHD or a high likelihood of PH-LHD at screening. Echocardiographic parameters obtained at screening V1 must demonstrate an intermediate or high probability of PH, as per the 2022 European Society of Cardiology (ESC)/European Respiratory Society (ERS) guidelines on PH,1 before proceeding to RHC (V2). At RHC, participants must meet the criteria of post-capillary PH (PAWP ≥ 15 mmHg and mPAP ≥ 20 mmHg). Caps on randomization have been established based on mPAP values. Participants with mPAP between 20 mmHg and 24.9 mmHg are capped at a maximum of 15% of the total study population. No cap exists for participants with mPAP values of 25 mmHg and above (Figure 3).
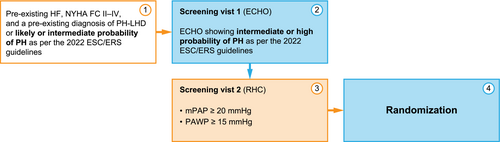
Exclusion criteria include the diagnosis of PH groups 1, 3, 4, or 5, or a clinically significant disease or disorder including myocardial infarction, stroke, transient ischaemic attack, coronary artery bypass grafting, and/or percutaneous coronary intervention within 12 weeks prior to V1, or decompensated HF, or any hospitalization within 4 weeks prior to V1. Furthermore, participants with severe mitral or aortic regurgitation or greater than mild aortic or mitral stenosis are not eligible. Full inclusion and exclusion criteria are given in Table 1.
| Inclusion criteria |
|
|
|
|
|
|
|
| Exclusion criteria |
|
|
|
|
|
|
|
|
|
|
|
|
|
- Abbreviations: b.p.m., beats per minute; DBP, diastolic blood pressure; FC, functional classification; FEV1, forced expiratory volume; HF, heart failure; ERS, European Respiratory Society; ESC, European Society of Cardiology; mPAP, mean pulmonary artery pressure; NYHA, New York Heart Association; PAWP, pulmonary artery wedge pressure; PH, pulmonary hypertension; PH-LHD, pulmonary hypertension associated with left heart disease; RHC, right heart catheterization; SBP, systolic blood pressure.
- a ESC/ERS guidelines on pulmonary hypertension.1
- b Randomization of participants with mPAP between 20 and 24.9 mmHg is capped at a maximum of 15% of the total study population. No cap exists for participants with mPAP of ≥25 mmHg.
2.3 Conduct and ethics
This study is being conducted in accordance with the protocol and international guidelines, including the Declaration of Helsinki and the Council for International Organizations of Medical Sciences, and all applicable ethical and regulatory requirements. All patients provide written informed consent before participating and can withdraw their consent at any time. The study is registered with ClinicalTrials.gov (NCT05737940).
2.4 Study assessments
- Medical history and concomitant medications.
- Physical examination, including vital signs and body weight.
- Laboratory assessments, including pharmacokinetics, clinical chemistry, urinalysis, coagulation, and serum for anti-drug antibodies/neutralizing antibodies (ADAs/NABs), and plasma and urine biomarkers associated with inflammation, fibrosis, and water–electrolyte balance.
- 12-lead electrocardiogram.
- Transthoracic echocardiography: This will be performed to assess left heart, right heart, and pulmonary circulation parameters. Echocardiography will be performed at screening V1 (baseline) and at the end of the study (V15). Imaging staff and equipment will undergo certification by a central core laboratory. Echocardiographic images will be assessed locally for study eligibility only. All images will be sent to the core laboratory and will undergo quality control checks. Echocardiogram images will be assessed by the core laboratory for endpoint analysis. The analysed parameters will include, but are not limited to, SV (Stroke Volume), EF (Ejection Fraction), LVGLS (Left Ventricular Global Longitudinal Strain), PASP (Pulmonary Artery Systolic Pressure), RV/LV ratio (Right Ventricle/Left Ventricle ratio), RVOT AT (Right Ventricular Outflow Tract Acceleration Time), TRV (Tricuspid Regurgitation Velocity), and TAPSE/PASP (Tricuspid Annular Plane Systolic Excursion to Pulmonary Artery Systolic Pressure ratio).
- RHC: This will be performed at baseline (V2) only after the participant has met echocardiographic eligibility criteria, and at the end of the study (V15). RHC parameters will be assessed locally for study eligibility and endpoints in accordance with the standard operating procedures and training provided by the core laboratory. The interpretation of the PAWP will require manual interpretation of the PAWP waveform to estimate pressure at end expiration over a minimum of three respiratory cycles. RHC tracings will be sent to the central core laboratory for exploratory endpoint analysis. PVR will be calculated according to the following formula:
- Assessment of NYHA functional class.
- 6-min walk test: This will be conducted based on the American Thoracic Society (ATS) guidelines (ATS 2002)25 at V3 before dosing and at the end of the study (V15).
- Kansas City Cardiomyopathy Questionnaire (KCCQ)26: This 23-item test will be conducted at V3 before dosing and at the end of the study (V15). Total symptom score will be calculated to assess the frequency and burden of clinical symptoms.
2.5 Study endpoints
The primary study endpoint is the change in PVR from baseline to Week 25 (after 24 weeks of treatment) compared with placebo, as measured by RHC.
Secondary endpoints include change from baseline to Week 25 in mPAP, PAWP, systemic vascular resistance, selected echocardiographic parameters including biventricular size and function and right ventricular-pulmonary artery coupling, 6-min walking distance (6MWD), biomarkers (such as N-terminal pro B-type natriuretic peptide [NT-proBNP]), and KCCQ total symptom score. Each change will be measured from baseline to Week 24 compared with placebo.
2.6 Safety assessment
The safety of AZD3427 in patients with PH-LHD will be warranted by continuous medical monitoring focused on observable and laboratory responses, including serious adverse events/adverse events, vital signs (blood pressure, heart rate, respiratory rate, body temperature, and pulse rate), body weight, electrocardiograms, and clinical laboratory assessments including estimated glomerular filtration rate, physical examinations, and injection-site reactions.
2.7 Statistical analysis
The primary endpoint will be the change from baseline in log-transformed PVR at Week 25. The mean logarithmic changes in PVR for each of the three active doses will be estimated using an analysis of covariance (ANCOVA) model with treatment as fixed effect, stratification factors as categorical covariates, and baseline PVR as a continuous covariate. The values will be back-transformed onto the original scale, and the comparison of each active dose versus placebo will be done using the geometric mean ratio. A treatment policy estimand will be used for the primary endpoint, which is using all data regardless of occurrence of intercurrent events. Detailed descriptions of statistical analyses of secondary and exploratory endpoints will be outlined in the statistical analysis plan.
For sample size calculations, 47 evaluable patients per arm yields approximately 80% power, assuming a two-sided significance level of ɑ = 0.05, a geometric mean ratio of 0.8 of an active dose versus placebo, and a standard deviation on the log-transformed scale of 0.38. In total, approximately 220 patients will be randomized to account for incomplete data (15%). Patients will be randomized at a 1:1:1:1 ratio between treatment groups, and there will be country-level stratification for China and Japan to ensure that their randomization is balanced across treatment groups.
For this dose-ranging non-confirmatory trial, no adjustments will be made to control for multiplicity. P values will be presented as two-sided and should be interpreted descriptively.
3 Discussion
In the context of HF, PH-LHD carries a high unmet medical need because patients experience higher mortality and hospitalization rates than the general HF population. PH-LHD is prevalent in patients with HF irrespective of left ventricular ejection fraction and is subdivided into distinct subgroups (i.e., IpcPH and CpcPH) based on haemodynamic characteristics.
Currently, there are no dedicated treatments available for patients with PH-LHD, leaving optimization of the underlying cardiac disease as the only treatment option.1 Importantly, while the current guideline-directed medical therapy for HF has been shown to improve left ventricular function, its direct effect on pulmonary vasculature and right ventricular function is debatable.27 Furthermore, drugs approved for pulmonary arterial hypertension, including phosphodiesterase 5 inhibitors and endothelin receptor antagonists, are not recommended for PH-LHD.1 Small randomized controlled trials with the phosphodiesterase 5 inhibitor sildenafil in patients with HFpEF and distinct haemodynamic phenotypes resembling IpcPH or CpcPH have yielded conflicting results.28, 29 Additionally, small non-randomized controlled trials have suggested that sildenafil may improve haemodynamics and exercise capacity in patients with PH and HF with HFrEF30 or HFpEF and CpcPH.31 Notably, endothelin receptor antagonists may have potentially detrimental effects in patients with PH-LHD, associated mostly with fluid retention.32 At present, several drugs, including proliferation modulators (such as sotatercept) and vasodilators (such as levosimendan), are being tested for PH-LHD. The high rate of morbidity and mortality events and the lack of established therapies for PH-LHD highlight the urgent unmet medical need in patients with HF and concomitant PH.
Based on available preclinical and clinical data, AZD3427, a long-acting mimetic of relaxin, is expected to exert both vasodilatory and anti-fibrotic effects. The vasodilatory, anti-inflammatory, and anti-fibrotic effects of relaxin have been under therapeutic investigation for many years. Preclinical studies showed that relaxin mimetics decreased mPAP and prevented increases in mPAP and PVR during hypoxia-induced vasoconstriction in sheep,33, 34 and inhibits ventricular arrhythmia in rats with PH.35 Furthermore, a clinical study involving 71 patients with acute HF demonstrated that serelaxin, a relaxin analogue, significantly decreased pulmonary pressure and resistance.24 However, the phase 3 RELAX-AHF-2 trial found no reduction in cardiovascular mortality or HF worsening at 180 days following a 48-hour serelaxin infusion, compared with placebo.23 The lack of clinical benefit could result from the short duration of treatment with the short-acting compound, which may be insufficient to harness the long-term benefits of activated relaxin signalling.
To overcome this obstacle, AZD3427 was designed as a long-acting relaxin mimetic enabling chronic treatment. A preceding single ascending dose/multiple ascending dose (SAD/MAD; NCT04630067) study indicated that AZD3427 was well tolerated, with no significant safety concerns in healthy volunteers and patients with HF. The half-life of AZD3427 in patients with HF was between 13 days and 14 days, and the overall pharmacokinetic profile justified bi-weekly dosing in the current study. While the SAD/MAD study was not designed or powered to detect significant pharmacodynamic effects, 5 weeks of once-weekly SC treatment with AZD3427 demonstrated sustained haemodynamic effects. Specifically, groups treated with AZD3427 displayed a trend towards increased CO, with no significant changes in heart rate and blood pressure, and an increase in estimated glomerular filtration rate.36
Re-PHIRE is the first study of a relaxin mimetic in PH-LHD. In Re-PHIRE, we will evaluate the safety, tolerability, and potential efficacy of AZD3427 versus placebo across a variety of HF and PH phenotypes. The insights gained from this study are expected to inform the further development of AZD3427 for the PH-LHD population, including identifying the most suitable PH-LHD phenotypes for treatment.
Several studies suggest that up to 80% of patients with HF may exhibit elevated mPAP,3-7 however, only a small proportion of patients undergo RHC, the gold standard for diagnosing PH-LHD. Notably, several studies are exploring echocardiographic parameters for diagnosing PH and predicting cardiovascular outcomes in this patient group. In our study, we are employing a stepwise approach for establishing diagnosis of PH-LHD and fulfilling inclusion criteria during screening. Initially (at V1), patients are screened with echocardiography, and only those who meet the echocardiographic criteria of intermediate or high probability of PH as per the 2022 ESC/ERS guidelines,1 proceed to RHC (V2). This approach is intended to minimize unnecessary RHC procedures and screening failures during RHC. Additionally, detailed echocardiographic phenotyping of the right and left ventricles, along with haemodynamic data from RHC, is being used for an exploratory analysis of the predictive value of echocardiography in PH-LHD diagnosis.
Selecting a surrogate outcome for phase 2 studies poses a significant challenge because no single surrogate endpoint is universally considered to be a reliable predictor of cardiovascular outcomes in HF populations. Therefore, a multidomain approach to decision-making concerning further drug development is often employed.
In this study, we designated the primary endpoint as the change in PVR following 24 weeks of treatment. However, it is important to stress that the secondary endpoints of this study, such as changes in mPAP, PAWP, systemic vascular resistance, 6MWD, biomarkers such as NT-proBNP, and KCCQ score, are also important from a drug development perspective. It may be expected that patients with IpcPH will not show meaningful reduction in PVR. Therefore, pre-specified subgroup analyses of participants with IpcPH and CpcPH, as well as the distinct HF phenotypes, are being planned.
Ample evidence suggests that PVR and mPAP are strongly correlated with cardiovascular risk and outcomes.5, 8-13 Given that the current study is PVR-agnostic and will include both IpcPH and CpcPH subgroups, relatively low baseline values of PVR can be expected. Demonstrating a significant change in PVR in a population with low baseline PVR could prove challenging. Despite this, the inclusion of patients with low PVR is important. This standpoint is supported by recent findings from a large cohort, predominantly with post-capillary PH. According to this study, PVR of 2.2 WU or higher was associated with adverse outcomes and deemed to be abnormal.11 Furthermore, the recent 2022 ESC/ERS guidelines have revised the haemodynamic definition of CpcPH to include patients with PVR higher than 2 WU. Consequently, even in a study within the population defined as CpcPH, a relatively low baseline PVR can be expected.
Considering the aforementioned limitations of PVR as a primary endpoint, mPAP might be a more appropriate choice for the primary endpoint in the present study. However, demonstrating a significant change in mPAP could also pose challenges. For instance, a decrease in mPAP may occur when RV function deteriorates, which would not be a desired effect. Moreover, a decrease in PVR might coincide with an increase in CO, resulting in no significant change in mPAP, as was the case in the LEPTH study.37 This consideration is particularly important given that the phase 1 study found AZD3427 to increase stroke volume and CO.36
The Re-PHIRE study will also evaluate the effect of AZD3427 on 6MWD and the KCCQ score, both of which are frequently used in clinical trials, guide further drug development, and sometimes serve as registrational endpoints. However, it needs to be stressed that there is ongoing debate regarding the reproducibility of both the 6MWD and the KCCQ across various HF subpopulations, as well as the magnitude of change in 6MWD or KCCQ scores that should be considered a clinically meaningful improvement. Finally, a broad panel of biomarkers will be tested in this study to gain a better understanding of the mechanism of action of AZD3427, specifically focusing on the expected anti-inflammatory and anti-fibrotic effects that should become evident after 6 months of treatment.
4 Conclusions
The Re-PHIRE study is expected to answer the question of whether AZD3427 can improve haemodynamic parameters known to be associated with mortality in patients with PH-LHD. The study design and patient selection provide a unique opportunity to capture the potential effectiveness of AZD3427 in different areas (i.e., systemic/pulmonary circulation, left/right heart). In this sense, insights gained from the Re-PHIRE study are expected to inform the further development of AZD3427 in this patient population, including identifying the phenotypes that may be most responsive to the treatment.
5 Acknowledgements
The authors thank all investigators, all trial teams, and participants for taking part in the trial.
Conflict of interest statement
MG, DB, JE, FG, MC, RLV, TA, RG, PJ, SW, KK, EB, ZCJ and SR serve as the national investigator lead and/or primary investigators and receive reimbursement for their work in the Re-PHIRE study.MU, KC, MM, and ES are employees and stockholders of AstraZeneca. DB reports speakers fees, research support and travel grants from Alnylam, Astra Zeneca, Boehringer Ingelheim, Novartis, Zoll, Bayer, BMS, Abbott, MSD. JE reports research support for trial leadership or grants from American Regent, Applied Therapeutics, AstraZeneca, Bayer, Cytokinetics, Merck & Co, Novo Nordisk, Otsuka; honoraria for consultancy from AstraZeneca, Bayer, Boehringer Ingelheim, Novartis, Novo Nordisk, Otsuka; serves as an advisor to US2.ai. FG serves as an advisor for Astra-Zeneca, Abbott, Pfizer, Bayer, Ionis, Alnylam, Pharmacosmos, AdjuCor, FineHeart and receives speaker’s fee from Novartis. TA reports consulting fees from BI and AZ and research grants from AZ, BI, Amgen. RG reports speaker or consultant fees from Abbott, Anacardio, Astra Zeneca, Boehringer Ingelheim, Boston Scientific, Novartis, Pfizer, Pharmacosmos, Roche Diagnostics, and research grants to his institution from Abbott, Boston Scientific, and Roche Diagnostics. PJ reports fees and grants from Janssen Pharmaceutical Companies of Johnson and Johnson, AOP Orphan, Bayer HealthCare, and MSD, outside of the article. SW reports research grant from AstraZeneca to research foundation ‘stichting Perfusie’, speaker and consulting fees from Astrazeneca, and reimbursement of expenses as national lead (NL) for REPHIRE. SR reports speaker or consultant fees from Abbott, Accelleron, Actelion, Aerovate Therapeutics, Altavant Sciences, AOP Health, AstraZeneca, Bayer, Boehringer Ingelheim, Edwards Lifesciences, Eli Lilly and Company, Ferrer, Gossamer Bio, Inari Medical, Janssen, MSD, and United Therapeutics, and research grants to his institution from AstraZeneca, Bayer, Janssen, and MSD.
Funding
This work was supported by AstraZeneca.




