Discovery of miRNAs unique to actively transcribed erythroparvovirus infection in heart failure patients
Abstract
Aims
miRNAs, small non-coding RNAs, play key roles in gene regulation, cell differentiation and tissue development. They influence viral infection outcomes by directly interacting with viral genomes or modifying the host microenvironment. This study demonstrates miRNAs' ability to selectively suppress transcriptionally active erythroparvovirus, highlighting their potential in antiviral therapies.
Methods and results
Seventy-five endomyocardial biopsy (EMB) specimens from patients with unexplained heart failure were analysed. The samples included 19 with dilated cardiomyopathy and inflammation (DCMi), 12 with dilated cardiomyopathy (DCM), 25 with inflammation and active erythroparvovirus infection, 13 with active erythroparvovirus infection only and 6 from undiagnosed patients as controls. miRNA expression was measured using TaqMan assays. miR-98, miR-222, miR-106b and miR-197 were significantly upregulated in patients with transcriptionally active erythroparvovirus infection, independent of inflammation (P < 0.005). These miRNAs differentiated these patients from all other groups with over 90% specificity.
Conclusions
These specific miRNAs offer a novel diagnostic tool for active erythroparvovirus infections and hold promise as therapeutic targets, providing safer alternatives to traditional antiviral treatments.
Introduction
Infections of the heart that spread widely are a significant contributor to heart failure, often progressing to the clinical manifestation of dilated cardiomyopathy (DCM).
The primary virus identified in endomyocardial biopsies (EMBs) of individuals with inflammatory dilated cardiomyopathy (DCMi) is human parvovirus B19 (B19V).1-3 B19V belongs to the erythroparvovirus genus within the Parvoviridae family. It carries a linear, single-stranded DNA genome encoding the nonstructural protein NS1, two structural capsid proteins (VP1 and VP2) and small accessory proteins of 11 and 7.5 kDa. Previous research has suggested that the presence of B19V in EMBs may be a nonspecific bystander phenomenon.4-6 However, this interpretation may be incomplete, given that the inclusion criteria for these studies were exclusively based on B19V DNA loads. From a contemporary virological perspective, a more nuanced consideration of B19V detection in the heart is warranted. Despite numerous studies, effective therapeutic interventions against B19V infection are yet to be established.
Accurately assessing the replicative status of the virus proves crucial in determining the transcriptional activity of B19V in the myocardium.7, 8 While latent B19V infection, indicated solely by the detection of viral DNA in EMBs, is presumed to have no significant impact on the clinical course, research indicates that transcriptionally active B19V—characterized by the detection of viral RNA—results in altered cardiac gene expression and is correlated with progressive cardiac dysfunction and compromised survival.9, 10 A disease-specific differential expression of microRNAs (miRNAs) has been described in the regulation of replicating viruses.
miRNAs—short noncoding ribonucleic acids (RNAs) measuring around 20–22 nucleotides in length—play a regulatory role in controlling the activities of various protein-coding messenger RNAs.11 This regulation is achieved through processes such as degradation of the target RNAs or inhibition of translation.
miRNAs have significant involvement in diverse physiological processes, functioning either throughout the body or with specificity to particular organs and cell types. Over the last 10 years, miRNAs have been associated with the underlying causes of different health disorders, notably cardiovascular diseases (CVD).12 Changes in miRNA expression, whether increased or disrupted, have been connected to a range of abnormal physiological states like cardiac hypertrophy, fibrosis, metabolic irregularities and angiogenesis.13 Tracking the levels of miRNAs present in the bloodstream offers valuable insights into the advancement and evolution of cardiovascular diseases, providing essential information about their onset and progression.
Within the cardiac context, miRNAs play a pivotal role in governing vital processes like growth, inflammatory responses and regeneration.14
Analysing patients with latent and reactivated B19V infection, 29 differentially regulated miRNAs have been already found.15 Correlations were identified between 92 miRNAs exhibiting differential expression in individuals with Dilated Cardiomyopathy (DCM) experiencing Chronic Heart Failure (CHF). This investigation unveils the expression profiles of miRNAs in plasma exosomes among DCM patients with CHF, shedding light on their potential roles in the pathogenesis of this condition. These findings offer a novel perspective for the clinical diagnosis and management of DCM patients experiencing CHF.16
For instance, miR-21 is a noteworthy miRNA which exhibits heightened expression in different cardiac cell types, particularly fibroblasts and macrophages, within both human and animal models of cardiac failure.17 Notably, inhibiting miR-21 using an anti-miRNA approach (antimiR-21) in a mouse model of cardiac hypertrophy effectively halted cardiac fibrosis, marking a significant advancement in miRNA-based therapy for cardiovascular conditions. Subsequent research demonstrated that miR-21 also governs the development of interstitial fibrosis in various noncardiovascular diseases,17 and the use of antimiR-21 reduced pathological fibrosis.18 miR-21 has been shown to increase in viral myocarditis, decreasing the expression of miR-21 can protect myocytes in the myocarditis process.19
AntimiRNAs function by specifically binding to the seed regions of target miRNAs, with their successful deployment to the target organ being a critical factor in determining their therapeutic effectiveness. However, delivering these innovative treatments primarily to the heart through local administration poses challenges, potentially impeding their translation to clinical medical practice when compared to other systemically delivered protocols. Encouragingly, the application of miRNA-based therapeutics directly into coronary arteries has become a viable option for patients,20 as evidenced by ongoing gene therapy clinical trials. Levels of miR-133, which is notably present in healthy cardiomyocytes, were observed to decrease in animal models of hypertrophy and in patients with cardiomyopathy.21 Conversely, elevating miR-1 levels was linked to irregularities in electrical activity in the heart.22 Consequently, strategies involving the reinstatement or targeted modification of miRNA expression hold great promise as therapeutic interventions.23 Currently, this approach forms a central objective in numerous preclinical and clinical investigations, primarily outside the realm of cardiovascular ailments.
The emergence of innovative tools designed to influence miRNA expression is forging pathways toward novel therapeutic prospects.
Materials and methods
Study population
In this retrospective study miRNA expression levels were evaluated in biopsy samples from 69 patients with clinically unexplained heart failure diagnosed by EMB. The diagnoses of EMBs were based on different disease entities and sent from different clinics in Germany to the CAP-accredited Institute for Cardiac Diagnostics and Therapy, Berlin, Germany (IKDT) for further routine diagnostics (Table 1). Six EMB samples from patients who had not received a formal diagnosis were used as control group.
| Characteristic | Inflammation and no virus—DCMi | No inflammation and no virus—DCM | Inflammation and transcriptionally active erythroparvovirus infection | Transcriptionally active erythroparvovirus infection and no inflammation | Healthy controls |
|---|---|---|---|---|---|
| Number | 19 | 12 | 25 | 13 | 6 |
| Age, mean (year) | 45 ± 15 | 50 ± 12 | 51 ± 15 | 42 ± 12 | 44 ± 12 |
| Male sex (%) | 15 (79) | 8 (67) | 20 (80) | 7 (54) | 4 (67) |
| LVEF (%), mean ± SD | 40 ± 14 | 33 ± 7 | 42 ± 16 | 41 ± 10 | - |
| EF < 55% (%) | 73 | 100 | 60 | 63 | 0 |
| LVEDD (mm), mean ± SD | 52.7 ± 21.3 | 63.3 ± 14.2 | 55.2 ± 21.0 | 48.2 ± 24.0 | - |
| Fibrosis (%) |
No fibrosisa = 82% Low fibrosisb = 18% |
No fibrosis = 85% Low fibrosis = 15% |
No fibrosis = 80% Low fibrosis = 20% |
No fibrosis = 88% Low fibrosis = 12% |
- |
- a Up to 3% of connective tissue.
- b 5%–15% of connective tissue.
Patients with coronary artery disease, other possible causes of myocardial dysfunction (e.g., valvular heart disease, hypertension, restrictive, or constrictive heart disease) diagnosed by angiography and echocardiography, and concomitant chronic inflammatory disease (e.g., rheumatological disorders) were excluded from this study.
Left ventricular ejection fraction (LVEF) was determined by echocardiography.
Assessment of inflammation (DCMi) and dilated cardiomyopathy (DCM) in EMBs
Using analyses on EMBs inflammation was diagnosed immunohistochemically by detection of inflammatory infiltrates of >14.0 lymphocytes/mm2, including >7.0 CD3+ lymphocytes/mm2 according to the European Society of Cardiology guidelines.24 Antibodies used, the immunohistochemical staining procedure and the evaluation of EMBs were as described previously.10, 25
The diagnosis of dilated cardiomyopathy (DCM) was based on morphological and functional characterization with significantly impaired LVEF and/or dilated LV. EMBs from DCM patients were negative for immunohistochemically detected inflammatory responses and for detection of cardiotropic viral genomes.
Detection of viral genomes in EMBs
Viral DNA and RNA were extracted from frozen heart muscle tissue probes as described previously.26, 27 Reverse transcriptase (RT)-PCR was performed for the detection of enteroviruses (including coxsackieviruses), adenovirus, parvovirus B19 (B19V) and human herpesvirus type 6 (HHV6) as previously outlined.26, 27 Additionally, DNA was extracted from peripheral blood cells to exclude a systemic infection with B19V and HHV6. As a control for successful extraction of DNA and RNA from heart muscle tissue, oligonucleotide sequences were chosen from the DNA sequence of the GAPDH gene.
miRNAs isolation
miRNAs from all patients were isolated using mirVANA™ PARIS™ RNA and Native Protein Purification Kit (Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA, USA; https://www.thermofisher.com/order/catalog/product/de/de/AM1561) according to the manufacturer's instructions.
miRNA reverse transcription, pre-amplification and expression analysis
Total RNA including miRNA fraction was initially reversely transcribed to cDNA using Megaplex stem-loop RT primer (Thermo Fisher Scientific, Waltham, MA, USA) for Human Pool A and B in combination with the TaqMan MicroRNA Reverse Transcription Kit (Thermo Fisher Scientific, Waltham, MA, USA) for low content samples. The entire procedure for quantification of miRNAs has been described elsewhere.28 U6 was used as reference miRNA for data normalization. All experiments were performed in minimum in duplicates.
Ethical approval
The study was performed within the CRC Transregio 19 (NCT02970227, 1 January 2004) and was approved by the local ethics committees of the participating clinical centers as well as by the committees of the respective federal states. The study complies with the Declaration of Helsinki. An informed written consent was obtained from each study patient.
Statistical analysis
All expression data were analysed and represented with GraphPad Prism 7 (GraphPad Software, La Jolla, USA). The Student's t-test and one-way-ANOVA were used to compare miRNA expression levels between all study groups (Figure 1).
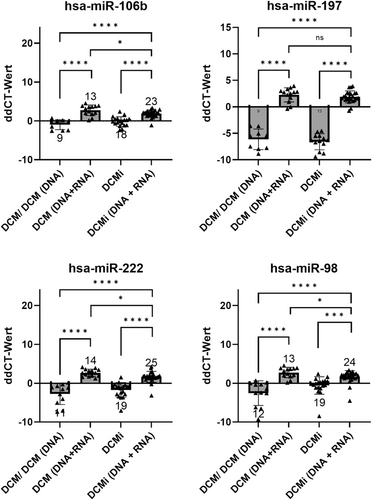
All data were presented as single values with mean and standard deviation (SD), with a significance level of *P < 0.05, **P < 0.005, ***P < 0.0005, ****P < 0.0001.
Results
Patient characteristics
In this retrospective study biopsy samples from 69 patients with clinically unexplained heart failure were analysed. EMB diagnosis showed that 19/75 patients suffered from inflammation without virus infections (DCMi), 12/75 patients showed no inflammation without virus infections (DCM), 25/75 patients had inflammation and transcriptionally active erythroparvovirus infection, and 13/75 patients were diagnosed with transcriptionally active erythroparvovirus infection without inflammation (Table 1).
miRNA analyses
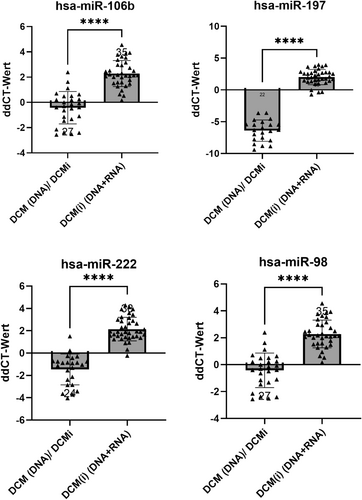
Based on the expression levels of selected miRNAs, four miRNAs—miR-106b, miR-197, miR-222, miR-98—were significantly deregulated in the biopsies of patients with transcriptionally active erythroparvovirus infection (P < 0.01) (Figures 1 and 2). Considering their significantly different expression levels in EMBs, these four miRNAs (here referred to has-miR-30a-3p) may have potential to be diagnostic biomarkers for transcriptionally active erythroparvovirus.
The relationship between the expression levels of miRNAs and viral load (DNA) and load of transcriptionally activated erythrovirus (RNA) has been further studied. We have not detected any significant dependence of viral load on the miRNA expression levels (Figures 3 and 4).
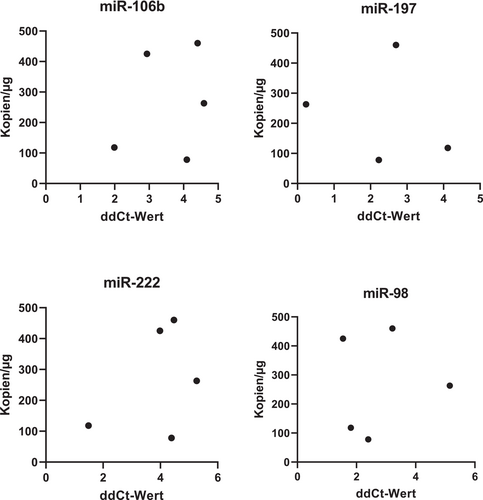
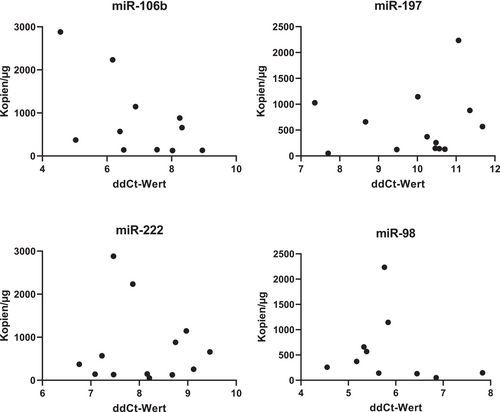
Discussion
MiR-98, miR-222, miR-106b and miR-197 were significantly upregulated in patients with transcriptionally active erythroparvovirus infection during this study, independent of inflammation (P < 0.005). These miRNAs differentiate these patients from all other groups with over 90% specificity, offering a novel diagnostic tool for active erythroparvovirus infections and holding promise as therapeutic targets.
MicroRNAs (miRNAs) serve as vital regulators in various biological processes and are often disrupted in human diseases.29 As demonstrated by their ability to orchestrate entire cellular pathways through interactions with a wide array of target genes, microRNAs (miRNAs) possess remarkable genetic regulatory capabilities. This attribute makes miRNAs intriguing candidates for therapeutic interventions aimed at restoring cellular functions disrupted in the context of disease. However, this potency of miRNAs also presents a vulnerability, as their multifaceted effects within cells make it challenging to completely avoid off-target consequences.
miRNAs can employ one of two distinct mechanisms to inhibit their target: either by cleaving and degrading the target mRNA or by suppressing protein translation.30 The specific mechanism that occurs depends on the degree of complementarity between the miRNA and the mRNA. A perfect match results in mRNA degradation, while imperfect complementarity leads to translational inhibition. Because miRNAs can bind with either perfect or imperfect complementarity, a single miRNA can regulate numerous different targets, enabling both precise and extensive control over translation.31 Furthermore, recent research indicates that miRNA binding to their target sequences on other non-coding RNAs, including pseudogenes, can modulate cellular miRNA levels, adding another layer of complexity to the regulation of the transcriptome.32
The study by Hinkel and colleagues demonstrated already the therapeutic promise of miR-21 inhibition in a pig model of heart failure through targeted delivery of antimiR-21.33 This study also validates the efficacy of a catheter-based delivery system for transporting antimiR-21 to the heart muscle, overcoming the challenge of administering anti-miRNAs within the heart. By directly applying antimiR-21 to the local area, the need for high doses in a clinical setting may potentially be reduced. This method exposes the affected heart tissue to concentrated therapeutic agents, resulting in improved uptake and enhanced therapeutic outcomes. This hypothesis gains support from the study's findings, which document sustained suppression of miR-21, reduced macrophage infiltration and decreased fibroblast proliferation over a 33-day observation period in a pig model of myocardial infarction.33
Gradually increasing doses of antimiR-21 therapy demonstrated a progressive restoration of cardiac function and sustained alterations in the myocardial transcriptome.33 Targeting miR-21 presents challenges due to its specific influence on nonmyocyte cells distributed throughout the body.34 However, this particular study effectively countered the detrimental effects of miR-21 while preserving its protective functions.
A significant drawback of anti-miRNA agents is their potential for off-target effects. Administering high doses of anti-miRNAs can carry risks of toxicity, stemming from their non-specific binding to similar nucleotide sequences (known as off-target effects) or due to chemical modifications within the oligonucleotides.35 Noticeable effects on miR-21 expression were observed not only in the heart but also in the liver and kidney. Although the monitoring period was limited to 33 days, no adverse changes in kidney and liver function parameters were reported. In such instances, targeted delivery using nanoparticle-based carriers could potentially prove advantageous in mitigating off-target effects in tissues beyond the heart.
The α-myosin heavy chain (αMHC) gene is one of the most important genes for determining cardiomyocyte contractility. Several years ago, it was discovered that this gene not only gives rise to a key protein, but additionally produces a miRNA, known as miR-208a.36 Although the expression level of miR-208a does not change significantly during cardiac stress, this miRNA appears to play a key role in the stress-induced induction of βMHC, the pathological counterpart of αMHC and is, thus, a relevant player during pathological remodelling that occurs during cardiac disease. Efficacy studies in animal models of heart disease using an LNA-modified antimiR targeting miR-208a indicated that subcutaneous delivery of antimiR-208a prevents disease-related cardiac remodelling, a decline in function and death during diastolic heart disease.37 These studies underscore the potential of antimiR-based therapies for modulating cardiac miRNAs and validate miR-208 as a therapeutic target for the modulation of cardiac function and remodelling during heart disease. Follow-up studies showed that long-term treatment with antimiR-208 prevented age-induced weight gain normally observed in mice.37 This effect occurred in the absence of detectable toxicity or observable cardiac effects. Inhibition of miR-208, in addition to blocking cardiac remodelling in the setting of heart disease, can have profound effects on metabolism and imply that the heart plays an unexpected role in the regulation of systemic metabolism and energy expenditure based on a miR-208-dependent mechanism.38
Analysis of miRNA microarray results indicated a noteworthy elevation in the expression levels of miR-324-3p following infection with CVB3, both in cellular and murine models. Further investigation revealed that miR-324-3p played a role in the downregulation of TRIM27, leading to a reduction in CVB3 replication in both in vitro and in vivo settings.
In the in vitro context, a detailed examination of the downstream signalling pathways associated with TRIM27 elucidated that miR-324-3p exerted an inhibitory effect on CVB3 infection. This effect was manifested by a decrease in cytopathic effects and diminished viral plaque formation, which could be attributed to the downregulation of TRIM27 expression.
In the in vivo scenario, miR-324-3p was found to suppress the expression of TRIM27, resulting in decreased cardiac viral replication and load. This, in turn, had a profound mitigating impact on cardiac injury and inflammation, thereby contributing significantly to the overall attenuation of the pathological consequences of CVB3 infection.39
MiR-425-3p exhibited low expression levels in the myocardial tissues of mice afflicted with viral myocarditis. However, the overexpression of miR-425-3p led to notable improvements in cardiac function, mitigated pathological manifestations, reduced cardiomyocyte apoptosis, lowered the expression of Bax and cleaved Caspase-3, elevated Bcl-2 expression, diminished the levels of inflammatory factors and enhanced the survival rate of mice grappling with viral myocarditis.
The luciferase activity assay confirmed the binding ability of miR-425-3p to TGF-β1. Furthermore, the overexpression of miR-425-3p was demonstrated to suppress the expression of TGF-β1, as well as phosphorylated Smad2/Smad2 and phosphorylated Smad3/Smad3. In complementary in vitro experiments, the overexpression of miR-425-3p was shown to inhibit apoptosis in CVB3-HL-1 cells, with the intriguing observation that the addition of TGF-β1 could reverse this effect.40
Coronaviruses, exemplified by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the causative agent of the 2019 coronavirus disease (COVID-19) pandemic, pose a significant menace to human well-being, inflicting a wide array of health complications and even fatalities. While conventional therapeutic approaches often entail the administration of small molecules to combat viral infections, a promising innovative strategy involves miRNAs that have the potential to serve as a novel antiviral tactic by virtue of their capacity to modulate host-virus interactions through mechanisms such as RNA degradation and translational inhibition. Exploring the interplay between miRNAs and SARS-CoV-2 can unveil fresh therapeutic avenues to combat this viral adversary. Multifunctional microRNA-155 (miR-155) elevation in malaria, dengue virus (DENV), the thalassemias and SARS-CoV-1/2 plays critical antiviral and cardiovascular roles through its targeted translational repression of over 140 genes.41 Eight miRNAs displayed altered expression in anterior nasal tissues from COVID-19 patients, with miR-142-3p, a negative regulator of interleukin-6 (IL-6) production, the most strongly upregulated. Supervised machine learning analysis revealed that a three-miRNA signature (miR-30c-2-3p, miR-628-3p and miR-93-5p) independently classifies COVID-19 cases with 100% accuracy.42 Given that current treatment options for SARS-CoV-2 remain constrained and lack substantial evidence of safety and efficacy in addressing COVID-19, the investigation into the interplay between miRNAs and SARS-CoV-2 offers a promising avenue.43
Antiviral therapy stands as a captivating frontier in virology, skillfully applying fundamental scientific knowledge to create remarkably effective treatments for severe viral infections. The realm of human immunodeficiency virus (HIV) infection therapy has shown the potential of antiviral agents to profoundly impact life-threatening, persistent infections, benefiting over 12 million individuals.44 This notable progress is on the verge of being mirrored in the context of hepatitis C virus (HCV) infection treatment. An example of this advancement is Miravirsen, an anti-miRNA compound targeting miR-122, which was among the pioneering miRNA-based therapies tested in patients with hepatitis C virus.45 In a phase II clinical trial involving 36 patients, this agent demonstrated a significant reduction in virus levels within the liver. Presently, the US National Library of Medicine database enumerates a total of 18 clinical studies assessing 8 distinct miRNA-based therapies.46 However, it is worth noting that none of these studies specifically focus on patients with cardiovascular disorders.
The paradigm for drug development is undergoing transformation. In the past, the primary thrust was directed toward viral targets, a strategy that remains notably fruitful. However, this approach is now being enriched with a broader array of methodologies. As of now, the strategies encompass: compounds aimed at generic viral elements like RNA or DNA synthesis, potentially effective against diverse virus types, and compounds designed to impede host cellular functions essential for virus replication, possibly targeting a single virus or a range of viruses. Moreover, the traditional techniques for drug discovery are being complemented by extensive databases and evolving computational biology techniques. Notably, there is an increased emphasis on repurposing drugs that have already received approval for human use. This shift is propelled by the significant time and costs associated with original drug development endeavours.
Acknowledgements
This work was supported by ProFIT grant of the Investitionsbank Berlin/EFRE (No. 10198849, Berlin, Germany) and by Zentrales Innovationsprogramm Mitterstand (ZIM) (No. KK5175802AP2). For their excellent technical assistance, we are grateful to K. Winter, S. Ochmann and J. Klostermann (IKDT).
Conflict of interest statement
None declared.




