Duration of sodium zirconium cyclosilicate treatment and continuation of RAASi therapy after a hyperkalaemia episode
Abstract
Aims
Renin–angiotensin–aldosterone system inhibitors (RAASi) are foundational in the management of heart failure (HF) and chronic kidney disease (CKD) but increase the risk of hyperkalaemia. To facilitate continuation of RAASi therapy, guidelines suggest managing hyperkalaemia using newer potassium binders such as sodium zirconium cyclosilicate (SZC). This observational study describes the likelihood of continued RAASi therapy by duration of SZC treatment.
Methods
The study population included non-dialysis-dependent adults diagnosed with HF and/or CKD who initiated outpatient SZC treatment while receiving RAASi therapy. Patients were identified using healthcare registers and claims data from the United States, Japan and Spain. SZC treatment duration was described using the Kaplan–Meier method. Hernán's clone–censor–weight (CCW) approach, using principles of trial emulation, was applied to evaluate the likelihood of continued RAASi therapy at specific time points by distinct SZC treatment durations, using a weighted Kaplan–Meier method and Z-tests.
Results
The study included 7980 patients, from the United States (n = 4849), Japan (n = 2759) and Spain (n = 372). Across the three countries, mean patient age was 73.1–75.0 years, 53.2%–66.4% of patients were male, 39.0%–75.0% had HF and 76.9%–95.3% had CKD. Between Days 30 and 120, the percentage of patients remaining on SZC treatment decreased from 36.5% to 12.8% in the United States, from 63.8% to 33.7% in Japan, and from 81.9% to 65.0% in Spain. In the United States, patients who continued SZC treatment beyond 30 days had a higher likelihood of continuing RAASi therapy for up to 90 days (P < 0.001), and continuing SZC treatment beyond 60 days was superior for continuing RAASi therapy for up to 6 months (P < 0.001), versus earlier SZC discontinuation. At 120 days, the likelihood of remaining on RAASi therapy was 69%–70% for SZC treatment durations exceeding 60 days, versus 59% for shorter durations (1–30 days) (P < 0.001). Similar patterns were observed in Japan. At 120 days, the likelihood of remaining on RAASi therapy was 86%–87% for SZC treatment durations exceeding 90 days, versus 82% for shorter SZC treatment durations (1–30 days) (P < 0.05). The CCW analyses were not deemed feasible in the Spanish dataset due to the smaller initial sample size and few patients having a relatively short SZC treatment duration.
Conclusions
Patients with longer SZC treatment experience sustained protection against RAASi discontinuation, and the risk of RAASi discontinuation resumes once SZC is discontinued.
Introduction
Renin–angiotensin–aldosterone system inhibitors (RAASi) are foundational therapy in the management of heart failure (HF) and chronic kidney disease (CKD) and have been shown to reduce the risk of cardiovascular mortality and morbidity and kidney failure.1, 2 International evidence-based guidelines recommend RAASi therapy at the maximally tolerated dose to optimize treatment benefits in patients with HF and/or CKD.1, 2
RAASi therapies however increase the risk of hyperkalaemia due to their potassium (K+)-sparing properties.3-5 Patients receiving RAASi therapy have twice the risk of developing hyperkalaemia as patients not receiving RAASi therapy.6 If left untreated, hyperkalaemia can lead to cardiac arrhythmias, cardiac arrest, and death.7-9 The risk of hyperkalaemia can be perceived as a barrier to achieving guideline-directed treatment with RAASi therapy, and RAASi therapy is often reduced or discontinued in patients experiencing hyperkalaemia.10-14 However, hyperkalaemia-related RAASi reduction is associated with an increased risk of cardiorenal adverse events, healthcare resource utilization and mortality, compared with maintained RAASi therapy.13, 15, 16
According to current international guidelines and a matching consensus from practicing clinicians, hyperkalaemia should no longer be a barrier to continued RAASi therapy; instead, newer K+ binders, such as sodium zirconium cyclosilicate (SZC), may be used to manage hyperkalaemia and facilitate continuation of RAASi therapy.1, 2, 17, 18
SZC has demonstrated beneficial effects in facilitating continued RAASi therapy, both in the clinical trial setting and in routine clinical practice.19, 20 In an open-label, single-arm, Phase 3 trial, 87% of patients treated with SZC continued their RAASi therapy or had their dose increased at 12 months.19 In routine clinical practice across three countries, the odds of maintained RAASi therapy at 6 months were 2.5-times higher in patients treated with SZC for at least 4 months, relative to patients with no K+ binder prescription.21 However, the impact of duration of SZC treatment, or any newer K+ binder, on the ability to facilitate continued RAASi therapy, has not been assessed to date.
The aim of this multi-country, observational study was to evaluate the likelihood of continued RAASi therapy by duration of SZC treatment after a hyperkalaemia episode in non-dialysis-dependent patients with HF and/or CKD. This study is part of ZORA, an observational study programme investigating the management and consequences of hyperkalaemia in patients with HF and/or CKD in routine clinical practice.13, 16, 21
Methods
Data sources
This observational, multi-country study used longitudinal data collected retrospectively from healthcare claims and registries from the United States, Japan and Spain. The data sources are detailed in the Supporting information S1. Briefly, the US data source, Optum's de-identified Clinformatics® Data Mart Database, captures health claims data from recipients of commercial health insurance and Medicare Advantage plans across the United States.22 The Medical Data Vision (MDV) database captures healthcare data from hospitals across Japan.23 The BIG-PAC database captures electronic medical records data from seven Spanish regions.24
The study period began when SZC became available in each respective country and ended at the date of last available data in each data source as follows: July 2019 to September 2023 in the United States, May 2020 to April 2023 in Japan and June 2021 to January 2024 in Spain.
The US data source included de-identified data in compliance with the Health Insurance Portability and Accountability Act requirements; neither informed consent nor Institutional Review Board approvals were required. According to the Japanese Ethical Guidelines for Medical and Health Research Involving Human Subjects, ethical approval and informed consent do not apply to the use of de-identified secondary data. In Spain, the study was approved by the Ethical Committee (Comité de Ética de Investigación con Medicamentos del Consorci Sanitari de Terrassa; 02-23-399-061), with a waiver of requirement for informed consent.
Study population
The study population included adult non-dialysis-dependent patients diagnosed with HF and/or CKD who initiated outpatient treatment with SZC while receiving RAASi therapy.
The index date was defined as the date of the first SZC prescription, with no SZC prescription in the preceding 6 months. Patients were required to be ≥18 years of age and have a diagnosis of HF and/or CKD, but to not be on dialysis during the baseline period (12 months prior to the index date). Patients were also required to have ongoing RAASi therapy at index, defined as having medication supply overlapping with the index date while applying a 30 day grace period. RAASi classes included were angiotensin-converting enzyme inhibitors (ACEi), angiotensin receptor blockers (ARB), angiotensin receptor-neprilysin inhibitors (ARNi) and mineralocorticoid receptor antagonists (MRA). At least 12 months of available look-back for baseline data prior to the index date was required for inclusion.
All potential index dates per patient were screened for eligibility; where multiple eligible index dates were available, one was chosen at random.
A preceding hyperkalaemia diagnosis or a recorded K+ value >5.0 mmol/L was not required for inclusion, based on1 hyperkalaemia being the sole indication for SZC,2 known under-recording of hyperkalaemia diagnoses in routine clinical practice, and3 the extent of missing data on laboratory K+ values in the Japan and US databases. A previous study that included patients treated with SZC, using the same data sources as the current study, found that baseline characteristics of SZC-treated patients with versus without a preceding documented hyperkalaemia diagnosis were similar.21
Statistical analyses
Patient demographics and characteristics at SZC initiation (i.e., the index date) were described using standard descriptive statistics.
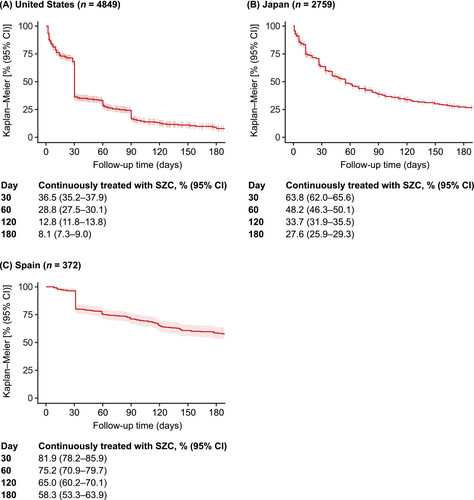
The duration of continuous SZC treatment was described using the Kaplan–Meier method. Discontinuation of SZC treatment was strictly defined as a gap in SZC supply of 7 days or more. In the United States, SZC stockpiling patterns were observed, and overlapping supply was therefore added to extend the SZC treatment durations accordingly. The discontinuation date itself was defined as the end of SZC supply. The proportions of patients [with corresponding 95% confidence intervals (CIs)] who were continuously treated with SZC were described at specific time points (30, 60, 120 and 180 days) after SZC treatment initiation.
Informed by prescribing patterns, discontinuation of RAASi therapy was defined as a gap in RAASi medication supply that exceeded 30 days in the United States and Spain and 21 days in Japan. The discontinuation date itself was defined as the end of supply. During hospitalization periods in the United States and Japan, patients were assumed to be supplied RAASi medication from the hospital rather than from their own supply.
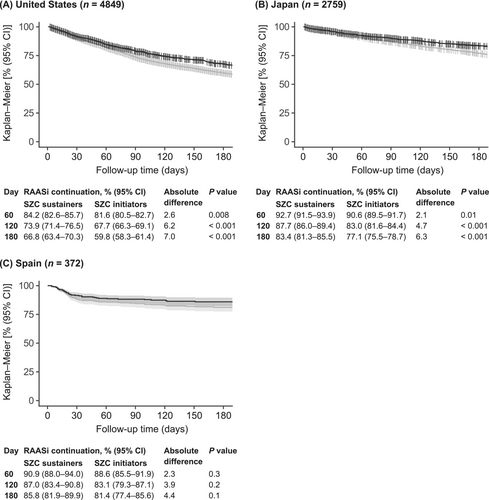
The association between SZC treatment duration and continuation of RAASi therapy was first analysed descriptively using a standard Kaplan–Meier method with versus without censoring at SZC discontinuation. RAASi continuation was thus described among all SZC initiators (without censoring, i.e., including both patients who remained on continuous SZC treatment and those who discontinued SZC treatment early), and among SZC sustainers (censoring at SZC discontinuation, i.e., including patients who remained on continuous SZC treatment). This approach therefore resembles an analysis of intention-to-treat (SZC initiators) versus per-protocol (SZC sustainers) and provides limited distinction between different SZC treatment durations. The proportions of patients (with corresponding 95% CIs) who continued RAASi therapy were described at Days 60, 120 and 180 after SZC treatment initiation among the SZC initiators and SZC sustainers. Differences in the proportions of patients who continued RAASi therapy between the SZC sustainers and SZC initiators were summarized with absolute differences; Z-tests were used to evaluate if the proportions differed statistically at specific time points (Days 60, 120 and 180).
To evaluate the likelihood of continued RAASi therapy by distinct SZC treatment duration strategies in routine clinical practice, without imposing immortal time bias, Hernán's randomized trial emulation methodology using cloning, censoring, and weighting (CCW) was applied.25-27 The CCW approach has been described in detail previously.25-27 The Supporting information S1 provides further details on the specific definitions applied in the current study. Six distinct SZC treatment durations were defined: 1–30, 31–60, 61–90, 91−120, 121–150 and 151–180 days.
To estimate the likelihood of continued RAASi therapy by the six distinct SZC treatment durations, a weighted Kaplan–Meier method with bootstrapping was used; Z-tests were used to evaluate whether the likelihood of continued RAASi therapy at specific time points (Days 60, 120 and 180) differed statistically between the distinct SZC duration strategies.
Follow-up time was censored at the end of available follow-up or at the end of the study period, death, initiation of dialysis, initiation of another K+ binder or re-initiation of SZC after discontinuation. Details of handling informative versus non-informative censoring are provided in the Supporting information S1.
Results
Patient characteristics
This study included 7980 patients who initiated outpatient treatment with SZC in the United States (n = 4849), Japan (n = 2759) and Spain (n = 372). In the United States, Japan and Spain, respectively, mean patient age was 73.1, 75.0 and 73.3 years; 53.2%, 66.4% and 64.5% of patients were male; 41.1%, 75.0% and 39.0% of patients had HF; and 95.3%, 76.9% and 91.9% of patients had CKD (Table 1). The most commonly used RAASi class at baseline was ARB, followed by ACEi in the United States and Spain; MRAs were used by about a fifth of patients.
| United States | Japan | Spain | |
|---|---|---|---|
| (n = 4849) | (n = 2759) | (n = 372) | |
| Age, years, mean (SD) | 73.1 (9.9) | 75.0 (11.6) | 73.3 (9.8) |
| Male, n (%) | 2581 (53.2) | 1831 (66.4) | 240 (64.5) |
| HK severity at index,a n (%) | |||
| Mildb | 265 (17.0) | 58 (15.9) | 75 (20.2) |
| Moderateb | 759 (48.8) | 145 (39.8) | 169 (45.4) |
| Severeb | 533 (34.2) | 161 (44.2) | 128 (34.4) |
| Missing | 3292 | 2395 | 0 |
| CKD, n (%) | 4622 (95.3) | 2122 (76.9) | 342 (91.9) |
| Stage 3b | 2094 (48.3) | 119 (15.0) | 168 (54.2) |
| Stage 4b | 1670 (38.5) | 262 (33.0) | 89 (28.7) |
| Stage 5b | 571 (13.2) | 413 (52.0) | 53 (17.1) |
| Unknown stage | 297 | 1328 | 30 |
| HF, n (%) | 1995 (41.1) | 2070 (75.0) | 145 (39.0) |
| Type 2 diabetes, n (%) | 3741 (77.2) | 1706 (61.8) | 193 (51.9) |
| RAASi, n (%) | |||
| ACEi | 2176 (44.9) | 366 (13.3) | 152 (40.9) |
| ARB | 2206 (45.5) | 2124 (77.0) | 254 (68.3) |
| ARNi | 324 (6.7) | 307 (11.1) | 11 (3.0) |
| MRA | 875 (18.0) | 617 (22.4) | 75 (20.2) |
- Abbreviations: ACEi, angiotensin-converting enzyme inhibitors; ARB, angiotensin receptor blockers; ARNi, angiotensin receptor-neprilysin inhibitors; CKD, chronic kidney disease; HF, heart failure; HK, hyperkalaemia; MRA, mineralocorticoid receptor antagonists; RAASi, renin–angiotensin–aldosterone system inhibitor; SD, standard deviation; SZC, sodium zirconium cyclosilicate.
- a HK severity is defined as follows, based on the highest potassium value recorded within 14 days prior to and including index date, when available: mild, >5.0 to <5.5; moderate, 5.5 to <6.0; and severe, ≥6.0 mmol/L.
- b Percentages were calculated using patients with non-missing/unknown data as the denominator.
Duration of continued SZC treatment
Across countries, SZC was frequently discontinued after Day 30 following treatment initiation (Figure 1). Between Days 30 and 120, the proportions of SZC initiators who remained on continuous SZC treatment decreased from 36.5% to 12.8% in the United States, from 63.8% to 33.7% in Japan and from 81.9% to 65.0% in Spain. At Day 180, 8.1%, 27.6% and 58.3% of patients in the United States, Japan and Spain, respectively, remained on continuous SZC treatment (Figure 1).
Continued RAASi therapy among SZC sustainers versus SZC initiators
According to the descriptive Kaplan–Meier methodology, providing limited distinction between different SZC treatment durations, the percentage of patients who continued RAASi therapy was higher among those who sustained SZC treatment versus among all SZC initiators (Figure 2), consistent across all countries. Absolute differences in the proportions of patients who continued RAASi therapy between the SZC sustainers and SZC initiators were statistically significant at 60, 120 and 180 days for the United States (P values ranging from P = 0.008 to P < 0.001) and Japan (P values ranging from P = 0.01 to P < 0.001), but not Spain (Figure 2). Furthermore, a trend of increasing absolute differences in the proportion with continued RAASi therapy between SZC sustainers and SZC initiators was generally observed, for example, between Days 60 and 180 from 2.6% to 7.0% in the United States, from 2.1% to 6.3% in Japan and from 2.3% to 4.4% in Spain.
Likelihood of continued RAASi therapy by distinct durations of SZC treatment
Figure 3 shows the weighted Kaplan–Meier curves of the likelihood of continued RAASi therapy up to 180 days for each of the six distinct SZC treatment durations. The P values from the Z-tests at specific time points are displayed in Table S1.
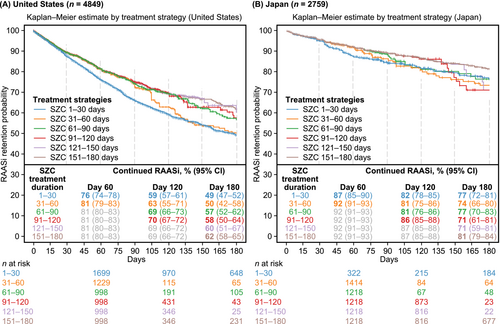
In the United States, the SZC treatment durations exceeding 60 days were consistently superior to the shortest SZC treatment duration (1–30 days) in continuing RAASi therapy during the full 180 days of follow-up (P values ranging from P = 0.02 to P < 0.001) (Figure 3A, Table S1). At 120 days, the likelihood of remaining on RAASi was 69%–70% for the longer treatment duration strategies (exceeding 60 days) compared with 59% for the shortest duration strategy (P < 0.001). The 31–60 day treatment strategy was superior to the 1–30 day strategy in continuing RAASi therapy for up to 90 days (P < 0.001), but not thereafter.
Similar patterns were observed in Japan. Relative to the United States, the probability of continued RAASi was consistently higher in Japan for all SZC treatment duration strategies at all time points, but there was limited statistical precision beyond Day 120 as few patients remained in follow-up in each treatment strategy (Figure 3B, Table S1). At 120 days, the likelihood of remaining on RAASi was 86%–87% for the SZC treatment durations exceeding 90 days compared with 82% for the shortest SZC treatment duration (1–30 days) (P < 0.05).
The Spanish dataset included a smaller initial sample size, and few patients had a relatively short SZC treatment duration; therefore, the CCW approach was not deemed feasible to apply with sufficient robustness.
Discussion
This multi-country, observational cohort study compared the likelihood of continued RAASi therapy following a hyperkalaemia episode according to duration of SZC treatment, in non-dialysis-dependent patients with HF and/or CKD. Overall, the findings show that a longer duration of continuous SZC treatment was associated with a higher likelihood of continued RAASi therapy.
Findings from clinical trials and routine clinical practice support that treatment with SZC increases the likelihood of maintaining RAASi therapy following a hyperkalaemia episode,19-21 in alignment with current international guidelines and a matching consensus from practicing clinicians.1, 2, 17, 18 Guidelines further advise that ongoing monitoring and management of hyperkalaemia are needed to prevent recurrent episodes.28 However, despite the continued risk of hyperkalaemia due to underlying disease or medication, K+ binders are often used only transiently in routine clinical practice, until the acute hyperkalaemia episode is resolved.10, 29 In the current analysis, SZC was frequently discontinued by Day 30 after treatment initiation; this pattern was consistently observed across all three countries. In a previous analysis, both recurrent hyperkalaemia and suboptimal RAASi dosing were observed upon cessation of K+ binders,30 emphasizing the risks of discontinuation of K+ binders and warranting exploration of the impact of longer durations of use. Therefore, the findings from the current analysis have clinical importance as they are the first to assess how the duration of continuous SZC treatment impacts the likelihood of continued RAASi therapy following a hyperkalaemia episode. The findings demonstrate that, compared with earlier discontinuation of SZC, continuing SZC treatment beyond 30 days following a hyperkalaemia episode is superior in continuing RAASi therapy for 3 months and continuing SZC treatment beyond 60 days is superior in continuing RAASi therapy for 6 months.
Two different methods were used to assess how SZC treatment duration associates with the continuation of RAASi therapy after a hyperkalaemia episode. One was a standard descriptive Kaplan–Meier analysis, which has limited ability to distinguish between different SZC treatment durations. Still, the findings showed that RAASi continuation was higher among those who remained on continuous SZC treatment (sustainers) than among the entire group of SZC initiators. The absolute differences in continued RAASi therapy between SZC sustainers and initiators increased over time and ranged from 4.4% to 7.0% across countries at Day 180. The absolute differences observed with this descriptive Kaplan–Meier approach are small, as the SZC initiators include both SZC sustainers and those who discontinued SZC treatment early. For context, in a previously published analysis from ZORA based on the same three countries, the absolute difference in maintaining RAASi at 6 months was 20% higher among patients treated with SZC for at least 120 days relative to no prescribed K+ binder treatment.21
To allow a distinction between different SZC treatment durations, without imposing immortal time bias, Hernán's randomized trial emulation methodology using CCW was applied. In the United States, patients who continued SZC treatment beyond 60 days had a significantly increased likelihood of continuing RAASi therapy for the full 6 months of follow-up, compared with those with SZC treatment durations of up to 30 days. The longitudinal patterns beyond 90 days further indicated that the likelihood of continued RAASi therapy increased with increasing duration of SZC treatment but did not consistently reach statistical significance, as low patient numbers in the longer SZC treatment strategies reduced the statistical precision for such comparison in the United States. Similar patterns were observed in Japan, although statistical precision beyond Day 120 was limited as few patients remained in follow-up. The dataset available in Spain included a considerably smaller initial sample size, and few patients were observed to have a relatively short treatment duration; therefore, the CCW approach was not deemed feasible to apply with sufficient robustness in the Spanish dataset.
The patterns observed in both the United States and Japan indicate that the beneficial effects on RAASi continuation become distinct when SZC use is continued beyond 30 days, and even more so beyond 60 days of treatment, and that the risk of RAASi discontinuation resumes once SZC treatment is discontinued. In the United States, discontinuation of RAASi therapy was generally observed to occur 1 or 2 months after SZC treatment discontinuation, rather than immediately afterwards. These couple of months could have encompassed the time needed for increased K+ values to be monitored at a subsequent healthcare visit following discontinuation of SZC treatment and the consequential decision to discontinue RAASi therapy, and subsequently being flagged as RAASi discontinuation in the analytical dataset. For the longest SZC treatment duration strategies, a similar effect may also have occurred beyond the end of the study period (i.e., at 6 months) and hence was not captured in these analyses. In Japan, RAASi discontinuation was observed more promptly following SZC discontinuation, which could indicate that concurrent prescribing of SZC treatment with RAASi therapy is more common. The two countries may differ further in terms of healthcare systems, such as practices for monitoring of K+, prescribing practices and frequencies and waiting times for physician appointments for follow-up visits.
A key strength of this study is the use of a purposeful statistical method that allows the analysis of how different treatment durations associate with the risk of an outcome in an observational study design setting, without imposing immortal time bias.25-27 Observational data can represent a valuable source to estimate causal effects among heterogeneous patient populations under routine care settings, but the lack of randomization may introduce various biases. While using observational data, the CCW approach used in this study emulates a randomized trial design in which patients are randomly assigned to one of several treatment duration strategies at the start of follow-up. This approach avoids the immortal time bias that would otherwise occur if patients had simply been categorized according to their observed treatment duration; only those who survive for long enough can be observed to have a long treatment duration. Because death is often preceded by disease and acute clinical events, this matters even when mortality is not the outcome of interest.
This analysis has limitations. First, discontinuation of RAASi therapy based on data on issued or dispensed drug prescriptions may have a lower specificity compared with a corresponding endpoint based on data collected prospectively in clinical trials. Although changes in RAASi dose (i.e., specifics of up- or down-titration) were not assessed in this particular analysis, a separate analysis of ZORA among patients with HF and/or CKD from the same countries as the present analysis (the United States, Japan and Spain), found that hyperkalaemia-related down-titration of RAASi dose occurred less frequently than RAASi discontinuation and was associated with a similar increased risk of cardiorenal events.21 Second, inherent to many observational studies, granular data on some of the baseline clinical characteristics, such as hyperkalaemia severity, CKD stage and HF subtype, were largely missing in the United States and Japan. Third, while data sources used in the United States and Spain are both based on closed systems, patients in the Japan dataset may have received prescriptions from hospitals or outpatient clinics outside the MDV system, which would not be captured in the data source. Based on local clinical experience, this may occur more frequently for RAASi therapies and may have contributed to dilute the patterns observed in Japan. In Japan especially, but also to some extent in the United States, the statistical precision declined over time as the number of patients remaining in follow-up decreased. This limitation applied to the dataset in Spain as well and precluded the application of CCW. Finally, the impact of treatment duration on RAASi discontinuation was only assessed for SZC, and so the findings may not be generalizable to other K+ binders.
Conclusions
Patients with longer SZC treatment experience sustained protection against discontinuation of RAASi therapy following a hyperkalaemia episode. The risk of RAASi discontinuation resumes once SZC treatment is discontinued. These findings have clinical importance as they demonstrate that patients with continued SZC treatment can continue to benefit from the cardio- and reno-protective effects of RAASi therapy after a hyperkalaemia episode, as recommended in current clinical guidelines for the management of HF and CKD.
Acknowledgements
Programming for the Spanish dataset was performed by Ignacio Hernández, Atrys Health. Medical writing support including assisting authors with development of the initial draft and incorporation of comments, fact checking, referencing and figure preparation was provided by Shaun W. Foley, BSc (Hons), and editorial support including formatting, proofreading and submission was provided by Jess Fawcett, BSc, both of Core (a division of Prime, London, UK), supported by AstraZeneca according to Good Publication Practice guidelines (Link). The sponsor was involved in the study design and collection, analysis and interpretation of data, as well as data checking of information provided in the manuscript. However, ultimate responsibility for opinions, conclusions and data interpretation lies with the authors.
Conflict of interest statement
C. V. P. has received consultancy fees from AstraZeneca. D. A. has received lecture fees and/or consultation fees from AstraZeneca, Bayer, Boehringer Ingelheim, CSL Vifor, Eli Lilly, GSK, Menarini, Otsuka and Viatris. E. K. reports no disclosures. I. J. S. L. is an employee of Hospital Universitari i Politècnic La Fe and has served on the speakers' bureaus of AstraZeneca and Novartis. E. L., S. F., C. M. G. and A. L. are employees and stockholders of AstraZeneca. T. M. has received honoraria for lectures from AstraZeneca. A. R. has received research grants and consulting fees from AstraZeneca.
Funding
This work was supported by AstraZeneca.




