Ferric carboxymaltose for the treatment of iron-deficient heart failure patients: a systematic review and meta-analysis
Abstract
Aims
Intravenous ferric carboxymaltose (FCM) has been shown to improve functional capacity and quality of life in iron deficient heart failure patients. However, FCM's effect on hospitalizations and mortality remains unclear as previous randomized controlled trials (RCTs) and their meta-analyses have been underpowered to detect significant differences. We sought to conduct an updated meta-analysis using recently published RCT data.
Methods and results
Online databases were searched from inception until November 2020 for RCTs evaluating the effects of FCM on clinical outcomes in iron-deficient heart failure patients. Outcomes of interest included heart failure hospitalizations, all-cause mortality, and cardiovascular mortality. Meta-analysis was performed using a fixed-effect model and estimates were reported as odds ratios (ORs), hazard ratios, or rate ratios (RRs) along with corresponding 95% confidence intervals (CIs). A total of 1947 patients (n = 1062 in the FCM group; n = 885 in the placebo group) were included. FCM, compared with placebo, significantly reduced the risk of the composite endpoint of time to first heart failure hospitalization or cardiovascular death (hazard ratio = 0.76; 95% CI = 0.63–0.90; I2 = 55%). FCM also significantly reduced the risk of recurrent heart failure hospitalizations (RR = 0.68; 95% CI = 0.54–0.85; I2 = 71%) and recurrent cardiovascular hospitalizations (RR = 0.71; 95% CI = 0.59–0.86; I2 = 56%). However, FCM had no significant effect on the risk of all-cause (OR = 0.97; 95% CI = 0.73–1.28; I2 = 0%) or cardiovascular mortality (OR = 0.93; 95% CI = 0.69–1.27; I2 = 0%).
Conclusions
Ferric carboxymaltose reduces heart failure hospitalizations and cardiovascular hospitalizations with no beneficial effect on all-cause and cardiovascular mortality in iron-deficient heart failure patients. These findings reinforce the role of FCM as a therapeutic option in heart failure patients.
Introduction
Iron deficiency is present in up to 50% of heart failure patients.1 In addition to impaired quality of life and functional capacity, higher rates of hospitalization and mortality are observed in heart failure patients with iron deficiency.1, 2 These findings remain consistent in patients with and without anaemia.2 Nanoparticulate iron–carbohydrate complexes of intravenous iron preparations are engineered to be taken up and processed by macrophages in order to quickly replenish depleted iron stores. Considering the important role of iron in metabolism of oxygen and cellular immune response, especially in cardiac myocytes, intravenous iron supplementation has emerged as a promising therapeutic target in heart failure patients, which may provide incremental benefit to beta blockade and renin–angiotensin–aldosterone system inhibition.3 Current guidelines give a Class IIA recommendation for the use of intravenous iron supplementation in these patients.4
Several randomized controlled trials (RCTs) have been published to evaluate the effect of intravenous ferric carboxymaltose (FCM) in iron deficient heart failure patients.5-8 While FCM has clearly been shown to improve the functional capacity and quality of life,9 the effect on hospitalizations and mortality has remained less clear.10 In the FAIR-HF study (Ferinject Assessment in Patients with Iron Deficiency and Chronic Heart Failure), no statistically significant benefit was observed with FCM on recurrent heart failure hospitalizations and all-cause or cardiovascular mortality.7 In the CONFIRM-HF study (Ferric CarboxymaltOse evaluatioN on perFormance in patients with IRon deficiency in coMbination with chronic Heart Failure), while a statistically significant benefit was seen in total heart failure hospitalizations, no reduction in all-cause or cardiovascular mortality was observed.8 However, these trials were underpowered to detect meaningful differences in hospitalizations and mortality.
The AFFIRM-AHF (A Randomised, Double-blind Placebo Controlled Trial Comparing the Effect of Intravenous Ferric Carboxymaltose on Hospitalisations and Mortality in Iron Deficient Subjects Admitted for Acute Heart Failure) was a recent large trial, which showed that FCM reduces the risk of first time and of recurrent heart failure hospitalizations with no clear benefit on cardiovascular death.11 In this systematic review and meta-analysis, we sought to pool the data from all randomized clinical trials comparing FCM with placebo in heart failure patients with iron deficiency to estimate the treatment effects on mortality and hospitalization, particularly including the very recently published data from AFFIRM-AHF.
Methods
Data sources and search strategy
This systematic review and meta-analysis is reported using the Preferred Reporting Items for Systematic Reviews and Meta-Analysis statement (PRISMA).12 The protocol for this study has been submitted to PROSPERO for registration (ID: 220248). Medline, Scopus, and Cochrane Central Register of Controlled Trials (CENTRAL) were searched from database inception through November 2020. To identify grey literature, online libraries www.clinicaltrialresults.org, www.clinicaltrials.gov, www.cardiosource.org, www.escardio.org, and abstracts and presentations from major cardiovascular proceedings were also searched. The sole manufacturer of this product, Vifor, was also asked and provided lists of all trials they were aware of that had evaluated IV FCM in heart failure. No restrictions were placed on time or language of the publication. A complete description of the search strategy used specific to each database is outlined in the Supporting information, Table S1. All citations retrieved from the search were transferred to EndNote X7.5 (Clarivate Analytics, Philadelphia, Pennsylvania) Reference Manager, and duplicates were removed.
Study selection
All the articles were screened by two independent investigators (MSK and MSU). Non-relevant articles were excluded on the basis of title and abstract. Full texts of references were then screened for inclusion based on predetermined criteria. Any differences were resolved by consensus. RCTs evaluating the effects of FCM on clinical outcomes in iron deficient heart failure patients were considered for inclusion. To avoid publication bias of small trials, we prospectively defined trials of interest to be double-blind RCTs of IV FCM or placebo with a minimum patient cohort of 100 patients and a minimum follow-up period of 24 weeks. These inclusion criteria were set based on author discussion and consensus. However, we also included data of FCM versus placebo from smaller unpublished trials in a secondary analysis. Data from these trials were incorporated by using an individual patient data meta-analysis published previously, which had reported results from these small trials in combination with FAIR-HF and CONFIRM-HF.13
Data extraction and outcomes of interest
Two authors (MSK and MSU) independently abstracted data from the selected studies. The data were abstracted on characteristics of the studies, crude point estimates, number of events, sample sizes, and follow-up durations. Outcomes of interest for this meta-analysis were time to first heart failure hospitalization, recurrent heart failure hospitalizations, number of patients hospitalized for heart failure, all-cause mortality, cardiovascular mortality, and time to cardiovascular mortality. The primary outcomes within each of the trials differed. Only AFFIRM-AHF had a clinical primary endpoint, that is, composite of recurrent heart failure hospitalizations and cardiovascular death.11 The primary outcomes of other trials were based on quality of life and functional capacity. The methodological quality of RCTs was assessed using the revised Cochrane risk-of-bias tool for randomized trials (RoB 2.0) across five domains (randomization, intended intervention, missing data, outcome measurement, and reported results).14
Statistical analyses
Odds ratios (ORs) were calculated for dichotomous outcomes. Hazard ratios (HRs) derived from Cox proportional hazard models were used for time-to-first-event endpoints, and rate ratios (RRs) were used for recurrent events. Heterogeneity was assessed using Higgins I2 statistic.15 Given the largely homogenous study designs and patient populations, we chose to use a fixed-effect model for the primary analysis.16 However, as there were some differences in enrolment, follow-up and dosing, sensitivity analysis using a random-effects model was also performed. Publication bias was not assessed as number of studies included were <10.17 Similarly, meta-regression or subgroup analyses could not be performed due to the limited number of the studies included. For this study, statistical significance was set at 5%. Review Manager (Version 5.3; Cochrane Collaboration, Oxford, UK) was used for all other analyses.
Results
Study characteristics
The literature search and study selection process are summarized in the PRISMA flow diagram (Figure 1). The search revealed a total of 6883 studies. After screening, five studies were included. FER-CARS-01 and EFFICACY-HF (NCT00821717) were RCTs evaluating the effect of FCM in heart failure patients; however, their results have not been published separately. These two studies together had a total of 50 patients on FCM and 29 on placebo with few events (three cardiovascular deaths, nine all cause deaths and seven heart failure hospitalizations). Results from these trials were included by using the individual patient data meta-analysis published by Anker et al. for the endpoints of composite of cardiovascular death and recurrent heart failure hospitalizations, and recurrent heart failure hospitalizations.13 The comparison of the trials and characteristics of the trial populations included in this meta-analysis are shown in Table 1. All studies enrolled patients with symptomatic HF with reduced LVEF only, and the cut-off for LVEF at inclusion varied from ≤35% to ≤50%. The follow-up time was 24 weeks in FAIR-HF and 52 weeks in CONFIRM-HF and AFFIRM-AHF. The risk of bias in FAIR-HF, CONFIRM-HF, and AFFIRM-HF was low, as determined by the Cochrane Risk of Bias tool (Figure S1). The risk of bias in FER-CARS-01 and EFFICACY-HF could not be determined.
| Variable | FER-CARS-01 | FAIR-HF | EFFICACY-HF | CONFIRM-HF | AFFIRM-HF |
|---|---|---|---|---|---|
| Randomization | 2:1 (FCM:placebo) | 2:1 (FCM:placebo) | 1:1 (FCM:placebo) | 1:1 (FCM:placebo) | 1:1 (FCM: placebo) |
| Number of patients (FCM/placebo) | 30/15 | 304/155 | 20/14 | 150/151 | 558/550 |
| Centres | Multi-centre | Multi-centre | Multi-centre | Multi-centre | Multi-centre |
| Study duration (weeks) | 12 | 24 | 24 | 52 | 52 |
| HF diagnosis and its severity | Ambulatory, optimally treated, systolic CHF with ID, NYHA Class II/III, eGFR < 60 mL/min per 1.73m2 | NYHA Class II/III ambulatory, optimally treated, systolic CHF with ID, NYHA Class II/III | Ambulatory, optimally treated, systolic CHF with ID, NYHA Class II/III | Ambulatory, optimally treated, systolic CHF with ID, NYHA Class II/III | Hospitalization for acute HF, treatment with intravenous furosemide at a dose of 40 mg, a LVEF <50% |
| Haemoglobin (g/dL) | 9.5–13.5 | 9.5–13.5 | <15 | <15 | <15 |
| Primary endpoint | PGA at Week 12 and NHYA Class from baseline to Week 12 | PGA at Week 24 and NYHA Class from baseline to Week 24 | Change in 6MWT and NYHA class | Change in 6MWT from baseline to Week 24 | Composite of recurrent events of HF hospitalization and cardiovascular death |
| Age (years) | NA | 68/67 | NA | 69/70 | 71/71 |
| Females (%) | NA | 52/55 | NA | 45/49 | 44/45 |
| NT-proBNP (pg/mL) | NA | NA/NA | NA | 2511/2600 | 4743/4684 |
| LVEF (%) | NA | 32/33 | NA | 37/37 | 33/33 |
| NYHA class | NA | 2.8/2.8 | NA | 2.5/2.4 | 2.5/2.6 |
| Ischemic HF | NA | 81/79 | NA | 83/83 | 47/47 |
| Ferritin (ug/L) | NA | 53/60 | NA | 57/57 | 84/89 |
| TSAT | NA | 18/17 | NA | 20/18 | 15/14 |
| Haemoglobin (g/dL) | NA | 11.9/11.9 | NA | 12.4/12.4 | 12.3/12.1 |
| Anaemia (%) | NA | 65/61 | NA | 53/48 | 53/57 |
- 6MWT, 6 min walk test; CHF, chronic heart failure; eGFR, estimated glomerular filtration rate; FCM, ferric carboxymaltose; HF, heart failure; ID, iron deficiency; LVEF, left ventricular ejection fraction; NA, not available; NT-proBNP, NT-proB-type natriuretic peptide; NYHA, New York Heart Association; PGA, physician global assessment; TSAT, transferrin saturation.
Analysis based on AFFIRM-HF, FAIR-HF, and CONFIRM-HF published data
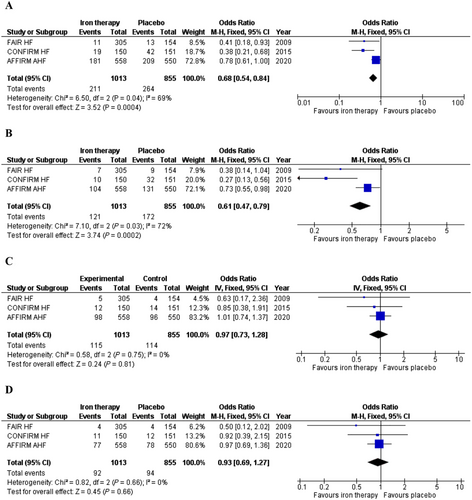
A total of 1868 patients were enrolled in the above three trials. The number of patients experiencing the composite endpoint of cardiovascular death or heart failure hospitalization was significantly lower in patients with FCM treatment (OR = 0.68; 95% CI = 0.54–0.84; I2 = 69%) (Figure 2). Similarly, the number of patients with heart failure hospitalizations during follow-up was significantly lower in the FCM arms (OR = 0.61; 95% CI = 0.47–0.79; I2 = 72%) (Figure 2). FCM did not significantly reduce the risk of all-cause (OR = 0.97; 95% CI = 0.73–1.28; I2 = 0%) or cardiovascular mortality (OR = 0.93; 95% CI = 0.69–1.27 l I2 = 0%) in iron deficient heart failure patients (Figures 2). All of these results remained consistent on sensitivity analyses based on random-effects model (Table S2).
Analysis based on AFFIRM-HF and previous individual patient data meta-analysis
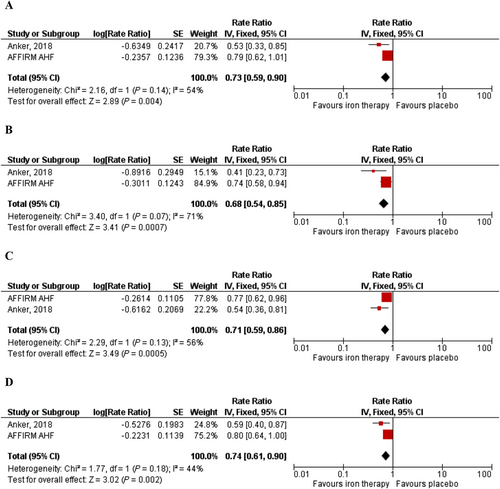
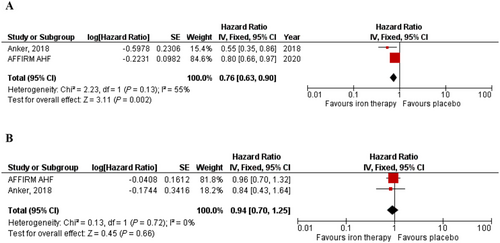
A total of 1947 patients were included in this analysis. FCM compared with placebo significantly reduced the composite endpoint of recurrent heart failure hospitalization and cardiovascular death (RR = 0.73; 95% CI = 0.59–0.90; I2 = 54%) (Figure 3). FCM compared with placebo also significantly reduced the risk of recurrent heart failure hospitalizations (RR = 0.68; 95% CI = 0.54–0.85; I2 = 71%) (Figure 3) and recurrent cardiovascular hospitalizations (RR = 0.71; 95% CI = 0.59–0.86; I2 = 56%) (Figure 3). FCM was also shown to reduce the composite endpoint of recurrent cardiovascular hospitalizations and cardiovascular mortality (RR = 0.74; 95% CI = 0.61–0.90; I2 = 44%) (Figure 3). While FCM was found to have a statistically significant benefit on time to first HF hospitalization or cardiovascular death (HR = 0.76; 95% CI = 0.63–0.90; I2 = 55%) (Figure 4), no significant benefit was seen on time to cardiovascular death (HR = 0.94; 95% CI = 0.70–1.25; I2 = 0%) (Figure 4).
On sensitivity analysis using a random-effects model (Table S2), the treatment benefit of FCM on the composite endpoint of recurrent heart failure hospitalization and cardiovascular death (RR = 0.68; 95% CI = 0.47–1.00; I2 = 54%), and time to first HF hospitalization or cardiovascular death (RR = 0.70; 95% CI = 0.50–1.00; I2 = 55%) became borderline significant. The benefit seen in recurrent heart failure hospitalization (RR = 0.59; 95% CI = 0.33–1.03; I2 = 71%) became non-significant upon using a random-effects model. The remaining results did not significantly change with the use of a random-effects model (Table S2).
Discussion
We report several key findings in this meta-analysis (Figure S2): first, FCM compared with placebo significantly reduced the composite endpoint of recurrent heart failure hospitalization and cardiovascular death in iron deficient heart failure patients. These results were mainly driven by reduction in recurrent heart failure hospitalizations. Second, while FCM strongly reduced the risk of recurrent heart failure hospitalizations, no beneficial effect on all-cause or cardiovascular mortality was observed in iron deficient heart failure patients.
Previous meta-analyses have also shown that FCM does not reduce all cause or cardiovascular mortality in iron deficient heart failure patients.6, 13 However, these meta-analyses were significantly underpowered due to low number of events (n = 34 for cardiovascular mortality and n = 38 for all-cause mortality) and sample sizes (n = 839) to detect any meaningful differences between the groups. This meta-analysis adds the results from the AFFIRM-AHF trial and hence approximately doubles the sample size and increases the number of events 10-fold. Our data confirm that FCM has no effect on all-cause and cardiovascular mortality.
Our results reinforce that FCM compared with placebo has a strong beneficial impact on total heart failure hospitalizations. Previous studies have also shown similar findings where FCM reduces the risk of heart failure hospitalization regardless of the presence of anaemia.13 Heart failure is one of the most common causes of hospitalizations worldwide and represents a growing public health challenge despite the increasing number of the drugs, which have shown to improve heart failure hospitalization risk.18, 19 These drugs are often not tolerated by patients due to borderline blood pressure and hyperkalaemia and are often not prescribed simultaneously.20-22 Moreover, even in heart failure patients who are receiving guideline directed medical therapy with target doses, readmission rates remain high suggesting that other mechanisms should also be considered in improving overall clinical status and prognosis of heart failure patients.23, 24
Iron deficiency can predispose to worsening of heart failure owing to mitochondrial dysfunction and abnormal cellular energy metabolism.1 Moreover, depleted iron stores can cause imbalance in erythropoiesis and metabolism of oxygen.3 While multiple studies have shown that FCM improves physical performance and quality of life in heart failure patients by targeting these abnormalities, our data signify that FCM also confers hospitalization benefits in addition to improving functional status, while being cost-effective at the same time.25, 26 In the absence of required trial data, it appears that other intravenous iron products may not confer the same benefit in patients with heart failure.27 These benefits persist even in patients without anaemia suggesting that reduced haemoglobin level is merely an end result of iron depletion and the dysfunction ensues soon after iron deficiency.
This study has several limitations, which should be considered. First, the inclusion criterion varied slightly among the trials. AFFIRM-AHF enrolled patients who were initially hospitalized with acute heart failure, while other trials enrolled chronic ambulatory heart failure patients.11 This could have led to some heterogeneity. Second, given the size of the AFFIRM-HF study the results of this meta-analysis were mainly driven by this study. Current ongoing large trials [FAIR-HF2 (NCT03036462), HEART-FID (NCT03037931) with FCM, and IRONMAN (NCT02642562) with iron isomaltoside] will confirm the impact of FCM on clinical outcomes in heart failure patients. Third, the optimal dosing of FCM in heart failure patients could not be determined in this analysis owing to lack of availability of data. Without head-to-head clinical studies proving the therapeutic equivalence of other intravenous iron products with FCM in the highly vulnerable population of heart failure patients, the extrapolation of results and substitution with a different intravenous iron product is not recommended.27
In conclusion, FCM strongly reduces total heart failure hospitalizations with no beneficial effect on all-cause and cardiovascular mortality in iron deficient heart failure patients. These findings reinforce the role of FCM as a therapeutic option in patients with heart failure and iron deficiency, which confers hospitalization benefits in addition to improving functional status as well.
Declaration of Interest
MSK has no disclosures to report. MSU has no disclosures to report. SVH has consulted for Vifor Pharma, Roche, Bayer, and Brahms and has received speaker fees from Boehringer Ingelheim, Novartis, Chugai Pharma, and Amgen. WD has no disclosures to report. AC reports honoraria and/or lecture fees from Astra Zeneca, Bayer, Menarini, Novartis, Nutricia, Servier, Vifor, Actimed, Cardiac Dimensions, CVRx, Enopace, Faraday, Gore, Impulse Dynamics, Respicardia, Stealth Peptides, V-Wave, Corvia, Arena, and ESN Cleer.
Conflict of interest
WD reports speaker fees and advisory honoraria from Aimediq, Bayer, Boehringer Ingelheim, Lilly, Medtronic, Pfizer, Sanofi-Aventis, Sphingotec, Vifor Pharma. WD reports research support from EU (Horizon2020), German ministry of Education and Research, German Center for Cardiovascular Research, Vifor Pharma, ZS Pharma.




