Pulmonary artery pressures and outcomes after MitraClip
Yonatan Rashi and Dan Haberman contributed equally to this work.
Abstract
Aims
We evaluated the impact of MitraClip on systolic pulmonary artery pressure (sPAP) and the effects of baseline sPAP on outcomes.
Methods and results
In a cohort of patients who underwent MitraClip implantation, three groups were defined according to pre-procedure sPAP levels. Clinical and echocardiographic data were compared. The study included 177 patients: 59 had severe pulmonary hypertension (PHT), 96 had mild to moderate PHT, and 22 had no PHT. In patients with pre-existing severe PHT, sPAP was reduced from 70.8 ± 9.2 to 56.8 ± 13.7 mmHg (P < 0.001), sPAP remained unchanged in patients with mild to moderate PHT but was significantly increased from 30.8 ± 4.3 to 38.6 ± 8.3 mmHg in the no-PHT group (P < 0.001). Improvement of sPAP was observed in 77% of severe PHT group, while worsening of sPAP was more common among patients with no-PHT [57% compared with 33% among the mild to moderate PHT and 7% in the severe PHT group, respectively, (P < 0.001)]. One year survival was similar among the study groups.
Conclusions
MitraClip decreases PHT among patients with severe PHT. A concerning finding is that most patients with no-PHT increase their sPAP.
Introduction
Mitral regurgitation (MR) is the most common valve disease and the second-most common indication for valve surgery in the United States and Europe.1, 2 Pulmonary hypertension (PHT) is a common finding among patients with MR and has been associated with increased mortality after mitral valve (MV) surgical intervention.3, 4 The percutaneous edge-to-edge MV repair with MitraClip (Abbott, Menlo Park, CA, USA) is a well-established treatment for high surgical risk MR patients with more than 100 000 procedures performed. However, the impact of MitraClip on systolic pulmonary artery pressure (sPAP) and the effects of baseline sPAP on MitraClip outcomes are unclear.
The haemodynamic effects of MitraClip are complex. Repair of MR may decrease the post capillary pulmonary pressure but might create relative mitral stenosis.5 Trans-mitral increased gradient and the haemodynamic effects of the iatrogenic atrial septal defect (iASD) may affect post-procedural sPAP, in relation with pre-procedure values.
The German Transcatheter Mitral Valve Intervention (TRAMI) registry showed that MitraClip decreased the sPAP among patients with severe PHT, while among patients with no PHT, there was no change in sPAP.6 This lack of improvement in sPAP after MV intervention was also shown in patients who underwent MV surgery.7 Although MitraClip might be beneficial in reducing sPAP among patients with severe PHT, their long-term survival remained low6, 8 and in fact, in the recent Cardiovascular Outcomes Assessment of the MitraClip Percutaneous Therapy for Heart Failure Patients With Functional Mitral Regurgitation (COAPT) trial, patients with sPAP > 70 mmHg were excluded.9 Furthermore, there is even disagreement regarding the definition of the severity of PHT, several studies defined severe sPAP as greater than 50 mmHg,6, 8 while others defined it as greater than 60 mmHg.7 Only few studies have examined the interrelations between PHT and MitraClip performance. The purpose of our study is to evaluate the impact of MitraClip in patients with pre-existing severe, mild to moderate, and no PHT.
Methods
Study design
An observational, retrospective cohort study was performed.
Study population
We studied a cohort of consecutive patients who underwent MitraClip implantation between September 2014 and September 2019, in two Israeli medical centres: Hadassah University Medical Center in Jerusalem and Kaplan Medical Center in Rehovot. Patients were followed for at least 6 months until 30 March 2020. We excluded from the analysis four patients who underwent combined MV and tricuspid valve (TV) clipping, seven patients who died within a week of the MitraClip implantation, 10 patient with unmeasurable TV pressure gradient or missing data. Six patients who underwent redo MitraClip procedure were considered as separate cases. A multidisciplinary heart team of non-interventional cardiologists, interventional cardiologists, cardiothoracic surgeons, and anaesthesiologists has assessed the patients, and all were deemed as high risk for surgery. The study protocol was approved by the institutional ethical committee, and all patients received thorough verbal and written explanations about MitraClip implantation procedure and signed informed consent.
Echocardiographic assessment
Transthoracic echocardiography and transesophageal echocardiography were performed to evaluate severity of MR and to assess suitability for MitraClip implantation. MR severity was initially assessed by integrated multi-parametric visual evaluation tool in accordance with standard clinical practice (incorporating 2D, spectral and colour Doppler images), using an ordinal scale (grading 0—no MR, 1 + mild MR, 2 + moderate MR, 3 + moderate to severe MR, and 4 + severe MR).10, 11
Systolic pulmonary artery pressure was estimated by calculating the amount of maximal tricuspid pressure gradient using continuous-wave Doppler and the estimated right atrial pressure based on inferior vena cava size and changes during inspiration. Three groups were defined according to pre-procedure sPAP levels: no PHT (sPAP ≤ 35 mmHg), mild to moderate PHT (35 < sPAP < 60 mmHg), and severe PHT (sPAP of ≥60 mmHg). In addition, we collected echocardiographic data of left ventricle (LV) end diastolic and systolic diameter, LV ejection fraction, mean MV gradient, tricuspid regurgitation (TR) severity grade, and LV and right ventricle (RV) dysfunction grades. All echocardiographic data were collected at three stages: before the procedure, before discharge from the hospital, and at the first follow-up visit at 6 month.
We evaluated baseline characteristics, clinical and procedural data including complications and New York Heart Association (NYHA) class at 6 months, and 1 year after the procedure. Procedural success was defined as a residual MR grade of 2+ or less after clip implantation in accordance with MVARC definition.12
After up to 6 months, and then every 6 months to a year, patients were invited for follow-up at the clinic that including general questioner, physical examination, and transthoracic echocardiography.
Invasive measurements
Pressure reading from the left atrium was measured before the procedure using the trans-septal Sheath, during the procedure using the MitraClip guide after clip delivery system removal.
Statistical analysis
Most of the analysis was performed by comparing the comparing three groups of baseline PHT severity. Age, sPAP, MV gradient, and other continuous parameters were expressed as mean ± standard deviation and compared with one-way ANOVA test in case of three groups and paired t-test in case of comparing two groups.
Sex, clinical background disease, NYHA class, PHT severity grade, and other ordinal or nominal parameters were expressed as percentage and compared with χ2 square test for nominal parameters and McNemar test for ordinal parameters. Kaplan–Meier method was used for survival analysis and the log-rank test to compare the groups. The SPSS software (Version 25) was used for analysis, and P value < 0.05 was considered to be significant.
Results
The study included 177 patients that underwent MitraClip procedure between September 2014 and September 2019 (Table 1).
| Variable | No PHT sPAP < 36 (n = 22) | Mild or moderate PHT 36 ≤ sPAP < 60 (n = 96) | Severe PHT sPAP ≥ 60 (n = 59) | P value |
|---|---|---|---|---|
| Age | 73.8 ± 10.7 | 73.5 ± 10.4 | 76.2 ± 9.4 | 0.270 |
| Gender (Male) | 46% | 57% | 64% | 0.291 |
| BMI | 26.1 ± 4.9 | 28.1 ± 5.6 | 26.5 ± 5 | 0.101 |
| Euroscore2 (%) | 8.4 ± 6.8 | 11.3 ± 11.3 | 8.4 ± 8.3 | 0.156 |
| Functional MR | 67% | 78% | 60% | 0.069 |
| NYHA class III/IV | 91% | 99% | 100% | 0.014 |
| CAD | 46% | 70% | 61% | 0.086 |
| Post MI | 39% | 62% | 43% | 0.062 |
| Post PCI | 33% | 57% | 46% | 0.162 |
| Pacemaker | 50% | 34% | 36% | 0.381 |
| S/P CABG | 27% | 31% | 19% | 0.248 |
| AF/flutter | 27% | 43% | 58% | 0.034 |
| Diabetes | 23% | 46% | 36% | 0.091 |
| Post CVA/TIA | 14% | 18% | 19% | 0.868 |
| oncological disease | 23% | 20% | 19% | 0.917 |
| HTN | 82% | 91% | 83% | 0.293 |
| Hyperlipidaemia | 86% | 81% | 72% | 0.283 |
| Smoking | 44% | 42% | 42% | 0.991 |
| CKD IV | 65% | 53% | 63% | 0.479 |
| COPD | 9% | 18% | 10% | 0.313 |
| OSA | 6% | 14% | 12% | 0.582 |
| Asthma | 6% | 3% | 2% | 0.795 |
| Haemoglobin (g/dL) | 11.3 ± 1.3 | 11.9 ± 1.9 | 11.7 ± 1.6 | 0.316 |
| Creatinine (mmol/L) | 155 ± 119 | 142 ± 113 | 140 ± 81 | 0.849 |
| GFR | 53 ± 32 | 57 ± 28 | 54 ± 38 | 0.788 |
| Albumin (g/L) | 38.6 ± 7.1 | 37.1 ± 4.6 | 36.8 ± 4.8 | 0.521 |
| Antiplatelets | 53% | 50% | 48% | 0.930 |
| ACEi or ARBs | 50% | 79% | 71% | 0.022 |
| Anticoagulants | 28% | 49% | 65% | 0.025 |
| Beta blockers | 82% | 89% | 88% | 0.681 |
| Digoxin | 9% | 11% | 12% | 0.919 |
- ACEi, angiotensin converting enzyme inhibitor; AF, Atrial fibrillation; ARB, angiotensin receptor blocker; BMI, body mass index; CABG, coronary artery bypass graft; CAD, coronary artery disease; CKD, chronic kidney disease; COPD, chronic obstructive pulmonary disease; CVA, cerebrovascular accident; GFR, glomerular filtration rate; HTN, hypertension; LV, left ventricle; LVEF, left ventricle ejection fraction; MI, myocardial infarction; OSA, obstructive sleep apnoea; PCI, percutaneous coronary intervention; PHT, pulmonary hyper tension; RV, right ventricle; TIA, transient ischemic attack; TR, tricuspid regurgitation; Significant P Value (P < 0.05) in bold.
Ninety-four per cent were an elective procedure, and 6% were an urgent MitraClip procedure. Mean age was 74.8 ± 10 years old, 58% of patients were men, and most patients had functional MR and were in NYHA class of III or IV. The median Euroscore2 was 6.4% with interquartile range of 4% to 11.8%. Fifty-seven patients were implanted with single clip, 108 with 2 clips and 22 with three clips. One hundred and seventy patients were implanted with either NT or NTR, and 17 were implanted with XTR.
Improvement in MR severity to grade +2 or less was achieved in 88.5% of patients. Five patients had peri-procedural complications resulted including tamponade, hemothorax, right atrial dissection, leaflet tear, and haemodynamic collapse. Stroke or clip embolization was not observed. Improvement in NYHA class was shown in 84% of patients, and 40% of them improved their NYHA class by at least 2 degrees 6 month follow-up.
The cohort was divided into three groups according to baseline sPAP values, 59 patients (33.3%) were classified as severe PHT group, 96 patients (54.2%) as mild or moderate PHT group, and 22 patients (12.5%) as no-PHT group. Most baseline characteristics were similar between the three groups apart of atrial fibrillation, which was more common among patients with severe PHT (Table 1). Baseline left atrial V-wave was not significantly different among PHT groups. The baseline echocardiographic findings and invasive measurements are presented in Table 2.
| Variable | No PHT | Mild to moderate PHT | Severe PHT | |
|---|---|---|---|---|
| sPAP < 36 | 36 ≤ sPAP < 60 | sPAP ≥ 60 | ||
| (n = 59) | (n = 96) | (n = 22) | P value | |
| LVEF (%) | 49 ± 17 | 39 ± 15 | 44 ± 18 | 0.119 |
| MR severity grade | 0.886 | |||
| Moderate (+2) | 4.5% | 7.1% | 3.8% | |
| Mod-severe (+3) | 18.2% | 21.2% | 17.3% | |
| Severe (+4) | 77.3% | 71.8% | 78.8% | |
| TR severity grade | 0.101 | |||
| Non-mild | 36.4% | 23.2% | 10.5% | |
| Moderate | 40.9% | 46.2% | 42.1% | |
| Severe | 22.7% | 30.8% | 47.4% | |
| RV dysfunction | 0.147 | |||
| Non | 63.2% | 69.8% | 53.7% | |
| Mild | 10.5% | 11.6% | 25.9% | |
| Moderate | 15.8% | 11.6% | 18.5% | |
| Severe | 10.5% | 7% | 1.9% | |
| LV dysfunction | 0.045 | |||
| Non | 33.3% | 23.3% | 48.1% | |
| Mild | 19% | 12.2% | 11.1% | |
| Moderate | 4.8% | 20% | 9.3% | |
| Severe | 42.9% | 44.4% | 31.5% | |
| V-wave (mmHg) | 30.1 ± 11.4 | 33.8 ± 14.1 | 33.5 ± 12.4 | 0.719 |
- LVEF, left ventricle ejection fraction; PHT, pulmonary hyper tension; RV, right ventricle; sPAP, systolic pulmonary artery pressure; TIA, transient ischemic attack; TR, tricuspid regurgitation.
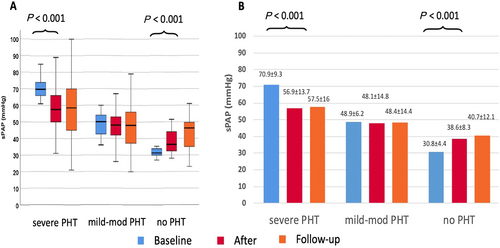
The average post procedure sPAP of the entire cohort was decreased from 54.1 to 49.9 mmHg (P < 0.001), an 8% decrease from baseline. The percentage of patients who were classified as severe PHT was reduced from 34% to 20% post-procedure (P < 0.001) (Figure 1).

Changes of post-procedural sPAP were correlated with baseline pulmonary pressures. In patients with pre-existing severe PHT, sPAP was reduced from 70.9 ± 9.3 mmHg to 56.9 ± 13.7 mmHg (P < 0.001), while in patients with mild or moderate PHT, the average sPAP (48.9 ± 6.2 mmHg) remained unchanged. In patients without pre-procedure PHT, there was a significant increase in sPAP from 30.8 ± 4.4 mmHg to 38.6 ± 8.3 mmHg (P < 0.001). In all groups, the initial trends in pulmonary blood pressure were remained unchanged during the follow-up period (Figure 2).
There was a significant V-wave reduction before and after the procedure (33.8 to 18.1 mmHg, P = 0.001) in the entire cohort. Higher V-wave at baseline was correlated with more prominent decrease in V-wave, R2 = 0.701.
The change in V-wave was least prominent in the No PHT group, compared with the moderate and severe PHT groups. This change, however, was not statistically significant (8.8, 15.8, and 16.9 mmHg, respectively, P = 0.215).
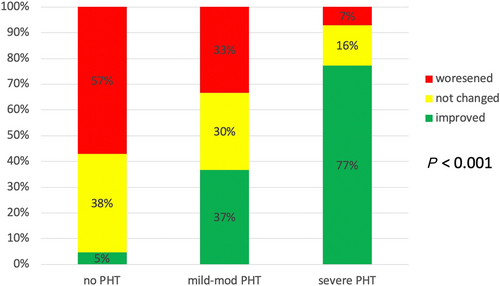
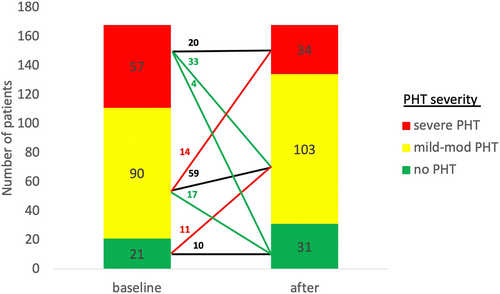
Significant changes in sPAP (defined as an increase/decrease of at least 5 mmHg compared with baseline pressures) occurred in most of patients and were related to baseline sPAP. Improvement of sPAP was observed in 77% of the severe PHT group, compared with 37% and 5% among the mild to moderate PHT and no PHT groups, respectively (P < 0.001). Worsening of sPAP was more common among patients with no PHT (57% compared with 33% among the mild to moderate PHT and 7% in the severe PHT group, respectively (P < 0.001) (Figure 3). The number of patients in each PHT severity group, pre-procedure, and post-procedure severity group is presented in Figure 4. Improvement in NYHA class was also associated with severity of baseline PHT, occurring in 92.5% of patients with severe PHT compared with only 80% in patients with non-severe PHT (P = 0.04).
To further explore the potential factors that might be associated with the increase in sPAP among the no PHT group, we evaluated the post-procedure mitral valve gradients (MVGs). The average MVG of the entire cohort was 4.1 ± 2.1 mmHg, and 31 patients (19%) had a MVG ≥ 6 mmHg. MVG ≥ 6 mmHg was significantly more common among no PHT group, 38% compared with 16% of the rest of the cohort, (P = 0.032) and was associated with less improvement in NYHA class. Only 72% of patients with MVG ≥ 6 mmHg had improvement in their NYHA class compared with 87% among the rest of the cohort (P = 0.048). LV, and RV functions, and the severity of TR were similar between the study groups and were not significantly changed after MitraClip (Table 3).
| No PHT | Mild to moderate PHT | Severe PHT | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Variable | Pre | Post | P | Pre | Post | P | Pre | Post | P | P value total |
| LV EF (%) | 49 ± 13 | 52 ± 16 | 0.35 | 38 ± 3 | 38 ± 3 | 0.86 | 46 ± 19 | 48 ± 18 | 0.16 | 0.31 |
| MR severity grade | <0.01 | <0.01 | <0.01 | <0.01 | ||||||
| Non-moderate | 5% | 86% | 7% | 7% | 2% | 88% | ||||
| Mod-severe | 18% | 5% | 21% | 21% | 18% | 5% | ||||
| Severe | 77% | 9% | 72% | 72% | 80% | 7% | ||||
| TR severity grade | 0.189 | 0.962 | 0.307 | 0.885 | ||||||
| Non-mild | 29% | 24% | 14% | 14% | 10% | 10% | ||||
| Moderate | 47% | 35% | 48% | 48% | 38% | 48% | ||||
| Severe | 24% | 41% | 38% | 38% | 52% | 42% | ||||
| RV dysfunction | 0.392 | 0.484 | 0.149 | 0.380 | ||||||
| Non | 50% | 64% | 66% | 66% | 50% | 70% | ||||
| Mild | 14% | 7% | 7% | 7% | 28% | 11% | ||||
| Moderate | 22% | 22% | 16% | 16% | 19% | 11% | ||||
| Severe | 14% | 7% | 11% | 11% | 3% | 8% | ||||
| LV dysfunction | 0.172 | 0.65 | 0.615 | 0.448 | ||||||
| Non | 37% | 56% | 24% | 24% | 56% | 56% | ||||
| Mild | 19% | 0% | 10% | 10% | 15% | 9% | ||||
| Moderate | 0% | 6% | 20% | 20% | 12% | 15% | ||||
| Severe | 44% | 38% | 46% | 46% | 17% | 20% | ||||
| NYHA | Pre | FU | <0.01 | Pre | Pre | <0.01 | Pre | FU | <0.01 | 0.045* |
| I | 0% | 15% | 0% | 0% | 0% | 8% | ||||
| II | 9% | 60% | 1% | 1% | 0% | 75% | ||||
| III | 55% | 20% | 56% | 56% | 47% | 17% | ||||
| IV | 36% | 5% | 43% | 43% | 53% | 0% | ||||
- LVEF, left ventricle ejection fraction; NYHA, New York Heart Association class; PHT, pulmonary hyper tension; RV, right ventricle; TIA, transient ischemic attack; TR, tricuspid regurgitation.
- P value calculated by McNemar test.
- * Non-PHT compared with both PHT groups.
To further evaluate the impact of post-procedural residual MR and baseline severe TR on pulmonary pressures, we repeated the analysis after excluding 22 patients with residual MR (>2) at the end of the procedure, and similar results were observed. The low rate of improvement in sPAP in the low PHT group was consistent. After excluding patient with severe TR (62 patients, 33%), there was no significant change in the results, and the impact of baseline PHT on outcomes remained unchanged.
Fifty-five per cent of patients had same degree of ASD at 6 month follow-up. The presence of ASD was similar in the three baseline PHT groups. sPAP was not statistically different between patient with or without ASD at 6 month follow-up.
One year survival was similar among the study groups (88%, 86%, and 83% in the no PHT, mild to moderate PHT, and severe groups, respectively, P = 0.33) in Kaplan–Meier analysis (Figure 5).
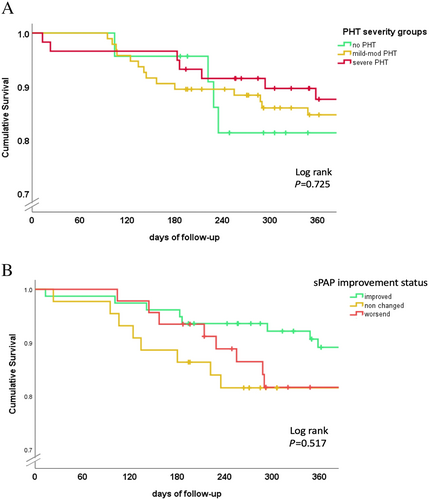
We also analysed outcomes according alteration in sPAP after the MitraClip. Patients who worsened their sPAP were less likely to improve their NYHA class (66% compared with 90% in the rest on the cohort, P = 0.003). There was no correlation between survival and post-procedure sPAP trends—decreased, not changed or increased (1 year survival 83%, 82%, and 88%, respectively; P = 0.71) (Figure 5).
When comparing no PHT with both PHT groups, NYHA class improvement was less significant in the no PHT group, P = 0.045 (Table 3).
Discussion
The study analysed the effect of MitraClip procedure on sPAP and outcomes in relation to pre-existing sPAP. We showed an improvement of sPAP among the entire cohort and a decrease in the number of patients with severe PHT (sPAP ≥ 60 mmHg) from more than one-third of the cohort at baseline to only one-fifth following MitraClip. Changes of post-procedural sPAP were directly related to baseline sPAP. Greater improvement in PHT was observed in patients who had severe pre-procedure PHT. In patients with mild to moderate PHT, sPAP remained unchanged. Although patients with no PHT at baseline worsened their sPAP following the procedure, this was not associated with effects upon survival. These results remained unchanged after excluding patients with significant post-procedural residual MR and patients with severe TR.
Pulmonary hypertension is common among patients with MV disease due to backwards transmission of elevated left atrial pressure. At early stages, pulmonary vascular resistance is normal (isolated post-capillary PHT), chronic left atrial pressure elevation might promote pulmonary vascular remodelling, which will develop to pulmonary vascular component of PHT. Our findings suggest that although the clinical baseline characteristics of study groups were similar, the reduction in PHT was observed only in patients with severe PHT. The explanation for these findings is not clear given the various determinants of PHT in patients with chronic MR who underwent MitraClip procedure. It is reasonable to assume that MR reduction improve the post-capillary component; therefore, patients with severe PHT at baseline (which have high post capillary pressure) decrease their sPAP and consequently improve NYHA class. Similar trend was shown in the TRAMI registry, a significant reduction of 10 mmHg in sPAP values in patients with severe PHT at baseline.
On the other hand, procedural factors like iASD and elevated MVG following clip implantation can promote an increase in pulmonary pressure.13-15 Post-MitraClip iASD often promotes immediate volume and pressure relief of the left atrium and marked decrease in pulmonary capillary wedge pressure.16 However, right to left shunt is also associated with RV dilatation and PHT17 as well as worse clinical outcomes and increased long-term mortality among patients after MitraClip. Elevated MV pressure gradient after MitraClip implantation might also impact outcomes and indeed Neuss et al.18 showed that the increased residual MVG > 5 mmHg was a significant outcome predictor. We showed that patients with no PHT at baseline had a significant increase of 8 mmHg in sPAP after MitraClip. It is possible that this concerning finding was caused by the haemodynamic effects of residual shunt, and increased trans-mitral gradient were translated to an increase in PHT. Indeed, our findings suggest that patients with no PHT are more likely to have ≥6 mmHg post-procedural MVG. The increase in MVG was associated with decrease improvement in NYHA class.
There was no significant difference in TV gradient or RV function between the three baseline PHT groups, which could have explained this increase in sPAP.
This kind of trend, baseline sPAP pressures below 36 mmHg population resulted in higher sPAP post-procedure, was also demonstrated in TRAMI registry but was not further elaborated.6
The increase in PHT as well as the relative modest improvement in NYHA class should be considered in the selection of patients for MitraClip. In addition, the quality of the implantation should be analysed carefully, and repositioning of the clip may be considered in the case of an elevated pressure gradient over the MV particularly in this population.
While other studies have shown lower survival in patients with pre-procedural severe PHT,8, 19 their survival was similar in our study. To further evaluate this issue, we performed a log-rank analysis for patients who had improved their pulmonary pressure compared with patients whose sPAP had worsened or remained unchanged and showed that survival was similar. This suggests that there was no association between alternations in pulmonary pressures and mortality. The similar survival might be related by the older age and the comorbidities of the cohort; however, we cannot exclude that our study was underpowered to show a survival difference between the study groups.
Limitations
There are several limitations to this study. First, the present study is a retrospective analysis of collected data from two medical centres in Israel, representing relatively small studied cohort of patients. Validation in a large multicentre prospective study is required. Second, invasive right heart catheterization—the gold standard of PHT diagnosis—was not performed.
Third, as mentioned before the cut-off for definition on severe PHT is undetermined, we chose to use 60 mmHg as the cut-off. Utilizing additional cut-offs (55 and 50 mmHg) in repeated analysis demonstrated similar results, assist to transcend this limitation.
Finally, similar mortality rate in our study might be related to the relatively small number of patients. The impact of PHT on mortality among patient who were treated with Mitraclip should be evaluated in larger studies.
Conclusions
Percutaneous MV repair improves sPAP. The reduction in sPAP was attributed mainly to patients with severe PHT at baseline. Increase in sPAP among patients with normal PHT at baseline and higher rates of elevated MV gradients are concerning findings. Additional studies are required to shed light on this phenomenon.
Impact on daily practice
MitraClip procedure reduces sPAP, especially in patients with pre-procedural severe PHT. In patients with no PHT, sPAP may increase significantly and result in clinical benefit attenuation.
Conflict of interest
None declared.




