Effect of sacubitril/valsartan on renal function: a systematic review and meta-analysis of randomized controlled trials
Abstract
A worsening renal function is prevalent among patients with cardiovascular disease, especially heart failure (HF). Sacubitril/valsartan appears to prevent worsening of renal function and progression of chronic kidney disease (CKD) as compared with renin–angiotensin system (RAS) inhibitors alone in HF patients. It is unclear whether these advantages are present in HF patients only, or can be extended to other categories of patients, in which this drug was studied. We performed a systematic review and meta-analysis to assess the consistency of effect size regarding renal outcome across randomized controlled trials (RCTs) that compared sacubitril/valsartan with RAS inhibitors in patients with or without HF. We searched Medline (PubMed), Scopus, and Thomson Reuters Web of Science databases until June 2020. We took into account RCTs that compared sacubitril/valsartan with a RAS inhibitor and reported data regarding renal function. We used random-effects models to obtain summary odds ratio (OR) with 95% confidence interval (CI). We extracted hazard ratios for renal outcomes, glomerular filtration rate slopes or rates of renal adverse events. Sensitivity analyses were performed by moderator analysis and random-effects meta-regression. The search revealed 10 RCTs (published between 2012 and 2019) on 16 456 subjects. Sacubitril/valsartan resulted in a lower risk of renal dysfunction as compared with RAS inhibitors alone [k = 10; pooled OR = 0.70 (95% CI 0.57–0.85); P < 0.001], with a moderate inconsistency between studies [Q(9) = 15.18; P = 0.086; I2 = 40.73%]. A stronger association was found in studies including older patients (k = 10; β = −0.047730; P = 0.020) or HF patients with preserved ejection fraction [pooled OR = 0.53 (0.41–0.68) vs. 0.76 (0.57–1.01) for studies on HF patients with reduced ejection fraction; P for comparison = 0.065]. The effect size did not change with different comparators (angiotensin-converting enzyme inhibitors vs. angiotensin II type 1 receptor blockers, P = 0.279). No significant association was found when the analysis was restricted to studies on non-HF patients [k = 3; pooled OR = 0.86 (0.61–1.22); P = 0.403] and studies with high risk of bias [k = 3; pooled OR = 0.34 (0.08–1.44); P = 0.143]. Our findings support the role of sacubitril/valsartan on preservation of renal function, especially in older patients and HF patients with preserved ejection fraction. However, evidence is currently limited to HF patients, while the renal outcome of sacubitril/valsartan therapy outside the HF setting needs to be further investigated.
Introduction
Chronic kidney disease (CKD) is characterized by a progressive decline in glomerular filtration rate (GFR) and is highly prevalent in the general population.1, 2 A tight link exists between CKD and cardiovascular disease (CVD). Major cardiovascular risk factors such as hypertension, obesity, diabetes, and dyslipidaemia are among the most common causes of both CKD and CVD,3 and CKD itself acts as a catalyst for both cardiovascular sequelae and death.4
A worsening renal function is found in at least 32% of heart failure (HF) patients,5 and renal dysfunction is closely associated with the HF status. An impaired cardiac function with reduced cardiac output decreases the renal blood flow (RBF) leading to renal haemodynamic changes.6, 7 A compensatory renin–angiotensin system (RAS) activation with angiotensin II-mediated vasoconstriction of the efferent arteriole leads to an increase in glomerular capillary hydrostatic pressure to maintain GFR despite the greatly reduced RBF. This compensatory mechanism has high costs in terms of glomerular damage and progression to glomerulosclerosis.
Sacubitril/valsartan is currently recommended for the treatment of HF patients with reduced ejection fraction (HFrEF).8 This first-in-class angiotensin receptor-neprilysin inhibitor (ARNI) was able to reduce both hospitalization and cardiovascular death in patients with EF below the normal range.9 Moreover, two large randomized controlled trials (RCTs) found that it can preserve renal function better than RAS inhibitors,10, 11 and we recently showed that sacubitril/valsartan is associated with a slower rate of decrease in estimated GFR (eGFR) even in the ‘real-life’ setting.12
Sacubitril/valsartan has also been evaluated in clinical settings other than HF, such as arterial hypertension13 and CKD.14 However, it is still unclear if the advantages on renal function of sacubitril/valsartan vs. RAS inhibitors alone can be extended to non-HF patients. Previous meta-analyses investigated the effect on renal outcome of the combined neprilysin-RAS inhibition (including omapatrilat) vs. RAS inhibition alone in HF patients.15, 16 However, an analysis of the specific renal effect of sacubitril/valsartan in different patients' categories is lacking.
We thus performed a systematic review and meta-analysis of RCTs that compared sacubitril/valsartan with RAS inhibitors in patients with and without HF to assess the consistency of effect size (ES) describing renal outcome.
Methods
This report adheres to the Preferred Reporting Items for Systematic Reviews and Meta-Analysis standards for reporting systematic review and meta-analysis studies.17
Eligibility criteria and search strategy
We searched Medline (PubMed), Scopus, and Thomson Reuters Web of Science databases to identify the published studies. The inclusion criteria were the following: the clinical trial had to be an RCT; the control group had to take a RAS inhibitor [angiotensin-converting enzyme inhibitor (ACEi) or angiotensin receptor blocker (ARB)]; the RCT had to report data regarding renal function. We excluded observational studies, case series and case reports, studies published in abstracts, literature reviews, editorials, studies not conducted on humans, and studies on patients aged < 18 years. The main search was run on 9 January 2020 and updated weekly until June 2020. The keywords regarding ‘sacubitril/valsartan’ and terms related to RCTs were typed in various combinations using Boolean operators (see the detailed search strategy in the Supporting Information). Hand searches of reference lists of articles and relevant literature reviews were used to complement the computer search. The search was limited to English-language studies published in peer-reviewed journals.
Study selection and data extraction
Two independent investigators (F. S. and F. G.) screened all identified records (title and abstract) and assessed the selected full-text articles for eligibility. Discrepancies at any step of the process (first screening, full-text screening, and data extraction) were resolved by consensus or by the opinion of a third investigator (R. S.). Descriptive, methodological, and outcome data were extracted from all the eligible studies by the two reviewers who worked independently using a predefined data extraction form. The following data were collected: publication year, number of subjects, mean age, sex, duration of follow-up, main inclusion criteria, renal function at randomization (creatinine or eGFR), systolic blood pressure (BP) at randomization, RAS inhibitor taken as comparator, exclusion criteria regarding the renal function, and definition of renal outcome. For the PARAMOUNT study,18 we took into account the post-hoc analysis performed by Voors et al.19 For the PARADIGM-HF study,20 we took into account the more conventional renal outcome proposed by Damman et al.10 The study authors were contacted to request additional information whenever a study did not report the necessary data for the ES calculation.
Assessment of risk of bias and study quality
Two trained reviewers (F. S. and F. G.) independently assessed the quality of the included studies. We used the Cochrane Collaboration's tool for assessing risk of bias in RCTs.21 The included RCTs were assessed for random-sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, completeness of outcome data, selective reporting, and other sources of bias. Each domain was assessed as low, unclear, or high risk of bias. The highest risk of bias for any criteria was used to reflect the overall risk of bias for the study (Table S1).
Statistical analysis
Data were synthesized using meta-analytic methods and statistically pooled by the standard meta-analysis approach; that is, studies were weighted by the inverse of the sampling variance.
The included studies used different definitions of renal outcome. For calculation of ES, we used a ‘hierarchical’ method/step-by-step method, based on the presence or absence of the renal data in each study. When studies reported renal function as a primary or secondary outcome, we extracted the number of renal events during follow-up in sacubitril/valsartan patients and RAS inhibitor patients or hazard ratios (HRs). When studies reported the variations of eGFR from randomization to the end of the study, we extracted changes in eGFR (or creatinine, if eGFR was not available) in both active and control groups. When studies took into account renal function as an adverse effect of sacubitril/valsartan administration, we collected the rate of renal adverse events.
Overall ES was expressed as odds ratio (OR) and its corresponding 95% confidence interval (CI). The DerSimonian and Laird random-effects model was used as a conservative approach to account for different sources of variation among studies. Forest plots were constructed to graphically represent the results. Q statistics were used to assess heterogeneity among studies. A significant Q value indicates a lack of homogeneity of findings among studies. Inconsistency analysis (I2) statistics were then used to quantify the proportion of observed inconsistency across study results not explained by chance.22 I2 values of <25%, 50%, and >75% represent low, moderate, and high inconsistency, respectively.22 Sensitivity analyses were performed in order to assess the influence of confounders on the pooled ES. Left ventricular ejection fraction, the comparator drug (ACEi or ARB), and the study population were taken into account as moderators, and the ES was assessed and compared across subgroups formed by these moderators. Continuous variables were examined as covariates using random-effects meta-regression (age, male prevalence, duration of follow-up, and baseline systolic BP). Subgroup analyses were performed to assess the effect of study quality on the calculated estimates. The presence of publication bias was investigated through funnel plots both visually and formally by trim-and-fill analysis and Eggers's linear regression method.23 A P value < 0.05 was used to indicate statistical significance. All analyses were conducted using a computer software package (ProMeta Version 2, Italy).
Results
Included studies
The study selection process is described in Figure 1. Among the initial 3483 records, 10 studies published between 2012 and 2019 met our inclusion criteria for a total of 16 456 subjects.11, 14, 18, 20, 24-29 The mean age across the studies ranged from 57.6 to 72.7 years, and 10 949 (66.5%) participants were men. The characteristics of the included studies are described in Table 1. The comparator was an ACEi in three studies20, 26, 28 and an ARB in the remaining studies.11, 14, 18, 24, 25, 27, 29 Most of the studies (n = 7) were conducted on HF patients,11, 18, 20, 26-29 one of them in the acute setting.28 One study was conducted on CKD patients14 and two studies on patients with hypertension.24, 25 Most of the studies reported an eGFR < 30 mL/min/1.73 m2 as exclusion criterion.18, 20, 25, 26, 28, 29
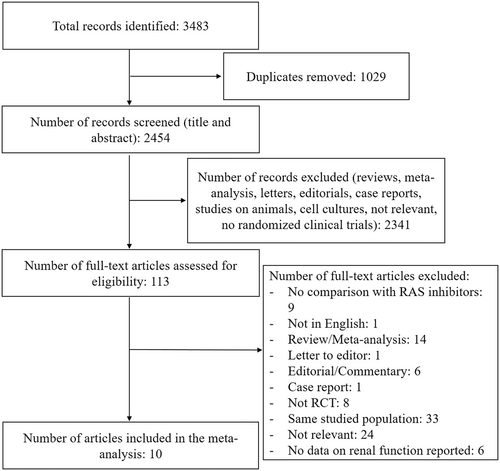
| Study | Inclusion criteria | Sample size | Follow-up | Age (years) | Sex (males) | Intervention | Comparator | Baseline creatinine (mg/dL) | Baseline eGFR (mL/min/1.73 m2) | Renal exclusion criteria | Definition of renal outcome |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Solomon 2012 (PARAMOUNT)18 | HF (NYHA class II–III, EF ≥ 45%) | 301 | 36 weeks | 70.9 ± 9.4 | 131 (43.5%) | LCZ696 | Valsartan | / | 67 ± 19.4 | eGFR <30 mL/min/1.73 m2 | eGFR change19 |
| McMurray 2014 (PARADIGM-HF)20 | HF (NYHA class II–IV, EF ≤ 40%) | 8399 | 27 months | 63.8 ± 11.5 | 6567 (78.2%) | LCZ696 | Enalapril | 1.1 ± 0.3 | / | eGFR < 30 mL/min/1.73 m2 | >50% reduction in eGFR or ESRD10 |
| Supasyndh 201724 | Hypertension | 588 | 14 weeks | 70.5 ± 4.7 | 294 (50%) | Sacubitril/valsartan | Olmesartan | / | / | / | Creatinine > 176.8 μmol/L |
| Haynes 2018 (UK HARP-III trial)14 | CKD (eGFR 20–60 mL/min/1.73 m2) | 414 | 12 months | 62.8 ± 13.7 | 298 (72%) | Sacubitril/valsartan | Irbesartan | / | 35.5 ± 10.9 | / | eGFR change |
| Cheung 201825 | Uncontrolled hypertension | 375 | 8 weeks | 57.6 ± 9.65 | 192 (51.2%) | Sacubitril/valsartan | Olmesartan | / | 80.0 ± 17.3 | eGFR < 30 mL/min/1.73 m2 | Creatinine > 176.8 μmol/L |
| Desai 2019 (EVALUATE-HF)26 | HF (NYHA class II–III, EF ≤ 40%), age ≥ 50 | 464 | 12 weeks | 67.3 ± 9.1 | 355 (76.5%) | Sacubitril/valsartan | Enalapril | / | 70 ± 22 | eGFR < 30 mL/min/1.73 m2 | Decrease in eGFR ≥ 35% or increase in creatinine ≥ 0.5 mg/dL and decrease in eGFR ≥ 25% (as adverse events) |
| Gao 201927 | HF (NYHA class II–IV, EF ≤ 40%), age>60 | 120 | 8 weeks | 70.5 ± 7.1 | 88 (73.3%) | Sacubitril/valsartan | Valsartan | 1.17 ± 0.3 | / | Serious diseases of the kidney | Severe renal insufficiency (as adverse events) |
| Solomon 2019 (PARAGON-HF)11 | HF (NYHA class II–IV, EF ≥ 45%), age ≥ 50 | 4796 | 35 months | 72.7 ± 8.3 | 2317 (48.3%) | Sacubitril/valsartan | Valsartan | 1.1 ± 0.3 | 63 ± 19 | / | Death from renal failure, ESRD, decrease in eGFR ≥ 50% |
| Velazquez 2019 (PIONEER-HF)28 | Acute decompensated HF, EF ≤ 40%, NT-proBNP ≥ 1600 pg/mL or BNP ≥ 400 pg/mL | 881 | 8 weeks | 61 ± 14 | 635 (72.1%) | Sacubitril/valsartan | Enalapril | 1.28 (1.07–1.51) | 58.4 (47.5–71.5) | eGFR < 30 mL/min/1.73 m2 | Increase in creatinine ≥ 0.5 mg/dL and decrease in eGFR ≥ 25% |
| Kang 2019 (PRIME Study)29 | HF (NYHA class II–III, EF 25%–50%) and significant functional MR | 118 | 12 months | 62.6 ± 11.2 | 72 (61%) | Sacubitril/valsartan | Valsartan | 0.98 ± 0.28 | / | eGFR < 30 mL/min/1.73 m2 | eGFR change |
- Normal continuous variables were expressed as mean ± SD. Skewed variables were expressed as median and interquartile range. / indicates data not available.
- CKD, chronic kidney disease; EF, ejection fraction; eGFR, estimated glomerular filtration rate; ESRD, end-stage renal disease; HF, heart failure; MR, mitral regurgitation; NT-proBNP, N-terminal pro-B-type natriuretic peptide; NYHA, New York Heart Association; RCT, randomized controlled trial.
Renal outcome
Five RCTs reported renal function as primary or secondary outcome of interest,11, 14, 18, 20, 28 while the remaining five RCTs only reported it as adverse event.24-27, 29
Overall, sacubitril/valsartan resulted in a lower risk of worsening renal function as compared with RAS inhibitors [pooled OR = 0.70 (95% CI 0.57–0.85), P < 0.001, Figure 2], with a moderate inconsistency between studies [Q(9) =15.18, P = 0.086, I2 = 40.73%]. The funnel plot showed asymmetry with a similar estimated ES [estimated ES = 0.70 (95% CI 0.58–0.84), P < 0.001; number of trimmed studies: 2], although the Egger linear regression test was not significant (P = 0.699) (Figure S1).
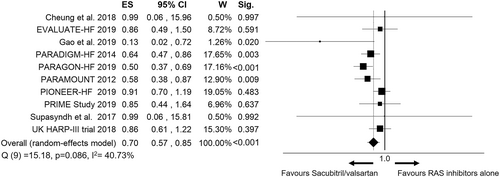
Sensitivity analyses
We performed the moderator analysis in order to search for possible sources of heterogeneity related to the different characteristics among studies and selected populations (Table 2).
| k | n | ES | 95% CI | P | Q | I2 | Pa | |
|---|---|---|---|---|---|---|---|---|
| LVEF in HF studies | 0.065 | |||||||
| LVEF > 40% | 2 | 5097 | 0.53 | 0.41–0.68 | <0.001 | 0.27 | 0.00 | |
| LVEF ≤ 40% | 5 | 9982 | 0.76 | 0.57–1.01 | 0.056 | 7.38 | 45.80 | |
| RAS inhibitor | 0.279 | |||||||
| ACEi | 3 | 9744 | 0.78 | 0.61–1.00 | 0.052 | 3.10 | 35.44 | |
| ARB | 7 | 6712 | 0.63 | 0.48–0.85 | 0.002 | 9.50 | 36.85 | |
| Study population | 0.231 | |||||||
| HF patients | 7 | 15 079 | 0.67 | 0.52–0.85 | 0.001 | 13.57b | 55.79 | |
| Non-HF patients | 3 | 1377 | 0.86 | 0.61–1.22 | 0.403 | 0.02 | 0.00 | |
| Risk of bias | 0.321 | |||||||
| Unclear/low | 7 | 15 373 | 0.71 | 0.59–0.86 | 0.001 | 11.30 | 46.92 | |
| High | 3 | 1083 | 0.34 | 0.08–1.44 | 0.143 | 2.35 | 14.99 |
- a P for comparison between subgroups
- b P < 0.05
- ACEi, angiotensin-converting enzyme inhibitor; ARB, angiotensin II type 1 receptor blocker; HF, heart failure; LVEF, left ventricular ejection fraction; RAS, renin–angiotensin system.
A lower risk of worsening renal function with sacubitril/valsartan was found in studies on HF patients with EF > 40% as compared with studies on HF patients with EF ≤ 40%. No difference in ES was found between studies using ACEi or ARB as comparators. The association between sacubitril/valsartan and preserved renal function remained robust after restricting the analysis to studies on HF patients or those with low/unclear risk of bias. On the contrary, no significant ES was found when considering only studies on patients without HF or those with high risk of bias.
In meta-regression analyses (Figures S2–S5), the ES decreased with increasing age, while sex, duration of follow-up, and systolic BP at baseline had no significant effect. After those studies reporting renal function only as an adverse event were excluded, the association between sacubitril/valsartan and preserved renal function was confirmed with a moderate inconsistency between studies [k = 5; n = 14 791; pooled OR = 0.69 (95% IC 0.54–0.87); P = 0.002; I2 = 61.86%].
Discussion
This systematic review and meta-analysis of 10 RCTs highlight the protective role exerted by sacubitril/valsartan on the kidney, in terms of lower risk of worsening renal function. Patients treated with sacubitril/valsartan showed a 30% lower risk of renal events and eGFR progressive decline than did patients treated with RAS inhibitors alone. Studies on older patients and HF patients with preserved EF showed an even greater risk reduction.
In our systematic review, we included studies on both HF and non-HF patients in order to gain a comprehensive view of the evidence available to date, given the potentially different pathophysiological mechanisms for decreasing renal function. Our analysis shows how most of the available evidence on the renal effect of sacubitril/valsartan is currently focused on HF patients, thus suggesting still a central role of the cardiac benefit of sacubitril/valsartan also in the nephroprotection. This innovative drug has also been studied in hypertensive patients, showing a greater consistent BP reduction as compared with olmesartan.24, 25 These studies however took into account renal function only as an adverse effect, thus challenging the reliability and robustness of data. Our work highlights the urgent need for studies that investigate the renal outcome outside the HF setting.
Only one RCT investigated the effect of sacubitril/valsartan on renal function outside HF in patients with advanced kidney disease. The UK HARP-III trial14 enrolled patients with advanced CKD, defined by an eGFR ≥ 20 and <45 mL/min/1.73 m2 or by an eGFR ≥ 45 and <60 mL/min/1.73 m2 and a urine albumin/creatinine ratio > 20 mg/mmol. At 12 months, eGFR progression and albuminuria did not differ between patients on sacubitril/valsartan and patients on irbesartan.14 Despite this, sacubitril/valsartan had the additional effect of lowering both BP and cardiac biomarkers (troponin I and N-terminal pro-B-type natriuretic peptide), indirectly suggesting a role in the cardiovascular risk reduction even in advanced CKD patients.30 However, this RCT had several limitations and was underpowered to detect eGFR changes in the medium/long period.30 Moreover, half of participants had causes of CKD in which the pathogenesis of disease progression was not predominantly mediated by glomerulosclerosis (i.e. hereditary nephritis and tubulointerstitial nephritis). Finally, much data were missing at the end of the study, and the study duration was too short. All these limitations do not allow to draw solid conclusions.30 Therefore, the effect of sacubitril/valsartan on renal outcomes in patients with advanced CKD, especially if having an eGFR < 30 mL/min/1.73 m2, remains a relevant unanswered question, although the drug is likely to be safe.14
The results of our meta-analysis are mainly driven by the positive renal outcomes of the two largest studies of sacubitril/valsartan in HF patients performed to date (PARADIGM-HF and PARAGON-HF).11, 20 In a secondary analysis of PARADIGM-HF, Damman et al.10 found that patients taking sacubitril/valsartan had a lower eGFR decrease during the follow-up than had patients taking enalapril, despite a greater BP reduction and independently of both CKD and albuminuria. Another sub-analysis of PARADIGM-HF showed that the reduction in eGFR decline in the sacubitril/valsartan group was greater in patients with type 2 diabetes mellitus (T2DM) compared with patients with no T2DM.31 Interestingly, at least part of the accelerated nephropathy and glomerular hyperfiltration typically found in T2DM could be mediated by both a cyclic guanosine monophosphate (cGMP) deficiency and an over-activity of sodium–hydrogen exchangers in the proximal renal tubule, which might be counteracted by neprilysin inhibition.31
In the PARAGON-HF,11 patients taking sacubitril/valsartan showed a lower risk of worsening renal function than did patients taking valsartan alone (1.4% vs. 2.7%, HR 0.50; 95% CI 0.33–0.77). Moreover, although the study failed to achieve statistical significance in the primary outcome on the entire study population, cardiovascular death and HF hospitalization reached the statistical significance in the sub-analysis on patients with baseline eGFR < 60 mL/min/1.73 m2 in favour of the sacubitril/valsartan group.
Pathophysiological considerations
The increase in renal perfusion, owing to the improved cardiac function, could partially explain the effects of sacubitril/valsartan on kidney, especially in the HF setting. Moreover, natriuretic peptides (NPs) affect several organs and systems, exerting multiple cardio-metabolic activities.32-35 The kidneys, together with the adipose tissue, are the organs where NP receptors are mainly expressed, as well as neprilysin, which is mostly expressed in the brush border of proximal renal tubular cells.36, 37 Neprilysin inhibition, mediated by sacubitril, likely increases the renal NP bioavailability. The preservation of renal function is a property in common with other drugs that stimulate the NPs system, such as omapatrilat or designer NPs.36, 38 Both ANP and B-type NP (BNP) infusion have been demonstrated to improve GFR in healthy humans,39, 40 hypertensive patients, and HF patients.41 Recent evidence suggests that sacubitril mainly acts by enhancing ANP rather than BNP.42 Moreover, also urodilatin, a splice variant of pro-ANP locally synthesized by renal cells, is a neprilysin substrate, although less susceptible than ANP.43 Urodilatin infusion led to significant increase in both urine flow and sodium excretion in rats.44 NPs, especially ANP, significantly decrease sodium reabsorption in the proximal tubule, through the activation of NPR-A/cGMP/PKG pathway, thus increasing sodium delivery to the distal nephron segment and interacting with the tubule-glomerular feedback.45 In diabetic rat models, ARNI was found to prevent segmental glomerulosclerosis and tubular injury as compared with ARB alone, independently of BP.46, 47 Increased NP activity exerted direct antioxidant, anti-inflammatory, and anti-fibrotic activities in experimental models.48-51 Moreover, sacubitril/valsartan prevented fibrosis, oxidative stress, mitochondrial damage, and apoptosis in kidney and heart tissues of rat models with cardio-renal syndrome.52
In our meta-analysis, duration of follow-up and baseline systolic BP did not affect the relationship between sacubitril/valsartan and renal impairment. In HF patients, especially those having lower BP values, the decrease in renal perfusion leads to adaptive mechanisms of the glomerular haemodynamics through the activation of the RAS and the consequent angiotensin II-mediated vasoconstriction of the efferent arteriole. In this condition, the RAS inhibition counteracts this renal auto-regulation, leading to a decrease in intra-glomerular pressure and consequently in GFR, which becomes more dependent on systemic BP, which is also reduced by these drugs.53 The concomitant inhibition of angiotensin II type 1 receptor and neprilysin partially maintains an adequate intra-glomerular pressure through a preferential vasodilatation of the afferent arteriole, mediated by the increase in ANP bioavailability,54, 55 and a relative persistent vasoconstriction of the efferent arteriole, despite a further reduction in systemic BP.53 In acute decompensated HF, the renal perfusion is further compromised, owing to the activation of several neuro-hormonal axes (mainly RAS and sympathetic nervous system) that maximize vasoconstriction of the afferent arteriole with a secondary increase in pre-glomerular resistances.56 In this setting, an increase in renal NP activity could be even more advantageous, by counteracting both these neuro-hormonal axes.41 One RCT (PIONEER-HF) investigated sacubitril/valsartan vs. enalapril in acute HFrEF patients, showing a good safety profile in terms of incidence of worsening renal function and eGFR decrease.28
An increase in albuminuria is usually found after starting sacubitril/valsartan in HF patients,10, 19 although an oppose effect was found in animal studies.46, 48 However, it is not likely due to glomerular hyperfiltration and podocyte damage, such as in diabetic nephropathy. Instead, previous studies showed a relaxation of contractile mesangial cells in the space between capillary endothelium and podocytes, contributing to the expansion of capillary surface area available for filtration, in addition to a possible attenuation of tubular protein reabsorption.57, 58 This explains why the worsening of albuminuria is not associated with a progressive loss of glomerular function in this setting but on the contrary to less renal disease progression.57
In conclusion, NPs provide several direct biological effects on kidney, affecting both glomerulus, by improving RBF and GFR while reducing renin release, and tubule, by decreasing sodium reabsorption, with a net benefit in terms of natriuresis, diuresis, and preservation of renal function.59 All these mechanisms could explain the preservation of the residual renal function during ARNI treatment, although the long-term renal effects of sacubitril/valsartan are still to be fully elucidated.
Study limits
The strength of our study is in the large sample analysed (16 456 patients). However, it has also several limitations. First, a substantial proportion of our data derived from PARADIGM-HF and PARAGON-HF, the two largest studies carried out on sacubitril/valsartan to date. However, in our meta-analysis (Figure 2), PIONEER-HF has the highest weight, and other two studies (UK HARP-III and PARAMOUNT) have a weight > 10%. Second, RCTs on patients with no HF are limited to only three studies (two on hypertension and one on advanced CKD) with high risk of bias and significant limitations. Therefore, we cannot draw any solid conclusion outside HF. Third, the included studies used different definitions of renal outcome and different ways to report renal function. This aspect may have affected our findings, although we tried to overcome this limitation by performing several sensitivity analyses. Finally, the heterogeneity of the comparators (molecule and dose) administered in each RCT is another limitation that needs to be taken into account.
Conclusions
Our findings support the role of sacubitril/valsartan on preservation of renal function, especially in older patients and HF patients with preserved EF. However, the evidence is currently limited to HF patients. There is an urgent need to investigate the renal outcome of sacubitril/valsartan therapy outside the HF setting, such as in T2DM and advanced CKD, where the evidence is still scarce and of low quality. Meanwhile, the available studies confirm also the renal safety of this innovative drug in different clinical scenarios.
Acknowledgements
Unconditional support for article publication charges was provided by Novartis Farma S.p.A.
Conflict of interest
None declared.
Funding
None.




