Drivers of Insect Diversity and Community Turnover in Protected Tropical Deciduous Forests of Mexico
Funding: This study was funded by grants given by CONAHCyT (now SECIHTI), (convocatoria Ciencia de Frontera 2019; project no. 58548) and PAPIIT-DGAPA-UNAM (convocatoria 2022, project no. IN201622) to AZR.
ABSTRACT
Tropical deciduous forests (TDFs) are among the world's most biodiverse yet endangered ecosystems. In the Neotropics, TDFs have experienced substantial range reductions due to land use changes, with profound implications for their insect diversity, much of which remains unknown. Here we conducted extensive Malaise trap sampling and metabarcoding of whole insect communities to investigate the influence of seasonality, anthropogenic disturbance, and habitat physical parameters on insect taxonomic diversity and community structure in two protected TDFs on the Pacific coast of Mexico, at Chamela (Jalisco) and Huatulco (Oaxaca), located approximately 1000 km apart. We recorded high insect species diversity, with 4626 and 3672 MOTUs identified at Chamela and Huatulco, respectively, of which only 18.5% were shared between the two regions. Nonmetric multidimensional scaling (NMDS), fourth-corner model, and zeta diversity analyses revealed a complex, multi-factorial community turnover driven by seasonal fluctuations and varying disturbance levels. Species turnover also was partially explained by the distance between sites and specific forest features (total deadwood volume, tree diversity, canopy coverage), suggesting that habitat heterogeneity shapes local insect diversity and community turnover. Our findings highlight the unexpectedly high local and regional turnover in insect communities in Neotropical TDFs, underscoring the importance of conservation of each remaining forest reserve and their semi-disturbed surroundings.
1 Introduction
The biodiversity crisis, characterized by the unprecedented and accelerating global loss of species, has been a critical issue on the international conservation agenda (Singh 2002; Slingenberg et al. 2009; Wagner, Fox, et al. 2021; Wagner, Grames, et al. 2021). Climate change and local anthropogenic disturbances are primary drivers of reduced species richness and community heterogeneity worldwide (Dornelas et al. 2019), particularly in tropical forests, which host the majority of terrestrial biodiversity (Lewis 2006; Alroy 2017; Hansen et al. 2020; Pillay et al. 2022). Tropical deciduous forests (TDFs) are among the most biodiverse ecosystems but are threatened worldwide (Janzen 1988; Miles et al. 2006). These forests exhibit significant seasonality, with deciduousness in the dry season and a temporarily persistent foliage in the wet season, shaping local species adaptations and ecological interactions (Mooney et al. 1995; Sánchez-Azofeifa et al. 2005; Dirzo et al. 2011). In the Neotropics, TDFs span from north-western Mexico to northern Argentina, with significant floristic turnover in both structure and composition across their geographic range, leading to the recognition of various community subtypes (Linares-Palomino et al. 2011; Dryflor et al. 2016). Notable differences in physiognomy and floristics can occur even within small geographic regions (Trejo and Dirzo 2002; Pérez-García and Meave 2005; Pérez-García et al. 2005; Durán et al. 2006), further highlighting the ecological complexity of these forests.
The TDF in the northern Neotropics, especially along the Mexican Pacific coast, is known for its high endemism due to historical isolation from other TDFs in Central and South America and from nearby biomes (Dryflor et al. 2016; Curry 2021). These TDFs have faced substantial range losses over the past 30 years, mainly due to the expansion of agriculture and tourism (Trejo and Dirzo 2000; Sánchez-Azofeifa and Portillo-Quintero 2011; Flores-Casas and Ortega-Huerta 2019), making conservation efforts critical. However, knowledge of species diversity and endemicity remains limited to vertebrates and a few specific arthropod groups (e.g., Hutto 1989; Allen et al. 1998; Sánchez-Velásquez et al. 2002; Álvarez-Yépiz et al. 2008; Andersen 2008; García-Marmolejo et al. 2015; Rocha-Ortega and Castaño-Meneses 2015; Boege et al. 2019).
The lack of biodiversity data is particularly acute for insects, which comprise over half of all known species on Earth, especially considering the precipitous global population decline affecting them worldwide (Hallmann et al. 2017; Lister and Garcia 2018; Seibold et al. 2019; Owens et al. 2020). Insect population trends remain largely undocumented across most tropical regions due to the limited taxonomic and ecological knowledge that hampers long-term studies for monitoring and understanding their decline. Whole-community DNA sequencing (metabarcoding) may overcome these limitations if species can be consistently identified in complex mixtures of specimens and the data used for direct large-scale biodiversity assessment (Ji et al. 2013; Creedy et al. 2018; Li et al. 2023). In insects, mitochondrial cytochrome oxidase I (COI) is widely used as barcode marker (Hebert et al. 2003) and combined with high-throughput sequencing (Liu et al. 2019) has proven effective for evaluating insect species diversity from bulk samples (e.g., Remmel et al. 2024; Souto-Vilarós et al. 2024).
Here we used metabarcoding to evaluate insect species diversity across two protected TDFs located near the Mexican Pacific coast, in the states of Jalisco and Oaxaca. Although part of the same biogeographical province, these TDFs are separated by approximately 1200 km and differ significantly in plant species composition (Trejo and Dirzo 2002; Lott and Atkinson 2006; Williams 2009). No comprehensive assessments currently exist in these reserves on insect species diversity, community differentiation, or the effects of disturbances on local faunas. We hypothesized that insect community structure exhibits significant spatio-temporal heterogeneity due to the pronounced climatic seasonality and complex habitat structure of TDFs, with higher species turnover occurring between seasons. Additionally, we predicted that anthropogenic disturbance influences both species richness and community composition, leading to shifts in diversity patterns. To test these hypotheses, we applied the zeta diversity framework (Hui and McGeoch 2014) to analyze species turnover, dissecting the contribution of rare and widespread species across disturbed and presumed pristine forest sites. This approach allowed us to assess the influence of climatic seasonality, anthropogenic disturbance, and habitat physical parameters on insect community structure. Specifically, we hypothesized that spatial and disturbance-related variation in insect diversity is primarily driven by changes in the relative contribution of rare versus widespread species. Our results revealed remarkably high diversity and levels of endemicity in both TDFs, with largely non-overlapping species assemblages. These findings highlight the need for targeted conservation strategies to preserve biodiversity within each of these ecosystems. The study also reinforces the power of metabarcoding as a tool for biodiversity assessments of insect communities in tropical forests, whose study has remained elusive with conventional taxonomic methods.
2 Methods
2.1 Study Sites
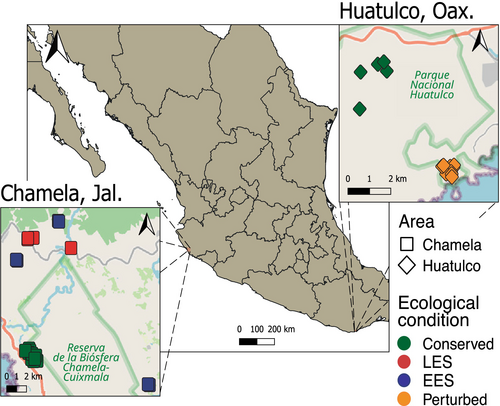
The study systematically monitored insect species diversity across two TDFs in México: the Chamela-Cuixmala Biosphere Reserve in Jalisco and the Huatulco National Park in Oaxaca (hereinafter referred to as Chamela and Huatulco, respectively) (Figure 1). These reserves are located in the Sierras de las Costas de Jalisco y Colima and the Costas del Sur subprovinces, respectively (Instituto Nacional de Estadística, Geografía e Informática 2004, 2017), at opposite edges of the lowlands adjacent to the Pacific coast within the Sierra Madre del Sur physiographic province (Quiñones 1987; Krasilnikov et al. 2013). Both regions have a warm climate (20°C–29°C), with annual precipitation ranging from 600 to 1200 mm and a marked dry season lasting from 5 to 8 months: October–May in Chamela and November–April in Huatulco (García 2004; Takano-Rojas et al. 2023). Surrounding areas experience varying levels of grazing, agriculture, and tourism activities.
2.2 Insect Sampling
We employed 20 and 14 Malaise traps in Chamela and Huatulco, respectively, over six and seven months (May–October, June–December 2020). Traps were placed in protected (conserved) sites (10 and 5 traps) and in surrounding disturbed sites (10 and 9 traps) (Figure 1; Table S1). Disturbed sites in Chamela had undergone disturbance between 15 and 30 years prior to the study, and thus we categorized them into late ecological succession (LES; 29–30 years; 4 traps) and early ecological succession (LES; 15–20 years; 6 traps). In Huatulco, traps in disturbed areas were situated in forest patches surrounded by agricultural fields (perturbed sites). Traps were placed at least 200 m apart from each other, with collector bottles half-filled with 95% ethanol, replaced every 15 days. Samples were stored at −20°C until further processing.
For each plot within a 10 m circular area around the trap, we recorded the distance between sites and six habitat physical parameters following Larrieu et al.'s (2018) typology for trees with a perimeter (at breast height) larger than 20 cm: (1) tree diversity (species number), (2) basal tree area calculated from tree diameter measured at breast height (dbh), (3) tree microhabitats diversity (see description of microhabitat types in Table S2), (4) microhabitat density (number of microhabitats per tree), (5) total deadwood volume (area × length), and (6) canopy coverage (percentage of vegetation in 96 spherical densiometer squares). Measurements were taken from 26th September–1st October in Chamela and 19th–23 October in Huatulco (Table S2). Monthly temperature and precipitation data were obtained from CONAGUA Automated Weather Stations in La Huerta, Jalisco, and Santa María Huatulco, Oaxaca.
2.3 Laboratory Procedure
All samples were grouped by month, resulting in 218 final insect bulk samples. Following Sire et al. (2022), specimens were size-sorted to avoid bias from large biomass and improve detection of small and rare specimens. Specimens larger than Apis mellifera were removed, and three of their legs or abdomen were dissected and reincorporated into the sample. Bulk samples were ethanol-drained, dried overnight, and ground into fine powder using an Ultra Turrax Tube Drive grinder (IKA) with sterile disposable tubes (IKA) and 10 steel beads. Two 25 mg subsamples from each ground sample were placed into 1.5 mL tubes. An empty control tube with 200 μL of lysis buffer was left open during the powder sampling process and was replaced every 60 subsamples to monitor cross-contamination. A total of 436 subsamples and controls were sent to the Canadian Centre for DNA Barcoding, University of Guelph, Ontario, Canada, for sequencing.
DNA was extracted following Ivanova et al. (2006), and PCR amplification targeted a 418 bp fragment of the animal barcode locus using the BF3 and BR2 primers (Elbrecht et al. 2019). Two PCRs were performed: PCR1 amplified the targeted fragment, and PCR2 added P5/P7 Illumina adapters with individual 6–8 bp barcodes attached to them to maximize multiplexing. PCR1 had an initial denaturation of 95°C for 5 min, followed by 30 cycles of 94°C for 30 s, 46°C for 30 s, and 72°C for 50 s, with a final extension at 72°C for 5 min. PCR2 had an initial denaturation of 2 min, followed by 15 cycles of 94°C for 40 s, 51°C for 1 min, and 72°C for 1 min, with a final extension of 72°C for 5 min. Each PCR reaction (25 μL) contained 2.5 μL of buffer (10×), 0.5 μL of dNTPs (10 mM), 1 μL of each forward and reverse primer (10 mM), 0.2 μL of mi-Taq (5 U/μL), 16.8 μL of extra pure molecular grade water, and 2 μL of DNA or 1× diluted PCR1 template. Negative controls were included every 96 subsamples. Each subsample was amplified three times, sequenced on 96-well plates with three different identification barcodes for tracking and contamination detection. PCR products were purified using magnetic beads (ratio 0.8:1 μL beads/product), ligated to adapters, and sequenced on the Illumina NovaSeq 6000 instrument.
2.4 Sequencing Reads Processing and Taxonomic Assignment
The obtained reads were first removed of any remaining adapter, then cleaned and quality-filtered using AdapterRemoval v.2.3.0 (Schubert et al. 2016) and Sickle v.1.33 (Joshi and Fass 2011). Sequencing errors were corrected using Bayes-Hammer via SPAdes 3.12.0 (Nurk et al. 2013) and paired-end reads were merged with PANDAseq v.2.11 (Masella et al. 2012). Reads were demultiplexed into samples using DAMe v.1.0 (Zepeda-Mendoza et al. 2016). PCR replicates per sample were filtered using filter.py from DAMe, and only reads present in all three PCRs with abundance equal to or larger than 10 reads were retained in the final output. The quality of filtering and trimming of reads was visually evaluated with FASTQC (Andrews 2010), and chimeras were identified and removed through VSEARCH (Rognes et al. 2016). Finally, reads were clustered into Molecular Operational Taxonomic Units (MOTUs) with SUMACLUST v.1.0.36 (Mercier et al. 2013) with a conservative species-level similarity threshold of 97%. The resulting MOTUs were quality-checked using the LULU package (Frøslev et al. 2017) in R v.3.6.1 (R Core Team 2017) to discriminate between rare and low-frequency MOTUs, which may be due to sequencing error but actually belong to more abundant MOTUs based on co-occurrence in the samples.
Taxonomic assignment was performed by similarity, comparing the nucleotide sequences with the BOLD Systems database (identification hits) using the BOLD package v.1.3.0 (Chamberlain 2020) in R v.3.6.1 (R Core Team 2017) and the BOLD_NCBI_Merger (Macher et al. 2017). We used a conservative approach, retaining taxonomic assignment at the order, family, genus, and species levels when MOTUs had sequence similarities ≥ 80, ≥ 85%, ≥ 95%, and ≥ 97%, respectively. Assignments with a similarity < 80% were discarded. The taxonomic assignment of each MOTU was retained only at the taxonomic level without ambiguities. When a MOTU had multiple identifications at a particular taxonomic level (i.e., a nucleotide sequence matched multiple entries in the BOLD database), the lowest taxonomic level with a unique identification was selected.
2.5 Statistical Analyses
All statistical analyses were performed in R v.3.6.1 (R Core Team 2017). Unless otherwise stated, plots were generated using ggplot2 v.3.5.2 (Wickham 2016). Reads were converted to incidence data to record MOTU presence/absence, assigning a value of 1 if a MOTU was detected in a plot, and 0 if it was not, regardless of read abundance, with MOTU diversity as a proxy for species diversity. Accumulation curves were generated using iNEXT v.3.0.0 (Hsieh et al. 2020) with incidence-based frequencies and Hill's number q = 0. The total number of MOTUs for Chamela, Huatulco, and both regions combined was plotted against sample units and coverage by month. The five most species-rich insect orders (Coleoptera, Diptera, Lepidoptera, Hymenoptera, and Hemiptera) and less represented orders (grouped) were plotted against sample units to assess collecting effort. Chao and first-order Jackknife biodiversity estimates were calculated using Vegan v.2.6.4 (Oksanen 2020) to evaluate sampling effort. MOTUs per month and their shared occurrences across months were visualized using UpSet plots generated with the UpSetR package v.1.4.0 (Conway et al. 2017; Lex et al. 2014).
Generalized linear model (GLM) analyses were used to correlate climate features (mean monthly temperature, mean monthly precipitation) and levels of anthropogenic disturbance (conserved, LES, EES for Chamela; conserved, perturbed for Huatulco) with taxonomic richness. A quasi-Poisson distribution was used in all GLMs to account for overdispersion in count data. Normality (Shapiro–Wilk's test; Shapiro and Wilk 1965) and homogeneity of variance (Levene's test; Levene 1960) assumptions were tested, with the corresponding alternative test used if violations were found (Kruskal-Wallis test; Kruskal and Wallis 1952; and Welch's ANOVA; Welch 1951). Moreover, the insect community composition of each TDF region and disturbance level were visually examined through NMDS analyses using the Vegan package, and statistical testing was performed using ANOSIM (Clarke 1993). Additionally, differences in insect diversity between the two TDF regions were assessed using GLM, whereas differences in community structure between regions were evaluated through ANOSIM.
The prevalence of the five most species rich insect orders across different levels of disturbance was evaluated using a Fourth Corner Model (Brown et al. 2014). A GLM predictive model was fitted to assess species presence/absence across all sites simultaneously, incorporating three explanatory variables: site disturbance levels, species traits, and trait-environment interactions (i.e., the “fourth-corner matrix”; Brown et al. 2014). This analysis was performed using the “traitglm” function from the mvabund v.4.2.1 package (Wang et al. 2020). Species traits were represented by the MOTUs' assignment to five most species-rich insect orders, and the analysis identified significant associations between these traits and disturbance levels.
Species compositional turnover for Huatulco and Chamela was evaluated using zeta diversity analysis (Hui and McGeoch 2014) implemented in the zetadiv v.1.2.1 package (Latombe et al. 2020). Zeta diversity (ζi) quantifies the number of species shared among i sites, where i represents the zeta order (Hui and McGeoch 2014). This approach sequentially calculates the number of species shared across an increasing number of sites (e.g., species shared by 2, 3, 4, 5 … n sites). By focusing on multi-site and cross-scale assemblage patterns, zeta diversity provides insights into the structure of biodiversity turnover, providing information about the contributions of both rare (shared by few sites) and common (shared by many sites) species to compositional change (Hui and McGeoch 2014). The metrics measures the “number of shared species” across sites, with higher zeta orders (i > 2) reflecting the influence of increasingly widespread species, i. e. those with larger spatial occupancy and shared across more sites.
We analyzed data combining all sites for Chamela and Huatulco, examining regions separately and grouping sites by disturbance levels. Two models were evaluated: one comparing all possible sites combinations (non-geographic) and another focusing on Nearest-Neighbor sites to account for distance. Model selection was based on the Akaike Information Criterion (AIC), with the lowest AIC value (ΔAIC ≥ 10) indicating the best fit. The selected model was fitted to exponential regression and power law functions to assess whether patterns were driven by stochastic processes or by ecological niche differentiation and rare species turnover (Hui and McGeoch 2014; McGeoch et al. 2019). A better power law fit suggests niche differentiation, where rare species (those found in one or few sites) drive compositional changes across sites, while an exponential fit indicates stochastic processes, with equal species occurrence probabilities (Hui and McGeoch 2014).
We plotted the zeta decline by graphing zeta values (number of shared species across sites) against zeta order (number of sites) to examine the pattern and rate at which the average number of shared species decreases as the number of sites increases. Moreover, the zeta ratio (ζi/ζi−1)—the probability that a species present in a sample will remain present when a new sample is added—was plotted against the zeta order (e.g., order 1 for ζ2/ζ1, order 2 for ζ3/ζ2, etc.). This generated a retention curve that reflects the rate (or probability) at which species shared by i−1 sites persist in the community as additional sites (i) are incorporated into the analysis. Since common species are more likely than rare ones to occur in additional samples, comparing zeta ratios at low and high orders highlights this phenomenon and improves visualization of turnover at higher zeta orders (McGeoch et al. 2019).
We used zeta multi-site generalized dissimilarity modeling (zeta.msgdm) in the zetadiv v.1.2.1 package (Latombe et al. 2020) to assess the contribution of site distance and the six recorded habitat physical parameters (tree diversity, basal area, tree microhabitat diversity, microhabitat density, deadwood volume, and canopy coverage) to species turnover at specific zeta orders (2, 3, 4, 5, …n). This model evaluated how species shared across sites respond to habitat physical parameters, with variable modeled three I-splines and the Sørensen-equivalent metric of zeta diversity (Ferrier et al. 2007). The combined I-spline for each variable represents its relationship with zeta diversity, showing how changes in the variable influence species composition.
The relative amplitude of an I-spline indicates the effect of an environmental/distance variable on species turnover compared to other variables, while its slope reflects the rate of turnover along the environmental gradient. Steeper slopes highlight points where small changes in the variable significantly impact species turnover (Brown et al. 2014). I-splines were plotted against the rescaled variable gradient. We conducted three analyses: merging all sites per region (20 for Chamela, 14 for Huatulco), categorizing conserved and disturbed sites in Chamela (EES, LES; 10 each), and including only perturbed sites in Huatulco (9 sites). Conserved sites in Huatulco were excluded due to limited sampling.
3 Results
3.1 Insect Species Diversity
A total of 470,645,713 raw 250 pb paired-end sequencing reads were generated. After demultiplexing and filtering, 166,101,095 reads remained (103,517-1,299,131 reads per sample; mean 326,817.21 ± 8345.85). All subsamples passed the 10-reads minimum threshold across three PCR replicates, yielding 218 merged samples. Clustering at 97% similarity identified 7000 insect MOTUs across the two examined regions, with 99.62%, 90.97%, 27.1%, 13.31% assigned to 18 orders, 332 families, 1048 genera and 932 species, respectively (Table S3).
Species richness was estimated at ~9300 MOTUs (Jackknife and Chao's estimation: 9310.35 ± 184.56 and 9413.29 ± 133.01, respectively) for both regions, with the species accumulation curve becoming stable (Figure 2). The 218 samples covered ~75% of the estimated species richness (Figure S1I). Chamela and Huatulco had ~6238 and ~5059 MOTUs, respectively (Jackknife and Chao's estimates: Chamela, 6200.77 ± 170.05 and 6275.56 ± 109.97; Huatulco, 5030.99 ± 162.43 and 5085.56 ± 162.43), with their species accumulation curves also becoming stable (Figure S1A,E). Similar results were observed across insect orders in both regions (Figure S2).
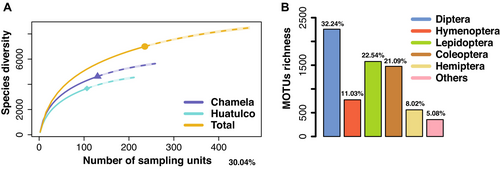
Species richness was significantly higher in Chamela than Huatulco (Table S4). Diptera, Lepidoptera, and Coleoptera were the richest orders, comprising ~76% of total MOTUs (Figure 2B and Figure S1C,G). Chamela had 4626 MOTUs, Huatulco 3672, and 1298 (18.5%) were shared (Figure S1J). Diptera and Lepidoptera contributed the most shared MOTUs (474 and 437, respectively), followed by Hymenoptera (158), Coleoptera (130), and Hemiptera (63). Temporal turnover was significant, with only 92 and 124 MOTUs persisting year-round in Chamela and Huatulco, respectively (Figure S1D,H).
3.2 Influence of Climate and Disturbance
Precipitation and temperature variation between the two regions was most pronounced during August and September (Figure S3B,D). In Chamela, mean monthly temperature was positively correlated with species richness for all insects and for each of the five most species-rich orders analyzed separately, whereas in Huatulco mean monthly precipitation was positively correlated with species richness for all insects, as well as for Coleoptera, Diptera, and Hemiptera separately (Table S4). Disturbed sites (LES, EES) had significantly higher species richness than conserved sites in Chamela for all insects, Hemiptera, and Lepidoptera (Table S4).
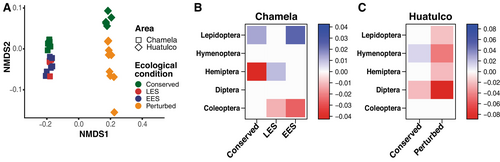
Community composition analyses revealed distinct species assemblages between the two regions, both for all insects and for the five most species-rich orders (Figure 3A; Table S5). The insect community assemblage in Chamela and Huatulco exhibited significant temporal species turnover, with species aggregating during months of higher precipitation, primarily from June to August. Precipitation had a moderate influence on community structure (envfit r2: Chamela = 0.40, Huatulco = 0.56; p < 0.001), whereas temperature had a stronger effect in Chamela (r2 = 0.88) compared to Huatulco (r2 = 0.44; p < 0.001 in both cases; Figure S3A,C; Table S5). Similarly, conserved sites in each region harbored unique communities (Figure 3A; Table S5). Disturbance levels influenced species assemblages in both regions. Significant differences were observed between conserved and disturbed sites in Chamela and Huatulco for all insects and the five most species-rich orders, except Hemiptera in Huatulco (Figure 3A; Table S5).
3.3 Species Turnover Patterns
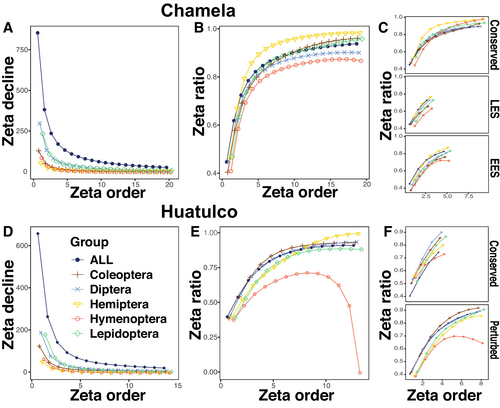
Our analyses of species turnover within each region revealed significant zeta diversity decline across sites using both the Nearest-Neighbor and the all-possible-sites combinations models. The latter model provided a substantially better fit to a power law distribution (AIC pl. = −140.39 for Chamela and −54.62 for Huatulco) compared to the Nearest-Neighbor model (AIC pl. = −67.41 for Chamela and −34.61 for Huatulco) (Figure S4A–D). Zeta diversity in Chamela and Huatulco exhibited a sharp decline between two and five sites, indicating turnover driven primarily by rare species (Figure 4A,D). Zeta ratio revealed that a few species were shared by six or more sites, as the zeta ratio approached one (ζi/ζi−1) = 1 with increased numbers of sites, indicating these species were widespread and consistently present across both regions (Figure 4B,E). Among the most species-rich insect orders, all but Hymenoptera in Huatulco exhibited this type of prevalence of common species. Hymenoptera displayed a bimodal curve, with an initial plateau followed by a decline, reflecting complete turnover across sites. When sites were analyzed by disturbance level, Chamela's conserved, LES, and EES sites maintained the general pattern of rapid species decline with few species shared across sites (Figure 4C). In Huatulco, perturbed sites showed a diminished but persistent decline for Hymenoptera (Figure 4F).
Simultaneous zeta diversity analysis for both regions revealed fewer shared species between sites compared to within-region analyses (Figure S4E). Among the five most species-rich insect orders, Coleoptera and Diptera had higher species prevalence, whereas Hymenoptera, Hemiptera, and Lepidoptera exhibited steep retention declines, with no species shared across 30 or more sites (Figure S4I). Despite differences in community composition between the two regions, Diptera and Coleoptera exhibited common species shared among all 34 sites, irrespective of disturbance level (Figure S4J).
The prevalence of insect orders—the proportion of sites with the presence of a particular order—varied with disturbance level and TDF region (Figure 3B,C). Significant differences between Chamela and Huatulco were observed in the insect orders associated with each disturbance level, particularly conserved sites. In Chamela, conserved sites were negatively associated with Hemiptera and had a marginally significant positive effect on Lepidoptera (Figure 3B). In contrast, conserved sites in Huatulco showed a negative association with Diptera and a positive association with Hymenoptera (Figure 3C). Perturbed sites in Huatulco had a substantial impact on the insect community, exhibiting a detrimental effect on the five most species-rich orders, except for Coleoptera (Figure 3C). By comparison, sites with different levels of ecological succession (LES, EES) in Chamela showed a detrimental effect on Coleoptera alone while also favoring the presence of Hemiptera and Lepidoptera (Figure 3B).
3.4 Habitat Physical Determinants of Multisite Compositional Turnover
Habitat physical parameters explained compositional turnover more effectively in Huatulco than in Chamela, accounting for 0.5 > R2 > 0.79 and 0.6 > R2 > 0.96 of the variances, respectively (Figure S5A,B). At the local scale (ζ2), where rare species dominate, the compositional variance across all sites in both Chamela and Huatulco was primarily explained by the distance between sites and tree diversity. However, when sites were categorized as conserved or perturbed, canopy coverage emerged as a key driver of community turnover in conserved sites in Chamela, while in perturbed sites in Huatulco microhabitat diversity became more influential than site distance (Figure 5 and Figure S5C,D). At larger zeta dimensions (ζ6–10), where common species dominate, the distance between sites remains a significant driver of assemblage composition except in disturbed sites in Chamela. Tree diversity continues to influence compositional turnover in disturbed sites of both regions, while total deadwood volume and canopy coverage become key determinants in merged sites from Chamela and Huatulco, respectively. This pattern intensifies at even higher zeta dimensions for the merged sites in both regions (ζ12–18), where total deadwood volume in Chamela and canopy coverage in Huatulco emerge as the primary factors driving species turnover (Figure S5C,D).

Changes in total deadwood volume across all sites combined and disturbed sites in Chamela (EES, LEES), as well as changes in microhabitat density and microhabitat diversity for perturbed sites in Huatulco, appear to play a more significant role in driving species compositional turnover when these variables have low values. This is evidenced by sharper increases in turnover (steeper slopes) associated with lower values of deadwood volume, microhabitat density, and microhabitat diversity (Figure 5 and Figure S5C,D).
4 Discussion
A comprehensive understanding of insect community dynamics requires a thorough characterization of species composition. Prior to this study, insect diversity in Mexican TDFs had only been investigated for few individual families and genera of Coleoptera (e.g., Martínez-Luque et al. 2016; Pérez-Flores et al. 2017; Corona-López et al. 2017), Lepidoptera (e.g., Boege et al. 2019; Cuevas-Mendieta et al. 2021), and Hymenoptera (e.g., Rodríguez et al. 2010; Zaldívar-Riverón et al. 2010), mainly focusing on taxonomic issues rather than aspects of distribution, rarity, and sensitivity to habitat alterations. Our study represents the first systematic assessment of insect species diversity at community level in Mexican TDFs, and to our knowledge, the first insect global metabarcoding study of this biome. The data generated provide a baseline for future molecular studies of insect diversity in northern Neotropical TDFs. They were used here to address key questions about the structure of species diversity along axes of spatial and environmental turnover that will help direct conservation efforts.
4.1 Geographic Turnover
At the largest spatial scale, the two sites at Chamela and Huatulco were highly differentiated, with only 18.5% of species shared between regions. This was confirmed by simultaneous zeta diversity analysis of both regions, which further emphasized this divergence, showing a sharp zeta decline with minimal species overlap across sites, underscoring the uniqueness of these insect communities (Figure S4E,I). Limited community similarity was driven by a subset of widely distributed species of mainly Coleoptera and Diptera that were universally present also in each site within the two main areas. These patterns likely reflect the contrasting biogeographical and ecological contexts of the two regions within the Sierra Madre del Sur morphotectonic province. Specifically, the distinct geological histories (Ferrusquía-Villafranca 1998), variation in plant structure and composition (Trejo and Dirzo 2002; Lott and Atkinson 2006; Williams 2009), and differing levels of anthropogenic disturbance (Trejo and Dirzo 2000; Sánchez-Azofeifa and Portillo-Quintero 2011; Flores-Casas and Ortega-Huerta 2019) all may have contributed to the observed divergence. Phylogenetic inferences can now be conducted on the DNA sequences and may help to discriminate between these evolutionary and ecological scenarios, to distinguish the causes of endemism and to develop conservation strategies that should either focus on the preservation of spatial patterns of local differentiation or on maintaining the ecological diversity that may differentiate these sites.
4.2 Temporal Dynamics of Community Turnover
A second striking feature of the diversity pattern was the strong seasonal variation and a very similar trajectory of gradual community turnover in both sites driven by the strong fluctuations in rainfall and temperature (Figure S3A,D). Notably, we observed not only a clear turnover in insect communities between the dry and wet seasons but also a significant monthly turnover in both TDFs, with greater community aggregation occurring during the wetter months (June–October). Metabarcoding studies in various ecosystems have documented temporal insect turnover patterns (Kirse et al. 2021; Klimova et al. 2023; Souto-Vilarós et al. 2024). In tropical biomes, such turnover often mirrors climatic shifts, with diversity peaking during periods of highest precipitation. However, research in TDFs has largely focused on specific taxonomic questions (e.g., Ávalos Hernández 2007; Toledo-Hernández et al. 2015; Corona-López et al. 2017; Martínez-Hernández et al. 2019, 2022), leaving broader community-level dynamics underexplored.
Species richness was higher during periods of elevated temperatures in Chamela and increased precipitation in Huatulco. These seasonal differences likely result from multifactorial influences, including increased resource availability (e.g., plant and prey abundance) and temporal synchronization across trophic levels (Young 1982). Moreover, precipitation drives key ecosystem processes such as primary productivity and organic matter decomposition (Wu et al. 2011; Pace et al. 2021). The current study provides baseline information about the effect of seasonality and the trajectory of individual species making up these complex communities and the ecological processes prevalent during different seasons. In this context, TDFs, with their pronounced seasonal fluctuations in temperature and precipitation, represent ideal systems for investigating how environmental variability drives community turnover across temporal scales and possibly predicts what the climatic factors constraining community composition and survival are, e.g., to predict how climate extremes at particular times and seasons may affect species diversity and loss.
The observed seasonal community turnover in both regions underscores the critical role of climatic factors in shaping biodiversity patterns in TDFs. Understanding these dynamics is essential for conservation, as not all taxonomic and functional groups respond uniformly to climatic fluctuations (Forrest 2016; Wallon et al. 2024). This differential resilience highlights the need to identify which groups are most vulnerable to changes, particularly in the context of climate change, where alterations in precipitation regimes and temperature extremes may disrupt ecological balances (García-Robledo et al. 2016). Insects play diverse functional roles in ecosystems, including pollination and nutrient cycling, among many others (e.g., Klein et al. 2007; Torabian et al. 2024). If certain groups decline or are unable to adapt to changing conditions, the cascading effects on ecosystem functions could be profound. Therefore, comprehensive community-level studies, such as the one performed here, provide invaluable insights for conservation strategies aimed at mitigating the impacts of climate change on TDF biodiversity and maintaining ecosystem services.
4.3 Community Turnover in Response to Anthropogenic Disturbance
Anthropogenic disturbance is a well-known driver of biodiversity change in TDFs (e.g., Johnson and Wedin 1997; Allen et al. 1998; Lawton et al. 1998; Sánchez-Azofeifa et al. 2009). Land conversion for agriculture and grazing has been found to reduce species diversity, deplete nutrients, and diminish vegetative cover (Maass 1995). Conserved sites in Huatulco had higher species richness than disturbed sites, while peak richness in Chamela occurred at sites with intermediate levels of ecological succession (LES sites; Table S4). Moreover, in terms of community composition, disturbance significantly altered insect assemblage structure in both regions, though their community responses differed.
The study site in Chamela is characterized by extensive areas of secondary succession in abandoned agricultural fields, which may serve as important biodiversity reservoirs (Chazdon et al. 2011). Consistent with studies in other tropical ecosystems, our findings highlight the potential of disturbed forests to harbor significant biodiversity. Both successional stages (10–20 years, SES; and 20–30 years, LES) supported high species diversity and a large proportion of species also present in the undisturbed area. The observed pattern also suggests that intermediate disturbance levels may enhance diversity by promoting coexistence among a broader range of species. This is consistent with the intermediate disturbance hypothesis (Connell 1978), which proposes that moderate disturbance supports both generalist and specialist species. However, this finding differs from a previous study in the same region, which reported similar lepidopteran species richness and composition across four successional stages over 8 years (Boege et al. 2019). This suggests that disturbance levels may affect taxonomic and functional groups differently. In Huatulco, the degree of alteration seems to be the primary factor influencing community structure and species richness. Habitat physical and spatial variables explained the compositional turnover more effectively in this region than in Chamela (Figure S5A,B), likely due to more pronounced differences between the conserved and perturbed sites. The disturbed sites in Huatulco are surrounded by agricultural landscapes, which may amplify the impact of anthropogenic pressures on community composition.
The zeta diversity analysis revealed a very high species turnover among sampling sites, likely driven by the presence of rare species, as indicated by the rapid zeta decline and low zeta ratio (Figure 4A–D). A similar pattern was observed when considering only the EES and LES succession sites (Figure 4C), although the limited number of data points is probably insufficient to detect any differences, such as higher homogeneity than in undisturbed sites. The detailed spatial analysis using the zeta diversity framework also showed that turnover among sampling sites fits an all-combined-sites model, indicating no specific spatial structure in community composition across the sampling sites. In sum, these analyses reveal the great conservation value of secondary forest sites, which may be comparable to primary forests in terms of species richness, as reported in other studies (Barlow et al. 2007; Chazdon 2008). Moreover, the high turnover and prevalence of rare species suggest that each sampling site contributes uniquely to the total diversity, highlighting the importance of preserving larger areas to incorporate the full set of species. Maintaining landscape connectivity will, therefore, play a pivotal role in reducing biodiversity loss and supporting ecosystem recovery (Leibold et al. 2004).
The implications of community turnover in disturbed TDF areas require further investigation, as shifts in community composition can alter ecosystem processes such as pollination, decomposition, and trophic interactions (Didham et al. 1996; Steffan-Dewenter 2002). For instance, Hymenoptera, which includes many key pollinators, did not maintain representative species across all perturbed sites in Huatulco. Disturbed areas may also facilitate the establishment of invasive species, reshaping community dynamics and potentially outcompeting native fauna (Hobbs and Huenneke 1992; MacDougall and Turkington 2005). Future research should focus on the long-term effects of disturbance on ecosystem succession, as exemplified by Chamela, and land use change, as observed in Huatulco, particularly their impact on functional diversity, and how they interact with global change drivers like climate change. The scale and intensity of disturbances, as well as their proximity to conserved patches, are critical factors. Thus, maintaining landscape connectivity is crucial for reducing biodiversity loss and supporting ecosystem recovery (Leibold et al. 2004).
Our study highlights the importance of conserving primary forests and managing disturbed areas to preserve biodiversity, especially in tropical regions where anthropogenic disturbance can alter community structure (Barlow et al. 2007; Chazdon 2008). Primary forests serve as irreplaceable biodiversity reservoirs for specialized and rare species (Gibson et al. 2011; Sinclair et al. 2024). Disturbed areas, particularly those undergoing secondary succession, can contribute to conservation by supporting a subset of biodiversity and enhancing landscape connectivity (Chazdon et al. 2011; Arroyo-Rodríguez et al. 2020). Integrated conservation strategies combining primary forest protection with the restoration and management of disturbed habitats are essential to mitigate biodiversity loss and sustain ecosystem processes (Lindenmayer et al. 2008; Chazdon 2014).
4.4 Environmental Determinants of Community Composition
The zeta diversity methodology also allowed us to investigate the specific habitat physical parameters related to habitat structure, including tree abundance and diversity, canopy coverage, and the availability of microhabitats, especially deadwood, among others (Didham and Lawton 1999). A greater proportion of variance was explained in Huatulco, suggesting potential differences in habitat structure or disturbance dynamics between the two regions (Figure S5A,B). Among the variables, distance between sites and tree diversity were the most influential determinants of community turnover, though their relative importance varied across regions and spatial scales (Figure 5A,C). In particular, tree diversity emerged as a key driver in disturbed sites in both Chamela and Huatulco. Meanwhile, total deadwood volume and canopy coverage exerted a more consistent influence when all sites were considered together in Chamela and Huatulco, respectively (Figure S5A,C), indicating that this effect is mediated through the widespread species.
The observed patterns—where distance between sites and some habitat physical parameters were found to be the dominant factors influencing turnover driving across zeta dimensions—remark the multifactorial nature of species turnover at local and regional scales. This finding strongly supports the role of habitat heterogeneity as a key determinant of biodiversity in TDFs, agreeing with previous theoretical frameworks (MacArthur and MacArthur 1961; Stein et al. 2014). Future studies should expand upon this research by incorporating broader environmental gradients (e.g., Basset et al. 2015; Song et al. 2023), conducting experimental manipulations (Haddad et al. 2001), and employing advanced analytical methods such as structural equation modeling (Scherber et al. 2010).
4.5 Understanding the Total Insect Diversity of TDFs
Metabarcoding of Malaise trap samples has become a standard tool for assessing insect diversity, consistently revealing vast numbers of previously unrecognized species, particularly Hymenoptera and Diptera, which are predominantly captured by these traps (Sire et al. 2022; Uhler et al. 2022; Chimeno et al. 2023). We identified over 7000 MOTUs during a 6–7 month sampling period in altogether 34 traps and 218 sampling events in Chamela and Huatulco combined. However, the sample composition was unusual, with Lepidoptera and Coleoptera dominating in species richness—a notable contrast to typical patterns observed in Malaise trap studies. Limited sampling in TDFs complicates broader comparisons, but it is conceivable that this ecosystem differs significantly in species composition from other Malaise sampling campaigns mainly conducted in temperate regions, either due to true variations in biodiversity or behavioral traits of the species inhabiting TDFs. Alternatively, the species composition may be skewed by primer bias in COI sequencing or the limitations of Malaise traps in effectively sampling microhymenopterans, which often represent the most diverse group within this order, though the set of primers selected here has been shown to recover most arthropod taxa in a diverse species assemblage (Elbrecht et al. 2019).
Further uncertainty about the species numbers arises from the use of sequence similarity for both MOTU clustering to approximate the species entities. These metrics are widely used in metabarcoding studies, but ultimately more precise approaches will have to be applied incorporating more robust species delimitation. Likewise, species identifications relied on sequence similarity but can be improved by expert taxonomists if specimens gathered in Malaise traps are imaged before DNA extraction, or if non-destructive methods preserve the physical specimens for taxonomic studies and the production of well documented barcode reference libraries (Creedy et al. 2018; Fujisawa et al. 2023; Sire et al. 2023). At the current state of knowledge, 13.31% of MOTUs already had a match to existing barcode databases, and a slightly higher proportion (14.97%) could be identified to genus level using a conservative 95% similarity criterion. Yet, our preliminary species clustering and taxonomic affiliation provide an approximation of a fully informed evolutionary system, showing the potential of metabarcoding to monitor poorly studied, megadiverse communities, even in the absence of comprehensive reference databases.
Beyond the lack of taxonomic precision, our sampling strategy likely represent only a fraction of the total diversity, as Malaise traps predominantly collect flying species in the understory (Uhler et al. 2022), leaving out ground and canopy-dwelling insects as well as non-flying species. Furthermore, the richness observed here may greatly underestimate the actual flying insect diversity. For instance, we recorded 56 species of Braconidae (Hymenoptera) in Chamela (Table S3), compared to 420 species identified in the region in prior barcoding studies for just three braconid subfamilies (Fernández-Flores et al. 2013; Aguilar-Velasco 2013; Gutiérrez-Arellano et al. 2015). Thus, insect metabarcoding results, particularly in hyperdiverse tropical regions, should be interpreted as temporal and spatial snapshots of community structure rather than definitive species counts. Nevertheless, these data are sufficient to establish the key parameters of community composition and turnover, especially for the common species, and here established the clear seasonal turnover in insect communities in TDFs, the differentiation of distant forest reserves, or the effects of various kinds of disturbance. These results align with an apparent broad ecological pattern observed in previous studies conducted across various seasons and ecological conditions. Specifically, while species richness appears to remain constant, community composition changes significantly, primarily due to the turnover of rare species (Thorn et al. 2020; Sire et al. 2022; Souto-Vilarós et al. 2024).
4.6 Implications for Insect Conservation
The contraction of Neotropical TDFs in recent decades poses major challenges for conserving their insect communities, highlighting the need for a comprehensive understanding of species diversity, ecological dynamics, species interactions, and responses to environmental change. Our findings underscore the critical role of conserved forests in preserving rare insect species and serving as propagule sources for colonizing disturbed areas. Non-managed forests should therefore remain a priority to national and international conservation strategies.
Metabarcoding has proven invaluable for biodiversity assessment, ecological monitoring, and understanding ecosystem functions (Taberlet et al. 2012; Evans et al. 2016; Turunen et al. 2021). When combined with ecological data, this approach becomes a powerful tool for evidence-based conservation, identifying key taxa, prioritizing protection areas, and detecting spatiotemporal patterns relevant to management actions (Taberlet et al. 2012; Ji et al. 2013; Cristescu 2014; Bohan et al. 2017; Ruppert et al. 2019; Steinke et al. 2022).
Our study represents the first comprehensive metabarcoding assessment of insect species diversity in northern Neotropical TDFs, establishing a critical baseline for future studies. Continued biodiversity monitoring in these reserves and surrounding areas will enable detection of community shifts or declines (Hardulak et al. 2020; Steinke et al. 2022). This information can guide adaptive management strategies, optimize resource allocation, and support more effective conservation decision-making (Lahoz-Monfort and Tingley 2018; Liu 2021; Mata et al. 2021).
Author Contributions
A.Z.R., P.B., and A.P.V. conceived the research; P.B., A.Z.R., E.d.V., A.H.L., F.E.Z.H., N.B.B., A.G.B., and E.R.G. participated in fieldwork; P.B., A.G.B., and N.B.B. contributed to sample preparation for DNA extraction and sequencing; P.B., A.G.B., N.B.B., F.E.Z.H., and D.P.Z.R. performed data curation and data analysis; P.B., A.Z.R., and A.P.V. wrote the manuscript. All authors reviewed and approved the final version of the manuscript.
Acknowledgments
We thank Abel Verduzco, Victoriano Godínez, José Eduardo Aragón, and Flávia Joele for their invaluable assistance during fieldwork; Karina Astudillo, Ernesto Samacá, and Leopoldo Islas for helping with the material processing; Abel Verduzco and Rafael Torres for their help with tree species identification; the Aragón family for their hospitality and for allowing us to collect in Cacaluta, Huatulco; Cristina Mayorga for her help with specimen curation; and Evgeny Zakharov for his help during the sequencing process at the CCDB. PB was supported by postdoctoral fellowships awarded by CONAHCyT (now SECIHTI), Mexico (Estancias Postdoctorales por México, convocatoria 2022-1; Estancia Postdoctoral de Incidencia 2024). APV's work on tropical insect diversity is supported by the SITE-100 charitable donation to the NHM. Fieldwork was conducted under SEMARNAT (Dirección general de Vida Silvestre) collecting permit no. SGPA/DGVS/01293/21. This study was funded by grants given by CONAHCyT (now SECIHTI), (convocatoria Ciencia de Frontera 2019; project no. 58548) and PAPIIT-DGAPA-UNAM (convocatoria 2022, project no. IN201622) to AZR.
Conflicts of Interest
The authors declare no conflicts of interest.
Open Research
Data Availability Statement
Raw metabarcoding data is available in GenBank Submission ID: SUB15348385; BioProject ID: PRJNA1269087.




