Environmental DNA Documents Ecosystem-Wide Biodiversity Within the Marine Protected Area Stellwagen Bank National Marine Sanctuary
Funding: This work was supported by Danaher Foundation (747428).
ABSTRACT
Marine protected areas (MPAs) can help ensure long-term conservation of natural resources and protect biodiversity, ecosystem services, and cultural values in the face of anthropogenic change. However, determining MPA effectiveness is often challenging due to the lack of comprehensive baseline data and/or biases associated with biodiversity survey methods. Environmental DNA (eDNA) represents a promising tool to overcome these challenges. Here, we used a suite of three metabarcoding targets—prokaryote-specific 16S, eukaryote-specific 18S, and vertebrate-specific 12S—to generate baseline data of all organisms, from bacteria to whales, within Stellwagen Bank National Marine Sanctuary (SBNMS). Surface water, bottom water, and sediment from 40 sites revealed three archaeal, 46 bacterial, 22 protistan, and 17 metazoan phyla. eDNA offers insight into the spatial resolution of biodiversity within SBNMS, potentially providing a new tool which could inform management practices to protect biodiversity. For vertebrate eDNA monitoring, most species were observed in bottom water, suggesting that less extensive sampling could be sufficient if targeting overall vertebrate richness. However, the inclusion of other sample types revealed patterns in relative sequence abundance that may be indicative of habitat use, particularly for Northern sand lance, a key forage fish. Microbial, phytoplankton, and zooplankton community composition differed dramatically between sample types, requiring all three to adequately capture species richness, providing data for potential indicator species such as those that cause harmful algal blooms. While future evaluations of cost, sampling scope, frequency, and how to incorporate data into management practices are needed, this study offers important baseline information for new hypotheses testing.
1 Introduction
Biodiversity is a key driver of ecosystem function, stability, and productivity (Tilman et al. 2014). In both terrestrial and marine ecosystems, anthropogenically induced climate change has led to increased rates of biodiversity loss (Ramírez et al. 2017; Habibullah et al. 2022), and this is expected to worsen under all potential warming scenarios (Beaugrand et al. 2015). In marine ecosystems, biodiversity loss can lead to decreased stability, potential for recovery, and water quality, with the potential for resource collapse; conversely, biodiversity restoration can increase productivity (Worm et al. 2006; Palumbi et al. 2009). For instance, protecting biodiversity through marine protected areas can enhance fishery stocks by protecting critical larval dispersal corridors (Fontoura et al. 2022), spawning and nursery areas, as well as supporting healthy age structure (Auster and Shackell 2000). This is particularly relevant as over 40% of the global population relies on aquatic animals for more than 20% of their animal protein supply (FAO 2024). As such, the need to protect biodiversity is not only an environmental issue but also one of social and economic importance.
International efforts such as the Global Biodiversity Framework have been established in recognition of the need to protect and monitor biodiversity on a global scale (CBD COP, 2022). Marine protected areas (MPAs) are often established with the goal of protecting and conserving areas of high biodiversity and productivity, as well as areas of historical or cultural significance (Giakoumi et al. 2018). However, the level of management and protection within individual MPAs is highly variable, ranging from fully protected, with no destructive or extractive activities permitted, to minimally protected, with extractive activities such as fishing and dredging allowed (Grorud-Colvert et al. 2021). For example, while 26% of marine waters in the US exclusive economic zone (EEZ) are within an MPA, only 3% of marine waters in the US EEZ are in fully protected (“no-take”) MPAs (Wenzel et al. 2020). Additionally, MPA management plans rarely incorporate strategies to adapt to shifting environmental conditions caused by climate change (Wilson et al. 2020), such as the climate adaptation plan for Greater Farallones NMS (NOAA 2016).
Stellwagen Bank National Marine Sanctuary (SBNMS), located in the Gulf of Maine (GOM) off the coast of Massachusetts, is one of 17 MPAs within the United States National Marine Sanctuary System. Designated in 1992 for both its ecological and cultural importance, the sanctuary is managed by the National Oceanographic and Atmospheric Administration (NOAA) and employs varied levels of protection within its boundaries. While SBNMS prohibits destructive or extractive practices such as waste disposal, drilling, dredging, or taking of any marine mammals, marine reptiles, or seabirds, recreational and commercial fishing are permitted throughout much of the sanctuary. Its mission is to “conserve, protect, and enhance the biological diversity, ecological integrity, and cultural legacy of the sanctuary while facilitating compatible use” (https://stellwagen.noaa.gov/). However, in recent decades, this rich ecological community has been under threat from numerous sectors including human development, fishery exploitation, and climate change (Office of National Marine Sanctuaries 2020; Pershing et al. 2021). The GOM is experiencing rapid and intense changes to both bottom and sea surface temperatures (Seidov et al. 2021; Whitney et al. 2022), reported to be warming faster than 99% of global oceans (Pershing et al. 2015). This warming has been associated with impacts on biodiversity including declines in zooplankton (e.g., Calanus finmarchicus), commercial species such as cod and the American lobster, and protected species such as the Atlantic puffin and northern right whales (Pershing et al. 2021). Additionally, warmer waters are causing northward shifts in species distribution including populations like small crustaceans (Lasley-Rasher et al. 2015) and plankton (Gregory et al. 2009), ultimately impacting local predator–prey interactions (Nye et al. 2013; Kleisner et al. 2017). The 2023 SBNMS Management Plan's Climate Change Action Plan identified the need to “evaluate climate change impacts on sanctuary resources and incorporate changing conditions in management decisions” (NOAA 2023). However, current monitoring efforts within SBNMS mainly focus on keystone or threatened species such as marine mammals and seabirds, and the scope of these monitoring efforts may not be broad enough to identify community-level shifts with widespread ecological implications. Thus, additional monitoring efforts aimed at understanding the broader impacts of these changes and the effectiveness of proposed or implemented management strategies are critical to forecasting and preparing for climate impacts on the distribution, diversity, and abundance of marine organisms.
Evaluating the effectiveness of MPAs such as SBNMS poses many challenges, particularly the lack of baseline knowledge of all species biodiversity within the MPA—from microorganisms to megafauna. The SBNMS Management Plan makes 22 references to biodiversity, highlights the need to increase biodiversity information, and states that the goal is “to develop an improved understanding of the condition of major habitat types within the sanctuary to understand their productivity and biodiversity” (NOAA 2023). Past environmental assessments of SBNMS have focused on the diversity of higher organisms (invertebrate and vertebrate species) (Balthis et al. 2011) using traditional means such as trawling, long line, and visual surveys. Despite their common use, these methods are expensive, time-consuming, and subject to bias (Harmelin-Vivien and Francour 1992; Breen and Ruetz Iii 2006; Kuriyama et al. 2019). For instance, trawling cannot be conducted in certain habitats (i.e., hard rocky bottom or near shipwrecks), limiting sampling locations. These assessment techniques are also impacted by both gear selectivity and species catchability, where certain species are not caught because of their size or ability to avoid the net. Further, biodiversity assessments that use these methods do not historically provide information on microorganisms, which are critically important to ecosystem function. Microorganisms form the base of the marine food web, mediate biogeochemical cycles, and constitute the life support system of the biosphere (Pomeroy et al. 2007), therefore, playing an important role in climate change biology. Shifts in microorganism biodiversity may have dramatic effects on abundance and diversity of higher trophic levels such as zooplankton, fish, and whales (Sumaila et al. 2011; Hollowed et al. 2013). Currently, Stellwagen Bank NMS Condition Reports only include limited microbial biodiversity indices in relation to water quality (e.g., HABs), largely mined from data collected within the larger Gulf of Maine region and/or in adjoining Massachusetts Bay and not comprehensively throughout the anctuary. Given the limitations of traditional methods and the importance of microorganisms, there is a need for comprehensive survey methods that evaluate the full diversity of organisms inhabiting marine sanctuaries.
Environmental samples, such as seawater and sediment, contain not only whole communities of prokaryotes and microeukaryotes but also extra-organismal DNA left behind by organisms (Gilbey et al. 2021). Advances in molecular techniques such as DNA metabarcoding provide alternative means to assess marine ecosystem diversity by utilizing this environmental DNA (eDNA). The use of eDNA is becoming more widespread, with published studies using eDNA monitoring approaches spanning the globe and covering many oceans and seas (Bernos et al. 2023; Capurso et al. 2023; Kelly et al. 2024). The addendum to the SBNMS 2020 Condition Report specifically highlights benthic biodiversity exploration using eDNA to expand the Sanctuary's knowledge of benthic ecology and biodiversity in SBNMS and highlight opportunities for future work and applications (Silva 2021). eDNA provides numerous benefits to biodiversity assessments, including increased taxonomic, temporal, and spatial resolution, improved time and cost efficiency, simple collection and laboratory protocols, and the ability to sample the ecosystem noninvasively (Rourke et al. 2022; Capurso et al. 2023). Additionally, eDNA outperforms many traditional monitoring methods for hidden/cryptic diversity identification, detection of larval and juvenile organisms, detection of rare or invasive species, and the ability to also monitor microbial communities (Feio et al. 2020). However, like all techniques, there are limitations and potential biases associated with eDNA, including potential for cross-contamination, primer bias, and PCR or sequencing errors (Capurso et al. 2023). Such biases must be taken into account when interpreting eDNA results. Additionally, a majority of eDNA studies simply report species presence/absence, with no recommendations of how this could be incorporated into management practices. Sanchez et al. (2022) proposed using the ratio of demerso-pelagic to benthic fish eDNA as an indicator of fishing pressure, simplifying complex data into a single numerical indicator. However, this method requires both metabarcoding and qPCR results, increasing per-sample costs, and has the potential to ignore changes occurring at a species level. The utility of eDNA may lie in its ability to augment, rather than replace, traditional survey methods that measure abundance (Stoeckle et al. 2021) and its application in areas where traditional methods are not feasible. As the use of eDNA metabarcoding assessments within MPAs continues to increase, novel methods for translating complex datasets into simplified metrics for management decision making will need to be prioritized.
Preliminary use of eDNA to assess biodiversity in SBNMS surveyed microbial diversity, both prokaryotic and eukaryotic, as well as a cursory view of higher eukaryotes (Polinski et al. 2019). However, while Polinski et al. (2019) highlighted the unseen diversity of microorganisms not previously reported within SBNMS, the study included only sediment from a small geographic area and provided little insight into fish and marine mammal communities due to sample type and primer bias. Given SBNMS's historical and current use as a valuable fishing ground and whale feeding ground, this study sought to address the question of whether eDNA could be incorporated into current monitoring efforts to provide new information, fill data gaps left by traditional monitoring methods, and augment current understanding of biodiversity within SBNMS. In light of this, we utilized eDNA to document and quantify biodiversity across all taxonomic groups in surface water, bottom water, and sediments collected from 40 sites spanning Stellwagen Bank, the underwater plateau and primary bathymetric feature within SBNMS. As previously done, we targeted prokaryote-specific 16S SSU rRNA and eukaryote-specific 18S SSU rRNA genes to cover a broad taxonomic range. To address the poor resolution for fish and marine mammal communities seen in previous 18S datasets (Polinski et al. 2019), vertebrate-specific 12S SSU rRNA genes were also targeted. Additionally, eDNA counts for species of interest were compared with data from visual surveys that were conducted simultaneously. These data represent a more comprehensive baseline for using eDNA to assess biodiversity, enabling further use of eDNA methods in SBNMS and incorporation of these genomic assessments into management practices.
2 Material and Methods
2.1 Sample Collection
Stellwagen Bank National Marine Sanctuary (SBNMS) has conducted surveys for sand lance, a key forage species (Silva et al. 2021), since 2013. Survey sites (n = 44) spanned northern, central, and southern regions of Stellwagen Bank (Figure 1) and were designed to sample potential sand lance habitat (Silva et al. 2021). During the 2021 summer survey on July 21–23, 2021, additional samples for marine microbiome and environmental DNA (eDNA) analyses were collected from 40 survey sites (Table S1). When possible, surface water, bottom water, and sediment were collected from each site. The U.S. Geological Survey's Seabed Observation and Sampling System (SeaBOSS) (Blackwood and Parolski 2001) was deployed from the R/V Auk at each site. When conditions allowed, video was captured during a 5-min drift that followed each deployment. Surface water was collected from the top 0.2 m using a niskin or bucket at the beginning of the drift period, transferred to a sterilized 0.5 L bottle, and placed on ice until processing. After the drift period, sediment was collected using the Van Veen benthic grab sampler (0.1 m2) on the SeaBOSS. Sediment samples were taken from the top 2 cm of each grab, transferred to sterile 15 mL tubes, and immediately stored on dry ice until returned to the lab, where they were stored at −20°C. Triplicate sediment samples were taken from each grab to account for potential microenvironments known to occur within sediments (e.g., Soltwedel et al. 2020). Sediment subsamples were homogenized by mixing with a sterile spatula within the collection tube. After sediment collection, the grab was emptied into a large container to check for the presence of sand lance. Bottom water samples were collected with a 1.5 L niskin immediately after the SeaBOSS was retrieved, transferred to a sterilized 0.5 L bottle, and placed on ice until processing. Immediately after sampling concluded at each site, 500 mL of surface and bottom water were vacuum filtered through 0.22 μm MCE filters. Water filters were stored on dry ice until transported to the lab. Filtered water for nutrient analysis was collected for a portion of sites and stored at −20°C until analysis. Filtering setups and collection bottles were sterilized between samples by rinsing first with 70% ethanol and then with sterile DI water. Temperature, salinity, and dissolved oxygen readings were measured for the surface and bottom water at all sites using a YSI ProDSS Multiparameter Water Quality Meter.

Visual observations of marine mammals and seabirds were documented by trained observers from the vessel's flying bridge at each site. All observed individuals were counted and identified to species level when possible. Seabirds were counted within 400 m of the vessel for 5 min and marine mammals within 800 m of the vessel for 10 min. Distances were estimated using a hand-held, fixed-interval range finder calibrated using laser range finders and a buoy at a known distance in relation to the horizon (Heinemann 1981).
2.2 Water Nutrient Analysis
Filtered seawater samples were analyzed for ammonium (NH4), nitrate/nitrite (NO23), phosphate (PO4), and silicate (Si) by the University of Maryland Center for Environmental Science Nutrient Analytical Services Laboratory (https://www.umces.edu/nutrient-analytical-services-laboratory) following center practices. Sediment samples were deemed too coarse for sediment chemistry analysis due to the large grain size of sand.
2.3 DNA Extraction
Nucleic acids were extracted from water filters and sediment samples using the Macherey-Nagel NucleoMag DNA/RNA Water kit and Qiagen DNeasy PowerSoil Pro kit, respectively. Extraction of water filters followed the manufacturers' instructions with the modification of using 1 mL of Lysis Buffer MWA1 in the initial lysing step and then splitting lysed cell solution into two reactions to extract and purify DNA and RNA simultaneously. Only DNA was used in subsequent steps within this study. Sediment extraction followed the manufacturer's instructions, using 0.2–0.4 g of sediment from each triplicate sample. Resulting DNA from both sample types was quantified using the broad range dsDNA assay with a Qubit 3.0 fluorometer (Thermo Fisher Scientific).
2.4 Metabarcoding Library Prep and Sequencing
The v4 region of the 16S SSU rRNA gene was targeted using the 515F and 806R primers to assess total prokaryotic diversity (Apprill et al. 2015; Parada et al. 2016). Total eukaryotic diversity was targeted with F04 and R22 18S SSU rRNA primers (Blaxter et al. 1998; Fonseca et al. 2010), and the vertebrate-specific primers targeted the v5 region of the 12S gene (Riaz et al. 2011). All primers were modified to include a linking sequence for addition of Illumina adapters and indices at their 5′ end. All metabarcoding primer sequences are provided in Table S2.
PCR reactions for 16S and 18S consisted of 25 μL 1X OneTaq Master Mix with Standard Buffer (New England Biolabs) with 0.2 μM forward and reverse primers. Water samples were standardized to 5 ng input DNA. Sediment samples were standardized to 10 ng input. PCR reaction conditions were as follows: denaturation for 3 min at 94°C, followed by 25 cycles of 94°C for 30 s, annealing at the appropriate temperature for 30 s, and 68°C for 30 s, with a final extension at 68°C for 5 min. Annealing temperatures were 62°C and 60°C for 16S and 18S reactions, respectively. Products were checked via gel electrophoresis on a 1% agarose gel with GelRed nucleic acid stain (MilliporeSigma), and a 1X cleanup with PCRclean DX beads (Aline Bioscience) was performed to remove excess primer.
12S reactions consisted of 50 μL containing 1.25 units AmpliTaq Gold 360 DNA polymerase (Applied Biosystems), 1X AmpliTaq Gold buffer, 2 mM MgCl2, 200 μM dNTPs, and 0.5 μM each forward and reverse primers. Reactions contained 7.5 ng and 15 ng of starting material for water and sediment samples, respectively. Reaction conditions were as follows: denaturation for 1 min at 95°C, followed by 35 cycles of 95°C for 30 s, 55°C for 30 s, and 72°C for 1 min, with a final extension at 72°C for 10 min. A 1.8X cleanup was performed with PCRclean DX beads prior to checking products on a 2% agarose gel with GelRed.
A second PCR reaction was performed on all samples to incorporate unique dual-index barcodes for multiplexing and adapters for Illumina sequencing. Each 20 μL reaction contained 5 μL cleaned PCR product in 1X OneTaq Master Mix with 0.2 μM each forward and reverse barcoded sequencing primer. Denaturation at 94°C for 3 min was followed by seven cycles of 94°C for 30 s, 60°C for 30 s, and 68°C for 30 s, with a final extension at 68°C for 5 min. A 1X PCRclean DX bead cleanup was performed to remove excess primer and primer dimer. Concentrations of adapter-ligated library were determined via qPCR using the NEBNext Library Quant Kit for Illumina (New England Biolabs), and samples for each target region were pooled in equimolar concentrations. Paired-end 2 × 300 sequencing of 16S and 18S library pools used Illumina MiSeq v3 reagents, with each library pool sequenced separately. Paired-end 2 × 150 sequencing of the 12S library pool used Illumina MiSeq v2 reagents.
2.5 Analysis of 16S and 18S Metabarcoding Data
Prokaryotic 16S and eukaryotic 18S data were processed separately but following the same analysis pipeline. Trimmomatic v0.39 (Bolger et al. 2014) was used to trim primers (HEADCROP:15), crop low-quality bases from read ends (CROP:200 for 16S, CROP:220 for 18S), and quality-trim cropped reads (TRAILING:15) with a minimum read length of 150 bp for 16S reads and 200 bp for 18S. Amplicon sequence variants (ASVs) were called using USEARCH v11 (Edgar 2016). Read pairs were merged, filtered with a maximum expected error of 1.0, dereplicated, and denoised using the unoise3 algorithm. Merged reads were then mapped to the denoised ASVs to generate a count table. The classify.seqs command in mothur v1.48.0 (Schloss et al. 2009) was used to assign taxonomy to ASVs. 16S ASVs were classified with the SILVA v138.1 database. 18S ASVs were first classified with PR2 v5.0.0, and unclassified sequences were also compared with SILVA v138.1 to classify non-protist sequences. Count tables, taxonomy results, and metadata were used as input into the R package phyloseq for downstream analyses (McMurdie and Holmes 2013). All code used to generate ASV count and taxonomy tables is available for reference on the project GitHub repository (https://github.com/jmpolinski/SBNMS-2021_Biodiversity). The relative abundances of reads are presented in subsequent results and displayed as the total percentage of all reads in each sample type.
2.6 Analysis of 12S Metabarcoding Data
FastQ files were processed using nf-core/ampliseq (v2.8.0) (Straub et al. 2024) from the nf-core workflows (Ewels et al. 2020) within reproducible software environments [singularity v3.10.3; Bioconda (Grüning et al. 2018), Biocontainers (Da Veiga Leprevost et al. 2017)]. Data quality was assessed with FastQC (Andrews 2010) and viewed using MultiQC (Ewels et al. 2016) to choose optimal trimming parameters. Cutadapt (Martin 2011) was used to trim primers and DADA2 (Callahan et al. 2016) was used to examine reads as a pseudo pool (sample_inference:pseudo). DADA2 further trimmed reads with a minimum of 100 bp length (trunclenf: 100; trunclenr 100), minimum quality of 25 (trunc_qmin:25), and < 2 expected errors (max_ee:2). Reads pairs were then merged prior to removal of PCR chimeras, then predicted ASVs were filtered to a length of 100–115 (min_len_asv:100; max_len_asv:115). The resulting 158 predicted ASV sequences were compared to three databases to identify ASVs to species level: GMGI-12S database (v1; Zenodo DOI: 10.5281/zenodo.13909873), MitoFish (https://mitofish.aori.u-tokyo.ac.jp/), and NCBI nt using ncbi-blast+ (v2.13.0)—and the blastn function with 98% or 100% sequence identity (perc_identity:98 for GMGI; 100 for MitoFish and NCBI) and 95% query cover (qcov_hsp_perc:95). The GMGI-12S database was adapted from Stoeckle et al. (2021) by progressively adding new vertebrate sequences that have since entered the public domain and are commonly detected in New England, including sequences from species included in the 2010 SBNMS Management Plan and Environmental Assessment (Appendix J) (NOAA 2010). Of the 160 vertebrates in the SBNMS Management Plan species list, sequences were available for 144. Taxonomic identification was performed using a hierarchical approach that accepted blast hits from GMGI, then MitoFish, and finally NCBI. Reads less than 0.1% of total ASV reads per sample were removed. Read counts from triplicate sediment samples from each site were combined and all ASV counts were collapsed by species name.
2.7 Statistical Analyses
For each dataset (16S, 18S, and 12S), triplicate sediment sample counts for each site were combined, and count tables were subsampled to even sequencing depth using the command rarefy_even_depth from the phyloseq package in R for the following statistical tests performed in R. Community Bray–Curtis dissimilarity matrices were analyzed with a PERMANOVA test using adonis2 from the vegan package (v2.6–4) and pairwiseadonis2 from the pairwiseAdonis package (v0.4.1) to assess region and sample type differences. Alpha diversity metrics (species richness and Shannon indices) were calculated using the BiodiversityR package (v2.16.1) on ASV count tables. Type III ANOVA and Tukey Post hoc statistical tests from the car package (v3.1–2) were used to assess significant effects of sample type and bank region for both biodiversity and environmental data. Code to perform all statistical tests is available on the project GitHub, and results of all statistical tests are in Table S3.
3 Results
3.1 Biogeochemistry
YSI environmental data (temperature, salinity, dissolved oxygen, and depth) and dissolved nutrients (NH4, NO23, PO4, and Si) for each site are reported in Tables S1 and S4, respectively. Surface waters were significantly warmer (19.92 ± 0.55 vs. 8.83°C ± 0.79°C; average ± standard deviation), less saline (31.38 ± 0.26 vs. 32.19 ± 0.09 PSU), and had lower levels of dissolved oxygen (8.77 ± 0.18 vs. 9.09 ± 0.18 mg L−1) than bottom waters, respectively (ANOVA type III: p < 0.05; Tables S1 and S3, Figure 2). Surface waters collected in the northern region (31.12 ± 0.14 PSU) were also significantly less saline than either central (31.67 ± 0.05 PSU) or southern (31.48 ± 0.13 PSU; ANOVA type III: p < 0.0001; Tables S1 and S3, Figure 2). Similarly, dissolved oxygen levels in the surface were significantly lower (ANOVA type III: p < 0.001) in the south (8.61 ± 0.19 mg L−1) than in the central (8.81 ± 0.10 mg L−1) or north (8.88 ± 0.08 mg L−1; Tables S1 and S3, Figure 2). Dissolved nutrient concentrations were below detection limits for NH4, PO4, and Si except for two surface stations which displayed relatively high levels of ammonium (S4 and S14, 5.00 and 6.42 μM as N, respectively; Table S4). Lastly, dissolved NO23 levels were higher in bottom waters (3.10 ± 1.05) than surface (1.02 ± 0.48) but these were not significantly different (ANOVA type III: p > 0.05; Tables S3 and S4).

3.2 Prokaryotic 16S Diversity
After trimming and merging, 30,883 ASVs were identified in 7,678,157 16S reads (Table S5). Significant differences in prokaryotic community composition were seen between sample types (PERMANOVA: p = 0.001; Figure 3A; Table S3). Additionally, species richness and Shannon diversity differed by sample type (ANOVA type III: p < 0.001 and p < 0.001, respectively; Table S3), increasing from surface water to bottom water to sediment (Figures 3B, 4). Water samples were dominated by ASVs in the class Alphaproteobacteria, making up 51.7% of all surface water reads and 41.7% of all bottom water reads (Figure 4). In surface water, additional abundant taxa included Cyanobacteria (18.0%), Gammaproteobacteria (15.2%), Bacteroidota (8.5%), Verrucomicrobiota (2.1%), and Planctomycetota (1.6%) (Figure 4). Archaea were more abundant across all bottom water samples, including Crenarchaeota (15.8%) and Thermoplasmatota (4.3%), as well as bacterial taxa Gammaproteobacteria (14.7%), Bacteroidota (8.3%), Cyanobacteria (4.2%), Planctomycetota (3.0%) (Figure 4). Overall, sediment samples were dominated by Gammaproteobacteria (25.4%), Planctomycetota (23.5%), Crenarchaeota (17.7%), Bacteroidota (6.4%), Acidobacteriota (4.2%), and Alphaproteobacteria (3.8%) (Figure 4).


Region also significantly affected bacterial community composition (PERMANOVA: p = 0.026; Table S3), and there was a significant interaction between sample type and region (PERMANOVA: p = 0.029; Table S3). To examine the effects of location, surface water, bottom water, and sediment results were subset and tested separately. In surface water, prokaryotic communities differed across the three regions of the bank (pairwise PERMANOVA: p = 0.001 north vs. central; p = 0.001 north vs. south; p = 0.003 south vs. central; Table S3). In contrast, only the central and southern regions differed in bottom water (Pairwise PERMANOVA: p = 0.048 central vs. southern; Table S3), although the northern region included only three sites from which bottom water was collected. Sediment communities in the southern region differed significantly from both northern and central regions (pairwise PERMANOVA: p = 0.001 and p = 0.024, respectively), but northern and central regions did not differ from one another (Table S3).
3.3 Eukaryotic 18S Diversity
From the 13,615,971 18S reads passing initial trimming and merging, 8292 ASVs were identified (Table S5). Eukaryotic community composition, based on 18S read abundances, differed significantly across sample types (PERMANOVA: p = 0.001; Table S3), as did species richness and Shannon diversity (ANOVA type III: p < 0.001 and p < 0.001, respectively; Table S3, Figure 3C,D). Shannon diversity increased from surface to bottom to sediment (Figure 5), as did the number of taxa unique to a particular sample type (Figure S1).
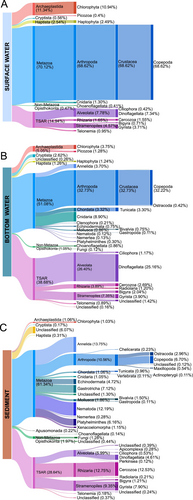
Due to the widely varied gene copy number of 18S across species (Martin et al. 2022), these data cannot be considered accurate representations of abundance within a sample. Therefore, all reported abundances are sequencing read abundance, and comparisons are only made across sample types and regions of the bank. All sample types were dominated by reads assigned to metazoan taxa, comprising 70.12% of all reads in surface water samples, 51.08% in bottom water, and 61.34% in sediment (Figure 5). However, dominant metazoan taxa at lower classification levels differed greatly between sample types. Copepod reads made up 97.8% of the metazoan reads and 68.62% of all surface water sequencing data (Figure 5A) but decreased to 63.07% of metazoan reads (32.22% all reads) in bottom water samples (Figure 5B) and only 10.92% of metazoan reads (6.70% all reads) in sediment samples (Figure 5C). Cnidaria were most abundant in bottom water, representing 8.90% of all reads (Figure 5B), and less abundant in surface water and sediment, with 1.30% and 1.05% of reads assigned, respectively (Figure 5A,C). In addition to Arthropoda and Cnidaria, bottom water and sediment also contained sequences assigned to the metazoan taxa Annelida, Chordata, Mollusca, Echinodermata, Platyhelminthes, Ctenophora, Nemertea, and Nematoda (Figure 5B,C). Additionally, sediment samples contained Gastrotricha, Platyhelminthes, Echinodermata, and Xenacoelomorpha (Figure 5C). The distribution of sequences across the metazoan phyla was more even within sediment samples than in water samples, and sediment exhibited the highest diversity at lower taxonomic levels.
Following metazoan taxa, reads assigned to the TSAR (Telonemia, Stramenopila, Alveolata, Rhizaria) supergroup were the second most abundant in all sample types (Figure 5). Within TSAR, Alveolata protists (mostly dinoflagellates) were most abundant in water samples, making up 7.78% and 26.40% of total reads in surface and bottom water, respectively (Figure 5A,B). Within the dinoflagellates, Tripos was the most abundant genus, representing 33.78%, 21.77%, and 4.66% of total reads in surface, bottom, and sediment, respectively. In addition, several harmful algal bloom (HAB) species common to the GOM were detected, including Alexandrium (only in sediments), Dinophysis (in surface and bottom waters), and Karlodinium (Figure S2). Rhizaria protists were most abundant in the TSAR supergroup within sediment samples (12.75%, Figure 5C) and stramenopiles were variable across the sample set (Figure 5). Within the stramenopiles, the HAB species Pseudo-nitzschia was present across all sample types with low abundance (0.40% and 7.6%) of total diatom reads in surface and bottom waters, respectively (Figure S3). In addition to Metazoa and TSAR, a small percentage of reads (< 1.5%) were classified as Archaeplastida, Cryptista, Haptista, Choanoflagellata, Fungi, and Telonemia (Figure 5). Chlorophyta were found in all sample types with decreasing abundance from surface water (10.94%), bottom water (3.75%), and sediment (1.03%; Figure 5). A similar pattern was observed for Haptista protists, making up 2.54% of surface water reads, 1.26% of bottom water, and 0.31% of sediment (Figure 5).
Significant differences in 18S community composition were also seen between bank regions (PERMANOVA: p = 0.002; Table S3). When sample types were subset and tested separately, significant differences were seen between regions of the bank in both surface water (PERMANOVA: p = 0.001) and sediment (PERMANOVA: p = 0.01), but not in bottom water prokaryotic communities (PERMANOVA: p > 0.05; Table S3). Like the prokaryotic datasets, it is of note that bottom water samples were only available for three sites in the northern region. In surface water, communities from the southern region differed from both central and northern regions (pairwise PERMANOVAs: p = 0.003 and p = 0.001, respectively; Table S3). Significant differences were only observed between the southern and northern regions in sediment communities (pairwise PERMANOVA: p = 0.004; Table S3). Species richness differed significantly in surface water from the central region compared to northern and southern regions (pairwise PERMANOVA: p = 0.043 and p = 0.0012, respectively; Table S3), but was not significantly different for any other sample type or for the dataset as a whole (PERMANOVA: p > 0.05, Table S3).
3.4 Vertebrate 12S Diversity
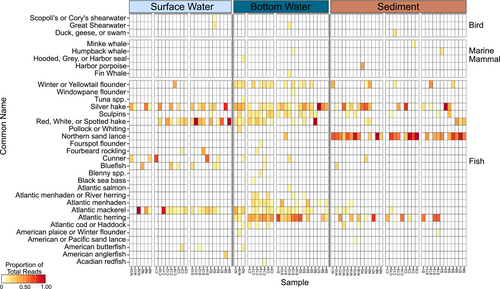
12S metabarcoding resulted in 9,589,313 filtered reads and 158 ASVs total (Table S5), with 9,587,715 reads (99.99%) and 143 ASVs (91.14%) that were taxonomically assigned to 41 unique species based on GMGI-12S (v1), MitoFish, and NCBI. Due to SBNMS's proximity to land and presence of active shipping channels, 2,393,912 reads and 64 ASVs were removed with taxonomic assignment as human or terrestrial vertebrates (Figure S4). Of the remaining 79 ASVs (7,276,328 reads total), there were 25 teleost fish species (81 ASVs; 7,136,659 reads), five marine mammals (eight ASVs; 92,313 reads), and three birds (5 ASVs; 47,356 reads). After filtering, the most common organisms were Northern sand lance (Ammodytes dubius; 17.83% ± 3.02% of each sample), Silver hake (Merluccius bilinearis; 15.19% ± 2.41% of each sample), Atlantic herring (Clupea harengus; 11.92% ± 2.17% of each sample), and red, white, or spotted hake (Urophycis sp.; 8.68% ± 2.08% of each sample). The remaining 29 species each comprised < 5% of each sample.
Bottom water samples contained significantly higher species richness than surface water and sediment (ANOVA Type III: p < 0.0001; Table S3; Figure 3F), with 27 out of 34 total species found and 12 of those unique to bottom water. Within bottom water samples, the southern region displayed significantly lower species richness compared to northern and central regions (ANOVA Type III: p = 0.0012; Table S3; Figure 3F) in contrast to no significant regional differences in the surface water samples (ANOVA Type III p = 0.189; Table S3; Figure 3F). In the sediment, the northern region had significantly more species richness than the central region (ANOVA Type III: p = 0.028; Table S3; Figure 3F). Sediment samples were dominated by the most abundant species groups, particularly A. dubius, M. bilinearis, and C. harengus (Figure 6). Like the sediment species composition, surface water samples displayed low species richness and were dominated by species of Hake, Atlantic mackerel (Scomber scombrus), Cunner (Tautogolabrus adspersus), and Bluefish (Pomatomus saltatrix). Five species of marine mammals were seen across bottom water and sediment samples, including Minke whale Balaenoptera acutorostrata), Humpback whale (Megaptera novaeangliae), Harbor porpoise (Phocoena phocoena), Fin whale (Balaenoptera physalus), and a taxonomic hit to either hooded, gray, or harbor seal (Cystophora cristata, Halichoerus grypus, or Phoca vitulina). Four species of birds were seen across all sample types, including Shearwaters (e.g., Ardenna gravis, Ardenna griseus, and Calonectris sp) and duck, geese, or swan (Anatidate sp). Overall, species composition was significantly different by sample type (PERMANOVA: p = 0.001; Table S4; Figure 3E) and within the bottom water samples, the central region was significantly different from both the southern and northern sites (Pairwise PERMANOVA: p = 0.001; Table S4).
3.5 Visual and eDNA Results for Species of Interest
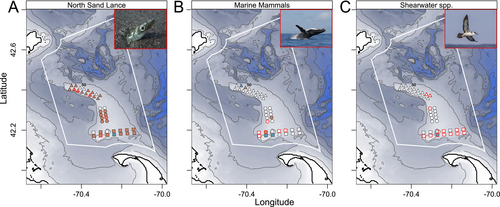
Sand lance, including adults and young of year, were visually identified at five sites—S13, C4, N11, N14, and N15 (Table S6, Figure 7A). 12S sequences identified sand lance presence in sediment from 26 sites, including the five sites where sand lance were visually identified, but were only identified via eDNA in bottom water at S11 (Figure 7A). Humpback whales were visually identified at sites S1 and C10 (Table S6, Figure 7B) but were only detected in eDNA from sediment collected at S2 (Figure 7B). Minke whales were observed at sites S2, S4, S5, and S14 (Table S6, Figure 7B) but only via eDNA in sediment at site C4 (Figures 6 and 7B). Fin whale sequences were detected in eDNA from bottom water at site S12 (Figures 6 and 7B) but were not visually observed (Table S6). eDNA detected seal DNA in bottom water from N2 (Figures 6 and 7B), although a gray seal was only visually observed at site S8 (Table S6). Lastly, eDNA detected harbor porpoise in sediment from N3 (Figures 6 and 7B), but no porpoises were visually recorded at any sites (Table S6). Great and Cory's Shearwaters were visually identified at 14 and two sites, respectively (Table S6; Figure 7C), while eDNA identified both species of shearwater only in surface water at site S3 and Great shearwater in bottom water at site S12 (Figures 6 and 7B).
4 Discussion
The intrinsic value of biodiversity is becoming recognized on a global scale, with international initiatives such as “30 × 30” (CBD COP, 2022) setting ambitious goals for the establishment of protected areas. However, MPA establishment has little impact without proper management and monitoring (Gignoux-Wolfsohn et al. 2024; Pike et al. 2024). As more MPAs are established, development of cost-effective methods of assessing MPA success is critical. eDNA is one such promising tool. While the use of rRNA gene sequencing to characterize marine microbes in environmental samples has been commonplace for several decades (e.g., Giovannoni et al. 1990), the utility of eDNA to assess diversity of higher organisms is a relatively new, rapidly developing application (Thomsen et al. 2012; Chavez et al. 2021). eDNA in biological monitoring has proven useful for detecting small or elusive species as well as providing early warning of nonindigenous or otherwise harmful species (Ruppert et al. 2019). It can also complement more conventional methods by providing insight into the presence of rare species within an environment or providing greater taxonomic resolution (Deiner et al. 2017). Further, eDNA is a valuable tool for detecting target species in hard-to-access environments, such as the deep-sea (Everett and Park 2018). Additionally, although a majority of eDNA studies focus on a single organism or taxonomic group of organisms, the ability to target multiple taxonomic groups using a suite of metabarcoding primers on a single sample distinguishes eDNA from traditional diversity assessment techniques. Here, a suite of three primer sets targeted a broad taxonomic range to assess biodiversity, from the smallest microbe to the largest whale, and showed the utility of eDNA as a monitoring tool that spans the tree of life and provides insights at all trophic levels.
4.1 Utilizing eDNA to Monitor Vertebrate Species
This first evaluation of vertebrate diversity in SBNMS using eDNA has provided essential baseline data for three environmental sample types, generating species distribution information and sample type considerations to guide future evaluations. The SBNMS Management Plan lists 160 vertebrates of interest, with over 80 fish, 22 marine mammals, 53 birds, and two sea turtles (NOAA 2010). The current study captured 33 of the 144 species with genetic references available in a single sampling period, representing organisms across numerous habitats, size classes, functional groups, and trophic levels that would be impossible to sample using any single traditional gear. The fish species detected in SBNMS using eDNA largely overlap with those detected in seasonal bottom trawls from 1970 to 1994, with 22 out of 25 eDNA fish species also detected in trawls (Auster et al. 2001). Fish species uniquely detected by eDNA were bluefish and tuna (pelagic or fast-swimming species that are not effectively sampled by a bottom trawl) and Atlantic salmon (a rare species). In addition to fish, 12S eDNA detected five species of marine mammals and three bird species that are also not typically vulnerable to bottom trawling gear.
A vast majority of vertebrate diversity was captured in bottom water samples, suggesting that a less extensive sampling design that includes only bottom water sampling may suffice for future evaluations that aim to capture as many vertebrate species as possible using one sampling method. This difference in biodiversity aligns with traditional survey findings that show the number of benthic fishing gears deployed in the GOM far outnumber the pelagic fishing gears (Stevenson et al. 2004), reflecting that teleost fish biomass in SBNMS is concentrated at the benthos. Cells and other organic matter settling to the bottom may also contribute to seeing the highest diversity in bottom water samples, particularly in the case of pelagic fish such as herring and mackerel. Sequencing reads and detections were more abundant in the surface water samples than bottom for only a few species like Bluefish and Atlantic mackerel that are typically associated with surface waters. In most cases where a species was only detected in the bottom or the surface water, it was detected at the bottom and usually was a benthic species with examples including Windowpane flounder (Scophthalmus aquosus), Fourspot flounder (Hippoglossina oblonga), Black sea bass (Centropristis striata), and Acadian redfish (Sebastes fasciatus), among others. In addition to habitat preferences of benthic species, the higher diversity seen in bottom water may reflect physical processes not measured here. Significantly colder bottom water and lower UV radiation may result in better preservation of extracellular DNA in bottom water, resulting in higher detections (Strickler et al. 2015). Additionally, unlike eDNA data for microbial and planktonic communities that originate from viable cells and whole organisms, the diversity recovered in 12S datasets originates largely from extracellular DNA or cells shed from organisms that eventually sink from the surface to bottom waters. However, while a single sample type captured the most diversity collectively, the inclusion of different sample types at each site revealed patterns in relative sequence abundance that may be indicative of habitat use.
The greatest number of 12S sequencing reads were assigned to Northern sand lance (Ammodytes dubious) among the vertebrate organisms detected, particularly in the sediment samples. The abundance of sand lance reads in the sediment samples could be influenced by a high primer-binding efficiency for the Northern sand lance sequence, but likely originates from a large quantity of sand lance DNA in the environmental samples relative to other species. Within SBNMS, the Northern sand lance is a key forage fish for commercial fish, mammals, and birds, including whales and shearwaters (Silva et al. 2021). Sand lance surveys are conducted in SBNMS annually to understand fluctuations in abundance and distribution and predator–prey interactions (Silva et al. 2021). The use of eDNA in sand lance monitoring was recently demonstrated in the Strait of Georgia, British Columbia, showing intertidal habitat use with potential utility in validating geospatial predictive models (Huard et al. 2022) in a logistically simple way (Robinson et al. 2022). When monitoring sand lance in SBNMS using eDNA metabarcoding, detections were distributed across all regions of the bank, including many sites where sand lance were not visually observed. Nearly all detections were limited to sediment samples, with only one detection in the bottom water and none in the surface water. Sand lance activity and movement vary throughout the day, with individuals exhibiting diel vertical migrations by moving up in the water column to feed during daytime and back down to benthic habitats at dusk (Friedlaender et al. 2009; Hazen et al. 2009). In addition, Northern sand lance activity varies seasonally, with individuals burrowing into the sediment for a dormant phase in the late summer for gonadal development (Suca et al. 2021). These dramatic shifts in activity both daily and seasonally may impact detection rates of eDNA for sand lance and certainly contribute to the results observed here, with most sand lance eDNA detections observed in sediment samples which were collected during the day in late July, likely during a burrowing phase. These results highlight the potential for eDNA to improve upon traditional survey methods, as well as the need for further sampling in SBNMS across all seasons to adequately understand the distribution and dynamics of sand lance, a critical prey source for the health of this MPA.
SBNMS is an established feeding ground for many marine mammal and seabird species, including protected and endangered species such as the North Atlantic right whale (Eubalaena glacialis) and Great shearwater (Ardenna gravis). Although several marine mammal species and Great and Cory's shearwater (Calonectris borealis) were detected with eDNA, these detections did not align well with visual observations. All marine mammal eDNA detections occurred in bottom water and sediment, with no detection in surface water. Shearwaters were only detected in two eDNA samples, one surface water and one bottom water. These discrepancies between eDNA and visual observation may be due to proximity. Observers recorded all sightings within 400 m and 800 m for seabirds and marine mammals, respectively. Distance from where surface water samples were collected, animal behavior, shedding rates, environmental factors such as temperature or pH, dispersion due to water movement, and, for seabirds, lack of contact with the water likely affected the presence of cells and extracellular DNA in the small parcel of sampled water. Similar to this study, Closek et al. (2019) found that visual marine mammal surveys outperformed eDNA metabarcoding in terms of the number of species detected. Marine mammals are generally rare in comparison to pelagic fish, and a higher abundance of eDNA from other vertebrate species in the sample would have affected the ability to detect low abundance sequences. A review of studies utilizing eDNA for marine mammal monitoring found that eDNA metabarcoding produces inconsistent detections when compared to traditional methods (Suarez-Bregua et al. 2022). More sensitive eDNA applications, such as qPCR, may be more appropriate for monitoring marine mammals and seabirds than metabarcoding, as the presence of other more abundant species will not affect the likelihood of detection. However, it is encouraging that despite these challenges, visually observed species were detected within the sample set.
Taken together, the vertebrate biodiversity results presented here highlight the value of eDNA for detecting a wide variety of species from forage fish like Atlantic herring, Atlantic menhaden (Brevoortia tyrannus), and American butterfish (Peprilus triacanthus) to commercially important predators like tuna (Thunnus spp.), flounder species, and Atlantic cod (Gadus morhua) and Haddock (Melanogrammus aeglefinus), along with the detection of rare and protected endangered species like Atlantic salmon (Salmo salar) and Fin whale. While the current evaluation of SBMS biodiversity using eDNA is limited to presence-absence interpretations, eDNA metabarcoding methods are continuing to evolve rapidly, indicating that reproducible quantitative metabarcoding is on the horizon (Shelton et al. 2023). Applying eDNA metabarcoding methods has been instrumental for understanding the baseline and distribution of biodiversity in SBNMS, but quantitative metabarcoding will expand the application of eDNA for macrofauna by providing abundance estimates for many species, potentially transforming the way MPAs are monitored in the future.
4.2 eDNA's Utility for Monitoring Microbial and Planktonic Organisms
The development of microbial indicators (e.g., monitoring changing biodiversity metrics or community composition, species related to wastewater contamination, species associated with eutrophication, etc.) could provide valuable insights into environmental quality (Caruso et al. 2016), which managers could incorporate into current assessment metrics. However, a lack of historical baseline data due to the exclusion of microbial and planktonic communities in traditional biodiversity survey methods presents an additional challenge studies like this seek to overcome.
Effective utilization of eDNA requires sampling at spatial and temporal scales that are appropriate for the targeted community or desired question to be answered, and these can differ dramatically between macro- and micro-organisms. Given that small-scale heterogeneity and microenvironments can play large roles in prokaryotic community composition (Stocker 2012), it is unsurprising that even at high taxonomic levels all three sample types were required to capture a full picture of the microbial communities on Stellwagen Bank. Differences seen in the surface and bottom water prokaryotic communities are likely a function of the environment. For instance, salinity is one of the main environmental factors defining the structure and function of aquatic organisms, with coastal waters having highly variable salinity gradients (Crump et al. 2004). In the current study, we observed significantly less saline waters in the northern section with subsequent corresponding significant differences in surface prokaryotic community diversity, suggesting even small changes in salinity may be an important factor here. Similarly, changes in salinity can create density gradients which can influence organism and eDNA dispersal (Jeunen et al. 2020) which may be driving variations between surface and bottom waters. Although water nutrients did not differ significantly between surface and bottom, photosynthetic organisms such as cyanobacteria were more abundant in surface waters, which were significantly warmer than bottom water and receive higher photosynthetically active radiation (PAR). It is notable that the microbial diversity seen in the sediment samples here is lower than what has been previously reported (Polinski et al. 2019), likely due to the bank's predominantly sandy bottom while the previous study sampled an area of the sanctuary with a muddy bottom. Sediment texture or permeability plays a large role in bacterial species richness in marine sediments, with highly permeable substrates such as coarse-grain sand exhibiting the lowest richness and impermeable sites the highest (Probandt et al. 2017; Aldeguer-Riquelme et al. 2022). Additional sampling across all bottom types within SBNMS is likely required to fully characterize benthic microbial communities.
Like the prokaryotic (16S) diversity, all sample types were required to capture a wider scope of eukaryotic (18S) diversity that included microeukaryotes, phytoplankton, zooplankton, and some macrofauna. Calanoida copepod abundance has been reported to increase between June and August in the Gulf of Maine (Manning and Bucklin 2005), and the eukaryotic (18S) data for surface water samples in this study were dominated by sequences identified as copepod. Observed diversity increased from shallow to bottom water, as copepod abundances decreased, and the highest diversity was seen in sediment, where protist diversity increased and sequences identified as metazoan originated from a wider range of benthic invertebrates, including echinoderms, mollusks, cnidarians, and crustaceans.
A targeted search for harmful algal bloom (HAB) species in the 18S dataset was conducted, due to the continued threat HABs present within coastal waters of the US (Anderson et al. 2021) as well as much of the world (Hallegraeff et al. 2021). As many current MPAs occur within 100 nautical miles of the coast, the negative impacts of these events can have consequences for biodiversity within MPAs. For instance, HAB events in California MPAs have impacted abalone and Dungeness crab fisheries (Murray and Hee 2019) and have been implicated in fish communities shifting to being dominated by planktivorous fish (Ziegler et al. 2023). Although not always specified, monitoring for harmful algae is considered a part of water quality monitoring within MPAs and within condition reports. Here, we specifically searched eukaryotic (18S) ASVs for regionally important HAB species within the Gulf of Maine, including Pseudo-nitzschia, Alexandrium, Dinophysis, Karlodinium, and Margalefidinium. Of these, detections of Alexandrium were only found in sediment samples, potentially representing cyst beds which are often monitored for bloom prediction (Anderson et al. 2005), with generally higher relative abundance in the south region. Dinophysis was only found in one bottom water sample, while Karlodinium was only found in one surface sample. Pseudo-nitzschia was detected in all sample types with most detections in bottom water. In addition, Clostridium, an anaerobic bacterium used to trace wastewater effluent influence in sediments (ISO 2013), is monitored within SBNMS due to the location of a sewage outfall 12 nautical miles from the western boundary. Within the prokaryotic (16S) dataset, only minimal detections were observed, all < 16 read counts, confirming prior observations of limited to declining risk within Sanctuary waters (Office of National Marine Sanctuaries 2020).
4.3 Considerations for eDNA Metabarcoding Biodiversity Assessments
While eDNA can provide a more holistic view of an ecosystem's biodiversity than traditional methods, there are still many challenges and limitations associated with this relatively new method. For example, the lack of fish diversity seen in the eukaryotic 18S dataset compared to the vertebrate-specific 12S results highlights the importance of primer selection and the benefits of using multiple targets when designing eDNA biodiversity studies. While the use of a primer set that targets all eukaryotes provided valuable data on microeukaryotes and zooplankton, the high abundance of zooplankton such as copepods in the nutrient-rich waters masked the signals from less abundant organisms and extracellular DNA, as seen here and in previous eDNA assessments within SBNMS (Polinski et al. 2019).
While the addition of more targeted primer sets can increase species resolution, reference availability in public databases still poses challenges to eDNA studies (Keck et al. 2023). For this study area, the region of the 12S gene covered by the Riaz et al. (2011) primer set has the highest percentage of reference availability for local marine vertebrate species (https://shiny.cefe.cnrs.fr/GAPeDNA/) (Marques et al. 2021), which was still only 48%. Even with a complete reference database, limitations arise. For example, despite having the highest reference availability, the 12S primer set used in this study does not amplify the 12S gene for elasmobranchs (Stoeckle et al. 2020), which are common inhabitants of SBNMS. Additionally, the utility of the 12S V5 region is limited by related species that exhibit identical sequences like the commercially important Atlantic cod and haddock. Nevertheless, in many cases, overlapping species assignments can be resolved by sequencing an additional mtDNA target or utilizing qPCR to distinguish among species, which is possible for Atlantic cod and haddock (Taylor et al. 2002).
Despite its limitations, eDNA shows promise in both documenting whole-ecosystem biodiversity and augmenting single species monitoring. With sand lance detections, not only could eDNA be used to detect species presence/absence within an area, but temporal evaluations could provide higher resolution on habitat usage and daily or seasonal dynamics of critical prey species. In addition, it can serve as a new tool for water quality monitoring, allowing for simultaneous evaluation of multiple species at once as well as monitoring co-occurring conditions which may promote HAB presence (Jacobs-Palmer et al. 2021). Although eDNA detections of whales and seabirds did not directly align with visual observations, the current study was designed to catalog biodiversity present within SBNMS and set baseline presence/absence data for the region. Like many eDNA methods, improved reference database availability and expanded sampling schemes are required to capture the full biodiversity within an ecosystem of interest (Somervuo et al. 2017; Mathieu et al. 2020; Kelly et al. 2024). However, at a basic level, these data represent a catalog of detected organisms, which, if converted to numerical value (e.g., species richness) and tracked over time, could be interpreted as an indicator of MPA condition.
5 Conclusions
eDNA is a unique tool in that it can be used to monitor organisms on all trophic levels, from bacteria to zooplankton up to apex predators. Although current eDNA methodology is not quantitative for macrofauna/flora, the information generated by eDNA can supplement existing biodiversity assessments, fill data gaps inherent in traditional survey methods, and reveal distribution patterns. The 2023 revision of the SBNMS Management Plan highlighted the urgent need to evaluate the impacts of climate change on sanctuary resources and take changing conditions into account when making management decisions (NOAA 2023). The impacts of warming temperatures on prey species, including sand lance and their zooplankton prey, raise concerns regarding bottom-up trophic interactions. This study provides a valuable baseline for future eDNA studies within SBNMS and highlights the promises of using eDNA to monitor total biodiversity, as well as species of particular interest, within MPAs. Despite current limitations still inherent in eDNA metabarcoding methodologies, this tool provides valuable presence/absence and spatial information for a broader range of organisms, from the microscopic to macrofauna, with a single sample.
Author Contributions
M.J.H. and J.M.P. conceptualized and designed the study. D.N.W. and T.L.S. organized ship time and sampling logistics. J.M.P. and T.L.S. conducted field sampling. J.M.P. completed laboratory work and sequencing. J.M.P. and E.L.S. analyzed amplicon sequencing data. J.M.P., E.L.S., T.P.O., and M.J.H. interpreted results and wrote the manuscript. All authors edited the manuscript.
Acknowledgments
This work was supported by a grant from the Danaher Foundation (grant ID: 747428). Conflict of Interest Statement: The authors declare that they have no conflicts of interest.
Conflicts of Interest
The authors declare no conflicts of interest.
Open Research
Data Availability Statement
All sequencing data associated with this study are available on the NCBI Sequencing Read Archive (SRA) under BioProject ID PRJNA1145320. Reproducible analysis code is available on the project GitHub (https://github.com/jmpolinski/SBNMS-2021_Biodiversity). 16S, 18S, and 12S results tables, the GMGI-12S database, and water nutrient data are available on Zenodo (DOI:10.5281/zenodo.13909873).




