Current Affairs: Examining the Use of Environmental DNA for Relative Abundance Monitoring in a Dynamic Tidal Habitat
Funding: The research was funded by a South Coast Biosciences Doctoral Training Partnership (SoCoBio DTP) by the Biotechnology and Biological Sciences Research Council (BBSRC) (Studentship No. BB/T008768/1 awarded to A.J.C.); the Blue Marine Foundation (through GreenTheUK) funded the boat time; NatureMetrics and the Sussex Wildlife Trust (through the Pebble Trust) covered the costs of eDNA analysis.
ABSTRACT
Assessing the abundance and spatiotemporal distribution of fish species is crucial for informing sustainable fishing practices and developing effective conservation management plans. Recently, environmental DNA (eDNA) has emerged as a promising tool for estimating not only marine species richness but also species abundance, with several studies demonstrating a positive correlation between eDNA concentration and species abundance. Consequently, eDNA surveys not only enhance the monitoring of species requiring conservation attention but may also serve as a method to quantify relative abundance, a critical indicator of ecosystem health. This study investigates the feasibility of using eDNA metabarcoding to estimate relative abundance of marine species in a tidal environment, using Sussex Bay, UK, as a case study. We compared eDNA relative abundance indices, estimated by the metabarcoding method, using two different primer pairs and found strong positive correlations between the results from each primer. Additionally, we evaluated the relationship between relative abundance estimates derived from eDNA metabarcoding index and those obtained from Baited Remote Underwater Video (BRUV) counts. Out of 14 species, only one significant positive correlation was found between eDNA index and BRUV counts. The BRUV surveys detected fewer species overall compared to eDNA, leading to a higher number of zero counts for several species, which may explain the lack of statistically significant relationships. This indicates that eDNA index and BRUV counts do not strongly correlate in dynamic marine environments, highlighting the differences between these survey methods. This study also estimated that eDNA can be detected at least 2–8 km from its assumed source in a marine tidal environment, with an average minimum travel speed of 1.8 km/h. Our findings illustrate the effectiveness of eDNA metabarcoding as a nondestructive biomonitoring method, while also highlighting its limitations as well as the challenges in comparing relative abundances of different methods in tidal environments.
1 Introduction
Assessing the distribution and abundance of fish species over spatial and temporal scales is crucial for monitoring fish stocks (Hill et al. 2014; Urban et al. 2024). These assessments are vital for maintaining sustainable fishing practices and developing effective conservation strategies to protect fish species (Baudron et al. 2020; Larson et al. 2022). Information on target fish species, particularly abundance and biomass data within commercial fisheries are often data poor (Fujita 2021; Geromont and Butterworth 2022). Fisheries have been in decline in the last 65 years, with more than 30% reductions in fish stocks, and 20% of fisheries in a collapsed state (Trindade-Santos et al. 2020; Britten et al. 2021). Trophic resilience has also declined, further impacting fishery longevity (Pelletier et al. 2020). To effectively maintain functioning ecosystems, fish biomass and sustained fishery economy is critical to have accurate assessments of catches to deliver confidence in setting total allowable catch limits within each fishery (Gebremedhin et al. 2021; Han et al. 2023).
Obtaining abundance estimates in marine environments is challenging. Traditionally, scientific surveys rely on nets, such as beach seines and trawls, to estimate species richness and abundance of fish (Murphy and Jenkins 2010; Laurel and Rogers 2020; Goethel et al. 2023). However, these methods are time-consuming, resource-intensive, and often destructive to the habitat, frequently resulting in the death of many fish (Steele et al. 2006).
Baited Remote Underwater Videos (BRUV) provide valuable insights into marine biodiversity and are widely used to assess fish abundance (Mallet and Pelletier 2014; Whitmarsh et al. 2017; Bacheler and Ballenger 2018). These systems are nondestructive and noninvasive, making them ideal for sampling marine biodiversity (Langlois et al. 2020). Consequently, underwater video systems are preferred in protected areas where the goal is to conserve the existing biodiversity (Langlois et al. 2020). Furthermore, BRUV systems provide a permanent sampling record that can be reviewed as needed (Cappo et al. 2009; Cheal et al. 2021). However, BRUV surveys can also be labor-intensive and time-consuming. They may also be biased by bait, which preferentially attracts meso-predators and affects fish behavior (Harvey et al. 2007). Additionally, their effectiveness is limited by underwater visibility (Harvey et al. 2001; Unsworth et al. 2014).
In recent years, environmental DNA (eDNA) has gained popularity as a biomonitoring tool. eDNA consists of genetic material shed by organisms into the environment through scales, skin cells, feces, mucous, and other excrements (Creer et al. 2016). This noninvasive and time-effective technique also eliminates the need for taxonomic expertise to identify species (Rees et al. 2014; Smart et al. 2015; Evans et al. 2017). Although eDNA can be more expensive than BRUV, it is often cheaper when accounting for the number of species detected, and more affordable than other traditional biomonitoring methods (Rees et al. 2014; Clark et al. 2024). One key advantage of eDNA metabarcoding is its ability to detect a wide range of taxa simultaneously, providing a snapshot of the species present within an ecosystem at a given time (Bohmann et al. 2014; Deiner et al. 2017). Additionally, eDNA has been shown to be more sensitive in detecting species richness compared to BRUV surveys (Port et al. 2016; Gold et al. 2023; Clark et al. 2024). More recently, the use of eDNA to indirectly estimate marine species abundance has been gaining momentum (Rourke et al. 2022; Yao et al. 2022). Studies have shown a positive correlation between eDNA concentration and abundance (Takahara et al. 2012; Thomsen, Kielgast, Iversen, Wiuf, et al. 2012; Pilliod et al. 2013; Rourke et al. 2022; Yao et al. 2022). Consequently, eDNA surveys may not only aid in monitoring of species requiring conservation attention but also serve as a method to quantify relative abundance, a key indicator of ecosystem health (Barnes and Turner 2016).
Most studies exploring the use of eDNA for estimating abundance in the field have employed quantitative PCR (qPCR), which measures the concentration of eDNA from a single species over space or time. As eDNA metabarcoding allows for a more comprehensive investigation into species diversity, using this method for fishery stock assessments, for example, would be highly advantageous. Harper et al. (2018) demonstrated that metabarcoding could detect the great crested newt at rates comparable to qPCR, particularly when strict detection thresholds (i.e., ≥ 4 of 12 positive replicates) were applied to qPCR results. Along with this, several studies have found correlations between eDNA metabarcoding relative abundance and the relative abundance detected by other monitoring methods (Thomsen et al. 2016; Jeunen et al. 2020; Stoeckle et al. 2021), suggesting that eDNA metabarcoding may be an appropriate method to understand ecosystem recovery in marine protected areas or regions with distinct ecosystem functions.
Several factors are known to influence the ability of eDNA to reflect relative abundance. For example, each primer set has biases, which could affect its ability to reflect eDNA relative abundance (Kumar et al. 2022). PCR amplification biases are an example of this, as these can result in uneven amplification and false negatives (Kelly, Port, Yamahara, et al. 2014; Goldberg et al. 2016). Additionally, if PCR conditions enable nonspecific binding or if poorly designed primers allow widespread binding to various templates, the preferential amplification of the most abundant templates can result in sequencing runs being dominated by nontarget taxa, while underrepresenting less common target DNA. Species-specific DNA shedding and decay rates, biomass, distribution, reproduction strategies, migrations, water temperature affecting decay time, and variations in sample processing methodologies will also affect eDNA relative abundance (Sassoubre et al. 2016; Lamb et al. 2019, 2022; Rourke et al. 2022; Xing et al. 2022; Yao et al. 2022). Consequently, the reliability of eDNA in accurately assessing relative abundance in natural settings remains uncertain.
Tidal exchanges and currents likely influence the distribution of eDNA concentrations in marine environments. Andruszkiewicz et al. (2019) demonstrated through ocean modeling that eDNA in coastal regions could be transported tens of kilometers in just a few days, potentially compromising the fine spatial resolution of eDNA detections due to water movement. However, the impact of tides and currents varies across different geographical areas. For example, Kelly et al. (2018) found no significant differences in community structure attributable to tidal influence in an area with a 3 m tidal range. Similarly, Larson et al. (2022) observed minimal impact of tides on eDNA results in Alaska, where tidal ranges can reach up to 8 m. However, these studies did not evaluate the capability of eDNA to quantify relative abundance in these highly dynamic marine ecosystems. Further research is necessary to assess the accuracy of eDNA for estimating relative abundance in dynamic tidal areas.
In this study, we investigated the feasibility of using eDNA metabarcoding to estimate the relative abundance of marine species at sites along the Sussex coast (UK). In the first instance, we assessed whether the eDNA indices for individual species detected by two metabarcoding primers (MarVer3 16S and MiFish 12S) correlate with one another. These primers were specifically chosen to investigate whether their results are consistent for the same species, as biases inherent to different primers may lead to variations in the relative abundances they detect. Understanding these differences is critical for evaluating their suitability for estimating species' relative abundances. In the second instance, we assessed correlations between the eDNA indices detected by the primers and the relative BRUV abundance for individual species. Finally, this study also aimed to show the dispersal distance of eDNA in a marine environment. Coastal ecosystems, such as Sussex Bay, are among the most physically dynamic habitats on Earth (Helmuth et al. 2006). Therefore, examining the effectiveness of eDNA metabarcoding for assessing relative abundances and the dispersal of eDNA in a highly tidal zone will enhance our understanding of its potential application in quantitative ecological assessments and inform future monitoring and conservation efforts.
2 Methods
2.1 Study Area
The relative abundance of fish species from BRUV and eDNA surveys was compared at 28 sites in Sussex Bay, UK (between 50°43.325 N, 0°46.040 W and 50°48.981 N, 0°12.265 W; Figure 1). These sites correspond to those used by the Sussex Inshore Fisheries and Conservation Authority (IFCA) for towed video transects used to map the changes in habitat type over time since the introduction of the Nearshore Trawling Byelaw (Sussex IFCA 2019; Mallinson and Yesson 2020). The samples were collected between the 5th and 21st of July 2021 and between the 11th and 29th of July 2022.

2.2 Baited Remote Underwater Video Surveys
The steel BRUV rigs were built with two GoProHERO8 cameras positioned to face a bait canister. The bait canister was filled with two Atlantic horse mackerel (Trachurus trachurus), one semi-thawed and one frozen, with slits cut along their bodies to maximize scent dispersal. At each site, three BRUV rigs were deployed 150 m apart on the seabed (between 3 and 18 m) and left to film for up to 75 min. Footage length was standardized by trimming the first and last GoPro clips, resulting in a survey interval of 47 min and 8 s. Since most species present in an area typically appear within 40–60 min of deployment, this duration was deemed appropriate (Unsworth et al. 2014). Videos were reviewed to identify species to the lowest taxonomic level. Species abundance was determined using the MaxN statistic, which represents the maximum number of individuals of a single species observed in one frame of the video during its entire duration (Cappo et al. 2003; Langlois et al. 2010).
2.3 Environmental DNA Surveys
Although the BRUV systems were deployed, one eDNA sample was collected at each of the 28 sites 1 m above the seabed to target the demersal layer, where eDNA is most concentrated (Mynott 2020). Using a Kemmerer sampler, 2 L of water were collected at each site and passed through an encapsulated PES disk filter with a 5-μm glass fiber prefilter. To preserve the eDNA, a salt and detergent-based lysis solution (Longmire's buffer) was added to each filter.
The eDNA analysis followed the methods described in Clark et al. (2024) and is summarized here.
DNA was extracted from filters with DNeasy Blood and Tissue Kit (Qiagen, Hilden, Germany) following Spens et al. (2017) method for disc filters in buffer. The manufacturer's protocol was followed except the proteinase K was added directly to the filter housing to minimize the risk of contamination arising from handling of the filter. PCR inhibitors were removed using a DNeasy PowerClean Pro Cleanup Kit (Qiagen) following manufacturer's protocol and DNA concentration was measured with a Qubit 3.0 fluorometer (Thermo Fisher Scientific, Waltham, MA, USA).
Two primer sets were used to target and amplify gene regions focusing on teleost fish and marine vertebrate taxa: (1) MarVer3, developed by Valsecchi et al. (2020) to amplify a fragment of the 16S rRNA gene region (Forward-AGACGAGAAGACCCTRTG and Reverse-GGATTGCGCTGTTATCCC); and (2) MiFish, which was developed by Miya et al. (2015) to amplify a section of the 12S rRNA gene region (Forward-GYYGGTAAAMYTCGTGCCAGC and Reverse-CATAGYGGGGTATCTAATCCCRGTTTG). These primer sets were selected to compare their efficiency in detecting relative abundance compared to BRUV abundance. Although MarVer3 is vertebrate specific, and the main taxa targeted by this study are fish species, MarVer3 has been shown to complement the MiFish primer by detecting additional taxa missed by MiFish (Chiquillo et al. 2024; Clark et al. 2024). Therefore, using both primers enables a more comprehensive view of the biodiversity present and thus gives the opportunity to compare the relative abundances of a wider range of taxa.
The MarVer3 eDNA amplification mixture had a volume of 25 μL and contained 12.5 μL DreamTaq Green PCR Master Mix (Thermo Scientific), 1.25 μL of each primer, 1 μL of template DNA, and 9 μL PCR grade water (Thermo Scientific). MarVer3 assay PCR conditions had an initial denaturation at 94°C for 4 min; then 38 cycles of the following: 95°C for 30 s, four 30 s touchdown annealing steps (+1°C per cycle; 8, 10, 10, and 10 cycles total) starting at 60°C, then 72°C for 40 s; and a final elongation step at 72°C for 5 min. The PCR reaction for the fish assay included 4 μL Phusion Green PCR Master Mix (Thermo Scientific), 0.32 μL of primers, 1.5 mM of MgCl2 (Thermo Scientific), 0.6 mg/mL of BSA (Thermo Scientific), 3% DMSO, 0.9 μL of template DNA, and 1.74 μL PCR grade water (Thermo Scientific). The PCR mixture volume was 8 μL. The PCR conditions in Miya et al. (2015) were used for the MiFish assay except that we used a touchdown annealing step in between denaturation and elongation starting at 69°C (10 cycles), then a 10 cycle step down to 65°C (0.5°C per cycle) then 25 cycles at 65°C. The process concluded with a final elongation step at 72°C for 5 min. Amplicons generated using these primer sets are hereafter referred to as MiFish 12S and Valsecchi 16S. The libraries for both assays were processed identically. The final pooled libraries were sequenced on an Illumina MiSeq system using a V3 600 cycle reagent kit (Illumina) with a max read length of 2 × 300 bp. Sequences were demultiplexed with bcl2fastq (v2.20.0.422) and then two different pathways were followed to calculate zOTUs (zero-radius OTUs). Reads were binned using USEARCH with the paired-end FASTQ reads for every sample (Edgar 2010). The forward and reverse primers were removed using cutadapt (v2.6; Martin 2011). Sequences were then filtered based on assay-specific length. These sequences were subsequently quality filtered and dereplicated using USEARCH (v11.0.667). Quality filtering was applied using expected errors predicted by Phred (Q) scores, with reads discarded if their expected error rate exceeded 0.01% (fastq_maxee_rate = 0.01), ensuring high-confidence sequence retention. Unique sequences from all samples were de-noised using the UNOISE program (USEARCH v11.0.667; Edgar 2016).
Taxonomy was assigned using Blastn (Altschul et al. 1990; Camacho et al. 2009) with the NCBI nucleotide database (NCBI nt; downloaded on 28-09-2021). Minimum similarity thresholds of 99%, 97%, and 95% were used for species, genus, and higher level assignments, respectively. Final taxonomy was confirmed using GBIF, and sequences not assigned to a kingdom were removed. OTUs with low abundance were also excluded, including those with less than 0.02% of all sequences for the MiFish 12S assay and less than 0.025% for the Valsecchi 16S assay. The threshold for removing OTUs was set as a percentage of reads because the difference in total reads per sample is variable. The threshold percent was chosen to be as close as possible to 20 reads for all samples within the primer pair specific dataset and varied between 0.01% and 0.1%. Identified species were cross-referenced with fish distribution maps from FishBase (Froese and Pauly 2023), and two taxa (Hypophthalmichthys nobilis and Sicyopterus lagocephalus) with no occurrence in the United Kingdom were removed from the analyses. The BRUV bait species, T. trachurus, was also excluded from the analyses as it could not be determined whether the DNA from this species was from naturally occurring individuals or from the bait. Some nontarget species, such as birds and rodents, were detected in the samples and were removed from datasets before analysis; however, freshwater species were kept in to determine possible dispersal distances (see below).
In the 2021 samples, site 9 failed to amplify and was removed from the dataset. In 2022, five (sites 7, 8, 11, 14, and 15) and two sites (sites 11 and 14) did not yield any results for the Valsecchi 16S and MiFish 12S assay, respectively. The lack of amplification in these samples was likely caused by low concentrations of eDNA; however, PCR inhibition cannot be ruled out.
2.4 Data Analysis
The experimental design included four factors: depth, tidal stream, year, and sample site. Depth was measured at each site using the boat's sonar system. The tidal stream at the time of sampling was calculated by interpolating the actual tide strengths at the time of sampling from Dover charts (Admiralty Chart 1652) using the closest tidal diamond to each site (Towler and Fishwick 2020).
Variance-to-mean ratios for the eDNA index from both primer pairs ranged from 0.27 to 0.94 for the species detected by both eDNA and BRUV (Table S1), indicating slight underdispersion or near-random variation across samples. These results support the assumption that species abundances randomly vary around a stable mean (Kelly et al. 2019), thereby justifying the use of eDNA index in this study.
To test for spatial autocorrelation within our data, we performed a Mantel test with Spearman correlation coefficients for each sampling method and year (R software, package vegan, function mantel; Oksanen et al. 2022). To account for the positive spatial autocorrelation detected, we included the X and Y coordinates as environmental variables in our multivariate analyses.
All statistical analyses were performed in R with RStudio (version 4.3.1).
2.4.1 Metabarcoding Comparison
For each method, species diversity was assessed using the vegan package (Oksanen et al. 2022). Rank abundance curves were carried out to identify the species with the highest cumulative eDNA index for each primer (Kindt and Coe 2005).
Correlations of eDNA indices derived from the MiFish 12S and Valsecchi 16S amplicons were compared for individual species. The aim was to investigate whether, for example, a high relative abundance detected for a species by one primer corresponds to a similarly high relative abundance detected by the other, despite potential primer-specific biases. This comparison may provide insights into the primers' consistency and suitability for estimating species' relative abundances. Due to the high numbers of 0s in the datasets, Spearman's rank correlation coefficients were computed and visualized using the ggscatter function (Kassambara 2023), which enables simultaneous plotting and correlation annotation.
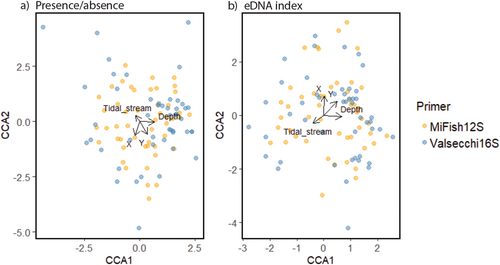
To further explore the relationship between the eDNA indices detected by the two primers and assess how covariates influence the community composition detected by each primer, canonical correspondence analyses (CCA) were performed. Two separate CCAs were carried out: one using eDNA index as a measure of relative abundance and another using presence/absence data. This comparison aimed to evaluate the influence of relative abundance on community composition. The variables used as covariates were depth, tidal stream, site, geographic coordinates (X and Y), primer pair, and year. The CCAs were conducted using the cca function in the vegan package (Oksanen et al. 2022). The first two axes of the CCA were plotted along with the environmental variables (Wickham 2016; Oksanen et al. 2022).
2.4.2 eDNA Index vs. BRUV MaxN
Generalized linear models (GLM) with beta regression were conducted between eDNA index and BRUV MaxN, accounting that the response variable (eDNA index) is bounded between 0 and 1. Given that the eDNA index included exact 0 and 1 values, a transformation using the Smithson and Verkuilen (2006) adjustment was applied to meet the assumptions of beta regression. The models were only conducted on species that were detected at a minimum of three sites by both methods.
A CCA was carried out on the BRUV data to investigate the relationship between community composition and environmental variables. The covariates used were: depth, tidal stream rate, sampling year, and geographic location (X and Y coordinates).
2.4.3 eDNA Dispersal Distance Estimations
To assess dispersal of eDNA in marine ecosystems, the freshwater species detected in our samples by the MiFish 12S primer were used. These were: European chub (Squalius cephalusi), gudgeon (Gobio gobio), roach minnow (Rutilus rutilus), Northern pike (Esox lucius), three-spined stickleback (Gasterosteus aculeatus), ninespine stickleback (Pungitius pungitius), ruffe (Gymnocephalus cernua), and stone loach (Barbatula barbatula). Knowing these species do not occur naturally at the sites sampled, it was assumed they came from the freshwater outlets in Sussex Bay (i.e., the rivers Adur and Arun, the Ferring Rife, Brooklands Lake or Pagham Harbor). Therefore, it was possible to map the closest source the freshwater species' eDNA may have originated from, providing an approximation of the distance eDNA is transported in a tidal environment.
The minimum dispersal distance was calculated by measuring the straight-line distance between the estuary mouth/outflow pipe and the site at which the freshwater species' eDNA was detected. Distance measurements were conducted using the “Distance to nearest hub (line to hub)” function in QGIS (QGIS Development Team 2025). The starting point was defined as the estuary mouth/outflow pipe, where freshwater flows into the marine environment, and the endpoint was the eDNA sample location at which the freshwater species were detected. Distances were recorded in kilometers.
The time the freshwater eDNA entered the marine system was also unknown; however, based on the tidal rate, the time the eDNA could have been transported from the source to the sample can be calculated. To estimate the fastest possible time for the DNA to reach the sampling site, the maximum tidal rate for each day was calculated and converted from knots to kilometers per hour. The minimum distance was then divided by the tidal speed to determine the shortest time (hours) it could have taken the eDNA to travel from the estuary mouth/pipe to the sampling site.
3 Results
The 2021 and 2022 MiFish 12S data, along with the 2021 BRUV data, showed significant positive spatial autocorrelation (Mantel statistic MiFish 12S 2021: r = 0.18, p = 0.006; MiFish 12S 2022: r = 0.17, p = 0.01, BRUV 2021: r = 0.17, p = 0.009). However, there was no indication of spatial autocorrelation in the remaining eDNA and BRUV datasets (i.e., Valsecchi 16S 2021, Valsecchi 16S 2022 and BRUV 2022).
Using the MiFish 12S primer, a total of 7,734,835 raw sequence reads were obtained across all samples. After quality filtering, 6,345,378 high-quality reads remained, and 6,170,416 reads were successfully assigned to taxa. Using the Valsecchi 16S primer, a total of 5,723,286 raw sequence reads were obtained across all samples. After quality filtering, 5,220,271 high-quality reads remained, and 5,214,792 reads were successfully assigned to taxa. No DNA was detected in lab controls based on visual bands in gels.
3.1 Relative Abundance Correlations Between eDNA Primers
MiFish 12S detected 74 species from 27 samples in 2021 and 60 species from 25 samples in 2022. The Valsecchi 16S detected 65 species from 27 samples in 2021 and 46 species from 22 samples in 2022. Across 2 years of sampling, the two eDNA primers detected 26 common species from 50 replicates (Table S2). The three most frequently detected species by the MiFish 12S were: European seabass (Dicentrachus labrax), European pilchard (Sardina pilchardus), and bib (Trisopterus luscus). In contrast, the Valsecchi 16S primer most frequently detected Atlantic mackerel (Scomber scombrus), European sprat (Sprattus sprattus), and European seabass (D. labrax; Figure S1). Notably, T. trachurus (the bait species) was the most frequently detected species by MiFish 12S and the second most frequently detected species by Valsecchi 16S, prior to its removal from the dataset.
Species-specific Spearman correlations revealed that 18 out of 26 species (69%) had a significant positive correlation in eDNA indices between the two primers (p > 0.05; Figures S2 and S3). However, after removing datapoints where both primers recorded absences (joint zeros), only 7 out of 26 species (27%) retained significant positive correlations (Table S3).
3.2 Community Composition and Comparative Analysis
Two canonical correspondence analyses (CCA) were performed on the MiFish 12S and Valsecchi 16S primer pair data. The first CCA used presence/absence data (Figure 2a) and the second used eDNA indices as a measure of relative abundance (Figure 2b). Both CCA models were found to be significant (presence/absence: anova.cca: F7,89 = 2.85, p = 0.001; eDNA Index: anova.cca: F7,89 = 2.63, p = 0.001). The CCAs explained a comparative proportion of the variation explained; the presence/absence CCA explained 13% while the eDNA index CCA explained 12.6%. With only a relatively small proportion of total variation explained by both analyses, the results should be interpreted with caution. In terms of species composition, the primer pairs detected different community compositions from one another in the presence/absence CCA (anova.cca: F1,89 = 2.1, p = 0.003). However, in the eDNA index CCA, the community compositions detected by the primer pairs were not significantly different from one another (anova.cca: F1,89 = 0.58, p > 0.05). Additionally, both CCAs indicated that communities were structured by tidal stream rate (presence/absence: anova.cca: F1,89 = 1.96, p = 0.01; eDNA index: anova.cca: F1,89 = 3.17, p = 0.001), depth (presence/absence: anova.cca: F1,89 = 6.76, p = 0.001; eDNA index: anova.cca: F1,89 = 5.75, p = 0.001), and geographic location (presence/absence: anova.cca: X: F1,89 = 3.01, p = 0.001; Y: F1,89 = 2.80, p = 0.001; eDNA index: anova.cca: X: F1,89 = 3.40, p = 0.001; Y: F1,89 = 2.60, p = 0.001).
3.3 Relative Abundance Correlations Between eDNA Index and BRUV
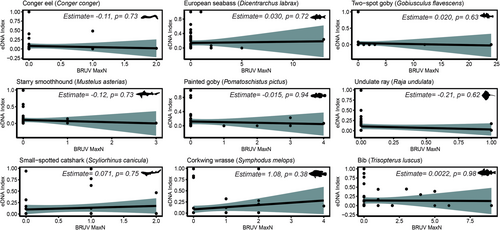
BRUV surveys detected a total of 26 species in 2021 and 24 species in 2022, whereas the MiFish 12S primer detected 77 species in 2021 and 80 in 2022. Across the 2 years of sampling, the two methods detected the same nine species at a minimum three sites, respectively. These were: Conger eel (Conger conger), goldsinny wrasse (Ctenolabrus rupestris), European seabass (D. labrax), two-spot goby (Gobiusculus flavescens), rock goby (Gobius paganellus), striped red mullet (Mullus surmullus), painted goby (Pomatoschistus pictus), black seabream (Spondyliosoma cantharus), and poor cod (Trisopterus minutus). Of these, only C. rupestris showed a significant positive relationship between BRUV MaxN and eDNA index (Estimate = 0.58, p = 0.014; Figure 3). No significant correlations were found for the other species (p > 0.05).

The Valsecchi 16S primer detected 69 species in 2021 and 48 in 2022. Over the 2 years of sampling, the Valsecchi 16S assay and the BRUV surveys detected nine species in common: Conger eel (C. conger), European seabass (D. labrax), two-spot goby (G. flavescens), starry smooth-hound (Mustellus asterias), painted goby (P. pictus), undulate ray (Raja undulata), small-spotted catshark (Scyliorhinus canicula), corkwing wrasse (Symphodus melops), and bib (Trisopterus luscus). Of these, no significant correlations were found between BRUV MaxN and eDNA index (p > 0.05; Figure 4).
The CCA model carried out on the BRUV data was significant (anova.cca: F5,49 = 1.72, p = 0.001). Only the first CCA axis was significant (CCA1: F1,49 = 2.41, p = 0.012) and explained 11% of constrained variation. The results should be interpreted with caution as only a small proportion of total variation is explained. The model revealed that sampling year (F1,49 = 2.97, p = 0.001) and depth (F1,49 = 1.67, p = 0.036) had an effect on the community composition detected by BRUV (Figure S4).
3.4 eDNA Dispersal Distance Estimations
Eight freshwater taxa were identified down to species level by the eDNA analysis. Metabarcoding yielded a total of 3705 sequence reads for freshwater species (G. gobio—832; R. rutilus—90; S. cephalus—345; B. barbatula—1067; E. lucius—162; G. aculeatus—633; P. pungitius—378; G. cernua—198). These species were distributed at five different sites along Sussex Bay. Five different species were detected at Site 8, which happens to fall in the middle of the freshwater sources; only one species was found at each of the other sites (Figure 5).
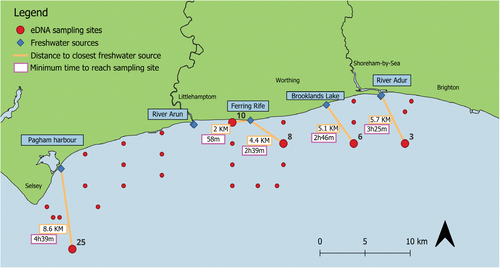
The freshwater species used to estimate eDNA dispersal distance were found at sampling sites between 2 and 8.6 km away from the closest freshwater source (Table S4). Based on the highest tidal rate on the day of sampling, it was estimated that the eDNA traveled from its source to the sampling point in approximately 1–4 h depending on the site location. The average speed at which the water could transport eDNA from source to sample site was 1.82 km/h (or ~0.98 knots).
4 Discussion
eDNA metabarcoding shows potential as a pivotal tool for tackling the need for scalable marine biomonitoring, thereby improving and expanding marine conservation efforts (Thomsen and Willerslev 2015). We compared eDNA indices for individual species detected by different primer pairs and found that the relative abundance detected by one had a strong positive correlation to the relative abundance detected by the other. On the other hand, our findings indicate that the relative abundance of fish detected from marine vertebrate eDNA does not correlate with marine vertebrate abundance measured using BRUV, potentially due to the much larger detection range of eDNA, especially in an area of high tidal range. Notably, and similarly to other studies (Thomsen, Kielgast, Iversen, Møller, et al. 2012; Deiner et al. 2017; Kelly et al. 2017; Taberlet et al. 2018; Jeunen et al. 2020; Gold et al. 2023), we found that eDNA analysis had a higher sensitivity than BRUV surveys, detecting up to three times as many species (depending on primer pair and sampling year). Notably, eDNA more frequently detected species that were also observed in BRUV surveys, but its higher sensitivity led to many species being recorded by eDNA but not by BRUV, resulting in a higher number of zero counts in the BRUV data. This discrepancy likely contributed to the lack of significant correlations between the two methods. In this study, we also estimated the minimum potential dispersal distances of eDNA and found eDNA can travel at least 2–8 km from its assumed source. This study demonstrates the potential for eDNA metabarcoding as an effective, nondestructive biomonitoring method, while also highlighting the challenge of comparing relative abundances of different biomonitoring methods in dynamic tidal areas.
4.1 Primers
Before assessing the ability of eDNA metabarcoding primers to quantify species' relative abundance, the quantitative correlation between the MiFish 12S and Valsecchi 16S primers was investigated to understand if one primer may be better at measuring relative abundance compared to another. We found the majority (69%) of the correlations were significantly positive. However, after removing joint zero detections, only 27% of the correlations remained significant, which was not unexpected. Even when the same species is detected by both primers, differences in primer specificity, binding efficiency, and amplification biases could lead to variation in the eDNA index values obtained (Kelly et al. 2019; Zhang et al. 2020; Banerjee et al. 2021). It is therefore likely that each primer set captures slightly different biological signals, contributing to the reduced correlation once joint absences were excluded. This highlights the limitations of comparing relative abundance across primer sets and supports the decision to compare each primer set to BRUV counts separately in the subsequent part of the study. Of note, before T. trachurus was removed from the dataset, it was among the top two most detected species for both primer pairs. As this species was used as bait for the BRUV systems, and eDNA samples were taken as the BRUV systems were deployed, we expected high eDNA index values for this species at all sites. Therefore, T. trachurus served as a positive control, validating the experimental design by confirming that high concentrations of eDNA correlate with a high eDNA index (Doi et al. 2015; Klymus et al. 2015).
The MiFish 12S assay demonstrated higher detection efficiency compared to the Valsecchi 16S assay, reflecting differences in the performance of the respective primer pairs. The MiFish 12S primer may have detected more species overall as it is specifically designed to target fish. However, using both primer sets allowed for the detection of a broader range of taxa, including species not captured by MiFish 12S alone, such as the elasmobranchs.
The differences in community composition detected by both primers and how abundance affects community composition were investigated by comparing ordinations from presence/absence data and relative abundance data. The qualitative (presence/absence) data showed significantly different community structures detected by each primer, whereas the analysis of the quantitative data (relative abundance from eDNA index) found no difference in community composition detected by the two primers. These findings are consistent with previous research (Nester et al. 2020; Banerjee et al. 2021; Cole et al. 2022). The results suggest that the communities are more similar when abundance is included, indicating that the primer pairs detect comparable quantities of eDNA. Additionally, including species relative abundance provided a more nuanced understanding of community composition. Relying solely on eDNA presence/absence data can lead to inconclusive results, due to inherent limitations of this approach (Wang et al. 2021; Guri et al. 2023). These results further confirm that despite both eDNA primers preferentially amplifying certain species over others, the relative abundance detected by both primers correlated. In addition, the multivariate analyses revealed that tidal strength, survey depth, and geographic location significantly affected species distribution or perhaps, species detection. This is expected, as fish activity varies with tide levels, and different species are associated with varying water depths (Smith and Brown 2002; Greenwood and Hill 2003; Jankowski et al. 2015; Viehman and Zydlewski 2017).
4.2 eDNA Index as a Proxy for Relative Abundance
One of the most valuable applications of eDNA would be the ability to monitor fish relative abundance for both the fishery industry and marine conservation (Kelly, Port, Yamahara, et al. 2014; Kelly, Port, Yamahara, and Crowder 2014; Miya et al. 2015; Thomsen and Willerslev 2015; Kelly 2016). We assessed species-level correlations between the eDNA index detected by each of our primer pairs and BRUV MaxN. Only one species, C. rupestris, out of the 14 analyzed showed a significant positive correlation between eDNA index and BRUV MaxN. The biology and behavior of this species may explain why its relative abundance estimates were more consistent between eDNA index and BRUV surveys compared to other species. Interestingly, Wilms et al. (2022) also found a positive correlation between eDNA detection and BRUV MaxN for wrasse, but not for the other taxa. C. rupestris is a reef-associated species known to be widely distributed along the Sussex inshore area (Williams and Clark 2010). It is territorial and conspicuous, making it more likely to be reliably observed and counted in BRUV footage (Darwall et al. 1992; Moen and Svensen 2004), which may help explain the observed correlation.
Similar studies have also reported a lack of correlation between eDNA relative abundance and that obtained through BRUV or other traditional methods (Hinlo et al. 2018; Deutschmann et al. 2019; Knudsen et al. 2019; Fraija-Fernández et al. 2020; Gold et al. 2023). This is not surprising, as correlations tend to be weaker in natural systems than in controlled environments (Yates et al. 2019). However, a review by Rourke et al. (2022) revealed that up to 90% of published comparative studies found a positive relationship between eDNA signal and fish abundance or biomass recorded by traditional methods. Though only 12 of the studies used eDNA metabarcoding to estimate relative abundance/biomass, whereas the other 51 studies used targeted species analysis with qPCR. Since metabarcoding data is compositional and composition affects detection probability, metabarcoding may generally be poorly suited for quantifying eDNA (Shelton et al. 2016, 2023). On the other hand, qPCR provides higher sensitivity and specificity compared to metabarcoding, allowing for the quantification of specific target DNA sequences and providing an absolute quantification of target DNA, which helps estimate the abundance of the target species (Buxton et al. 2017; Doi et al. 2017; Tillotson et al. 2018). Out of the 12 eDNA metabarcoding studies, eight were conducted in natural environments, and only three of those were carried out in marine environments. Freshwater eDNA generally degrades more slowly than marine eDNA due to differences in salinity, microbial community composition, UV regimes, and temperature (Lamb et al. 2022). Along with this, freshwater systems such as lakes and ponds are not influenced by tides and currents. Therefore, freshwater systems may be better suited for using eDNA metabarcoding for relative abundance than marine systems. Among the three marine studies in Rourke et al.'s (2022) review, one found no significant correlation between the relative abundance of eDNA and traditional methods (Fraija-Fernández et al. 2020) and another used presence/absence data rather than eDNA read counts/index (Stoeckle et al. 2017); therefore, only one study, out of these three, found a significant correlation (Thomsen et al. 2016). Future studies should test the use of eDNA metabarcoding for measuring relative abundance given that this review found only three studies comparing eDNA metabarcoding to a traditional method in the marine environment. Another study similar to ours, by Gold et al. (2023), compared relative abundances of individual species across visual surveys, seine nets, and eDNA and found few significant correlations between methods. Therefore, overall, our results align with previous studies using comparable methods to ours.
Another explanation for our findings could be that eDNA and BRUV surveys are not comparable sampling techniques for assessing abundance for most species. Consistent with past studies, we found that eDNA surveys were more sensitive than traditional surveys, detecting up to three times as many species as BRUV (Stat et al. 2019; Cole et al. 2022; Gold et al. 2023; Clark et al. 2024). Given the low overlap in species detected by both methods, comparing their ability to measure abundance was challenging, especially not knowing the true abundance of species (Kelly et al. 2017; Gold et al. 2023). eDNA was found to be more sensitive than BRUV surveys, detecting species that BRUV surveys did not, and leading to many zeros in the BRUV dataset that likely influenced the lack of relationship between eDNA index and BRUV MaxN. BRUV surveys are very widely used in marine biomonitoring and generally measure abundance more accurately than any other traditional biomonitoring method (French et al. 2021). However, eDNA and BRUV surveys target species detections in different ways. BRUV surveys have limitations which may influence the measure of relative abundance, for example, species identification being more challenging in low visibility (Harvey et al. 2002; Unsworth et al. 2014) and biases linked to bait choice (Harvey et al. 2007; Jones et al. 2020). The bait chosen for the BRUV sampling can preferentially attract certain species over others, which may also influence the results (Jones et al. 2020). The duration of the underwater video recordings (40–60 min) may be another limitation to the BRUV surveys. Although a recording time of just under an hour is considered an appropriate length (Unsworth et al. 2014; Harasti et al. 2015), increasing sampling duration can enhance the efficiency of surveys for certain species (Unsworth et al. 2014). The recording time may also represent a smaller sampling effort compared to eDNA, which integrates signals over a larger area and captures biodiversity on a much broader spatial scale. Deploying the BRUV surveys for a longer period of time may have made the methods more comparable. Nevertheless, there is a key difference between the two methods: BRUV surveys are localized, site-specific insights, whereas eDNA provides a more comprehensive view of biodiversity, particularly in regions with high tidal ranges (Deiner et al. 2016; Cole et al. 2022; Clark et al. 2024).
The highly dynamic tidal fluctuations at our study sites may also help explain the lack of correlation between BRUV and eDNA indices observed (Van Graafeil 2022). Tidal range has a strong influence on eDNA concentration (Baetscher et al. 2024) and was shown to affect community composition detected by eDNA surveys in this study. Although long-distance dispersal of eDNA is unlikely as eDNA degrades over a scale of days or weeks (Thomsen, Kielgast, Iversen, Wiuf, et al. 2012; Thomsen, Kielgast, Iversen, Møller, et al. 2012), it is also possible that eDNA will travel a number of kilometers within a few hours in coastal habitats, since these are some of the most physically dynamic areas in the world tidal area (Helmuth et al. 2006).
Similarly to BRUV surveys, the relationship between eDNA indices and species abundance can be influenced by many sources of error. Although we attempted to address primer amplification bias by transforming read counts to eDNA indices, the transformation does not account for the nonlinear factors affecting the relationship between true species abundance and the observed eDNA reads (McLaren et al. 2019; Shelton et al. 2023). The eDNA index for each species represents an unknown original number of eDNA sequences in the water, which is only a relative abundance of that species (Shelton et al. 2016). Correlation between eDNA indices and fish abundance depends on factors such as species, age, shedding rates, eDNA degradation time, and tidal strength. Abiotic factors such as sea temperature and salinity also affect eDNA degradation and detectability (Kelly et al. 2018; Lamb et al. 2022). These factors can skew the relationship between eDNA concentration and species abundance (Turner et al. 2014; Wilcox et al. 2018; Harrison et al. 2019). Previous studies that considered shedding and decay rates found more significant correlations between the relative abundance of eDNA and traditional methods (Harrison et al. 2019; McLaren et al. 2019; Guri et al. 2024). Although accounting for these mechanisms was not within the scope of this study, we recommend future research consider these mechanisms that can influence observed sequence reads (Barnes and Turner 2016; Harrison et al. 2019; Shogren et al. 2019; Yates et al. 2023). It is important that future studies address the impact of amplification efficiency on metabarcoding results by using joint models that integrate metabarcoding data, amplification efficiency estimates, and species-level abundance estimates (Kelly et al. 2019; McLaren et al. 2019; Shelton et al. 2019; Silverman et al. 2021).
4.3 eDNA Dispersal Distance Estimations
The eDNA dispersal estimates illustrated the spatiotemporal detection range of eDNA using metabarcoding. The dispersal distance estimations found that eDNA of freshwater species was detectable on average around 5 km from the source, with one species, B. barbatula, detected 8 km from the closest freshwater source. Previous studies in marine ecosystems have reported dispersal distances between 1 and 1.5 km, depending on the tidal range (Murakami et al. 2019b; Baetscher et al. 2024). However, the dispersal distances in river systems appear more similar to those detected in our study, ranging from 5 to 12.3 km (Deiner and Altermatt 2014; Laporte et al. 2020). The estimated average speed at which the water transported eDNA from source to sampling site in our study was 1.82 km/h, much faster than the 14–489 m/h found by Murakami et al. (2019a) in Maizuru Bay, which has a weaker current. These findings, in comparison with other studies, emphasize the dynamic nature of tidal movements in our study area, which resemble river water flow, and may explain the greater displacement of eDNA over longer distances.
Notably, the dispersal distance was calculated using the most direct route; however, water flow in coastal environments does not follow straight lines. Consequently, it is likely that the eDNA traveled farther and persisted longer than reported here. Our results only reflect the distance and amount of time the freshwater fish eDNA was in the marine environment before being collected in the sample, and do not account for the distance/time traveled within the freshwater system itself. The biology of the freshwater fish detected suggests that they tolerate different salinity levels, indicating that some species' eDNA may have traveled much further to enter the water system. For example, while G. cernua, G. aculeatus, and P. pungitius have a wide salinity tolerance (Hölker and Hammer 1994; Arai and Goto 2008; DeFaveri and Merilä 2014), S. cephalusi and B. barbatula occupy freshwater environments well above the seawater limit (Froese and Pauly 2023). Therefore, the eDNA of these species may have traveled much farther downstream before entering the coastal environment.
The possibility of contamination during sampling cannot be ruled out. However, other samples collected on the same days and under the same conditions as those with freshwater species detections did not show evidence of freshwater DNA. This suggests that contamination in the field and the laboratory experiment is an unlikely explanation for the detections. As the exact source of the freshwater fish species eDNA is unknown, it is also possible that freshwater fish predators, such as birds or marine fish that enter rivers or estuaries to feed, may have distributed DNA from prey items across marine localities through defecation. However, given freshwater species were detected at several different locations and on different days of sampling, and many of these species were found relatively close to freshwater outflows, our results likely reflect the dispersal of eDNA by currents up to 8.6 km from their source and persist in the environment for up to a minimum of 4 h 38 min. Nevertheless, as these results are estimations based on nontarget species which were found in the samples, future research should focus on controlled experiments to measure the dispersal distance and degradation times of eDNA in marine tidal environments.
5 Conclusion
This study demonstrates the potential of eDNA metabarcoding as a scalable and sensitive tool for marine biodiversity monitoring. We found a generally positive correlation between eDNA indices generated by MiFish 12S and Valsecchi 16S primers for jointly detected species. However, when joint absences were excluded, only 27% of these correlations remained statistically significant, reflecting the difference in primer specificity. Additionally, we found that using relative abundance in multivariate analyses provided a more nuanced understanding of community composition compared to using presence/absence alone. Furthermore, eDNA indices showed little correlation with BRUV counts (MaxN) for most species, highlighting the limitations of both methods for estimating relative abundance in highly tidal environments due to their differing spatial detection ranges. Notably, this study estimated that eDNA can be detected at least 2–8 km from its assumed source, emphasizing the importance of considering hydrodynamic context in future eDNA survey design and interpretation.
Author Contributions
A.J.C., M.P., V.S., I.W.H., and R.J.S. conceived and planned the study. A.J.C., V.S., F.S.M., and M.P. collected the data. A.J.C. carried out the analysis and wrote the manuscript. N.R.G. contributed to the eDNA analysis section of the methods. T.C. contributed knowledge on freshwater species. All authors discussed the results and contributed to the final manuscript.
Acknowledgments
We thank Neville Blake the skipper of the research vessel, along with his crew: Neil Frazer-Betts and Peter Everard, without whom we could not have carried out this research. We also thank all the University of Sussex Masters and Undergraduate students who helped with the data collection.
Ethics Statement
The authors have nothing to report.
Conflicts of Interest
The authors declare no conflicts of interest.
Open Research
Data Availability Statement
Data is available on Figshare: https://doi.org/10.25377/sussex.28417556.




