Temporal Dynamics of a Fish Parasite (Tetracapsuloides bryosalmonae) and Its Two Main Hosts in Pyrenean Streams: An Environmental DNA-Based Approach
Funding: The authors received no specific funding for this work.
Chiara Mercier and Eloïse Duval co-first authors
ABSTRACT
The success of parasite life cycles hinges on multiple factors, among which the temporal synchrony between parasites and their hosts holds a crucial role. Studying host–parasite interactions therefore requires investigating this synchrony, which is challenging in natural environments. Here, we delve into the temporal dynamics of the interaction between the myxozoan parasite Tetracapsuloides bryosalmonae and its two hosts—a bryozoan, Fredericella sultana, and a salmonid, Salmo trutta—employing an environmental DNA-based approach. We used digital PCR to quantify the molecular abundance of each species throughout an entire year and at eleven sites in the Pyrenees (France). We first characterized the seasonal dynamics of the parasite and its two hosts and their association with water temperature. Then, we assessed spatial synchrony between sites for each of the three species and temporal synchrony between the parasite and its two hosts. We revealed temporal fluctuations in the parasite and hosts DNA concentration, with peaks in abundance during the summer months. There were also significant associations between DNA concentrations and water temperature for the three species, but their form varied among species. We further found spatial synchrony for T. bryosalmonae and its hosts, with peaks of DNA of each organism occurring at the same time across sites. However, the strength of this synchrony varied across sites, a difference partially attributable to their geographical distance (the closer the sites the more synchronous the dynamics). We also showed a co-variation over time between the parasite and its two hosts. This co-variation was stronger in sites with fish showing high parasite infection prevalence, suggesting that synchrony facilitates the completion of the parasite's life cycle. These results open up new avenues for the examination of temporal synchrony between parasites and hosts and underscore the efficiency and usefulness of eDNA for further research into these mechanisms in natura.
1 Introduction
Parasites often exhibit complex life cycles involving the succession of multiple hosts. Each host plays a crucial role, providing specific environmental conditions or necessary resources for different parasite life stages. For example, the definitive host provides a suitable habitat for the reproduction of mature parasites, whereas intermediate hosts serve as nutrient sources for the growth of parasite larvae or as dispersal means (Combes 2001; Mazè-Guilmo et al. 2016). The transition from one host to another is a delicate step for parasites because parasites must encounter their hosts and adapt to new physiological and ecological conditions. Consequently, the number of parasites infecting a new host is often reduced at each step of the cycle, which limits their ability to complete their life cycle.
Parasites have evolved a variety of strategies to increase their chances of encountering their host in both space and time. For instance, some parasites can modify their host behavior (Hughes et al. 2012), such as Toxoplasma gondii, responsible for toxoplasmosis, which alters the aversion of infected rodents to cat odors, thus increasing the likelihood of its hosts encounters (da Silva and Langoni 2009). Other strategies are based on host–parasite temporal synchrony, where parasites are more abundant in the environment when their hosts are the most available (i.e., abundant, active…). Indeed, the strength of host–parasite interactions varies over time. Both hosts and parasites exhibit biological rhythms and behavioral patterns that can influence the likelihood and outcome of their encounters. For example, some parasites may synchronize the release of their infective stages or activity patterns with the physiological rhythms of the hosts, increasing the chances of successful transmission (Reece et al. 2017; Carvalho Cabral et al. 2019; Prior et al. 2020). Additionally, hosts may exhibit temporal variations in their susceptibility to infection, which can impact the transmission dynamics and prevalence of parasitic diseases. For example, the abundance and seasonal dynamics of fish and copepod host species can influence the encounter with the parasitic cestode Ligula intestinalis (Loot et al. 2006). Even though a circadian chronobiology has been established for some host–parasite systems such as Schistosoma mansoni metacercariae and its vertebrate hosts more than 40 years ago (Combes and Théron 1977; Théron and Combes 1988), the temporal dynamics of most host–parasite interactions are still understudied.
The study of temporal synchrony between hosts and parasites in their natural environment has been limited, likely due to the challenges associated with detecting parasites in their habitat. In addition to their microscopic, diluted, and often undetectable nature, the detection of parasites in their natural habitats frequently necessitates the use of invasive techniques that can harm their hosts. This is especially true for the study of endoparasites (Yusni et al. 2022; Xiao et al. 2004), for which host dissection is often needed (McAllister et al. 2016; Salman et al. 2018). These practices raise ethical concerns and are also poorly suited when the sampled host populations are already endangered. To address this issue, molecular methods have been developed (Beja-Pereira et al. 2009), such as the use of environmental DNA (eDNA), which refers to the DNA shed by organisms into the surrounding environment (Taberlet et al. 2012). The amplification of species eDNA enables their detection and/or quantification from soil, water samples, or other complex environmental matrices (Rees et al. 2014). This approach proves especially useful for determining the presence of parasites and their corresponding host species (Bass et al. 2015). Moreover, it holds significant potential for addressing questions of parasite–host synchrony in natural environments (Hundermark 2019).
Here, we used an eDNA approach to investigate the temporal synchrony between a heteroxenous parasite and its two main hosts in natural conditions. We focused on Tetracapsuloides bryosalmonae, a malacosporean endoparasite responsible for the Proliferative Kidney Disease (PKD), an emerging disease affecting several salmonid species, both within natural and farmed populations in Europe and North America (Burkhardt-Holm and Scheurer 2007; Okamura et al. 2011; Mo and Jørgensen 2017; Sudhagar et al. 2019). Brown trout (Salmo trutta) is the intermediate fish host of T. bryosalmonae, and the bryozoan Fredericella sultana is the final host (Sudhagar et al. 2019; Hartikainen and Okamura 2015). Various stages of this cycle, including spore shedding by the two hosts, have been demonstrated to be temperature and season-dependent (Ros et al. 2022). Once the water temperature reaches ~9°C in early spring, the spores are released from the bryozoan and dispersed into the environment (Gay et al. 2001). In vitro experiments also show that the increase in water temperature, around 15°C in early summer, accelerates the proliferation of T. bryosalmonae in the bryozoan host, allowing the transition from covert to overt infection (Tops et al. 2006). Furthermore, the activity of bryozoans like Fredericella sultana exhibits seasonality and correlates with temperature (Raddum and Johnsen 1983; Tops et al. 2009). We therefore expect bursts of parasite shedding to be temporally synchronized with bryozoan activity, and to a lesser extent with brown trout activity, for which biomasses should be more stable over time, and from which parasite release is expected to occur to a lesser extent and to be less seasonal (Carraro et al. 2017, 2018). A temporal synchronization among T. bryosalmonae and its hosts should ease parasite transmission within its host communities.
This study aims to characterize the seasonal dynamics of the parasite T. bryosalmonae and its two main hosts in Pyrenean rivers for an entire year, and to test whether their respective abundances were synchronous over time and space. Specifically, using targeted environmental DNA detection, we aim to describe the temporal patterns of the abundance of the three species involved in the interaction. The study was conducted at eleven sites exhibiting varying levels of infection prevalence within fish (Duval et al. 2022), from June 2019 to June 2020. First, we tracked the relative abundance of each species by measuring their water eDNA concentration over time and investigated seasonal variations for the abundance of each species separately. We further investigated whether seasonal changes in abundance were associated with water temperature, a key environmental parameter for the life cycle of T. bryosalmonae and its two hosts. Considering that bryozoans enter a dormant stage during the winter and that the proliferation of T. bryosalmonae mainly depends on their activity (Tops et al. 2009), we expected bryozoan and parasite DNA concentrations to be low during the winter sampling sessions and higher in summer when water temperatures start rising and become favorable to bryozoan growth and to spore liberation. As a consequence, we expected significant associations between the abundances of T. bryosalmonae, F. sultana, and water temperature, and more specifically positive associations (the higher the water temperature, the higher the eDNA abundance of T. bryosalmonae and F. sultana), although these associations may be non-linear. Conversely, we expected trout eDNA concentrations to be more constant over time and to be independent from water temperature. Given its generation time, trout biomass should indeed be relatively constant over time, although we may observe peaks in eDNA concentrations when adults begin to spawn at the end of autumn and the beginning of winter (September–December), and hence a negative association between water temperature and eDNA abundance. Second, we tested for spatial synchrony among the different sampling sites for each species to investigate whether peaks of DNA concentration occur simultaneously among sites. We did not expect strong spatial synchrony among sites as their environmental conditions may strongly differ; for instance, some sampled rivers are snow-fed, whereas others are rain-fed, which strongly impacts their thermal regime, with snow-fed rivers warming later in the season than rain-fed rivers. We went further by testing the environmental factors that could influence the patterns of spatial synchrony among sites. We then tested for interspecific temporal synchrony between the two hosts and the parasite to investigate whether peaks of DNA concentrations co-varied temporally among species within sites. We expected that eDNA concentrations of the parasite and its hosts would fluctuate in synchrony over time, that is, that large concentrations of DNA should be observed at the same time period for hosts and the parasite; especially for the parasite and the bryozoan host. Finally, we tested the association between parasite infection prevalence measured in fish within each site and levels of synchrony between the parasite and the hosts, as we may expect that a higher levels of synchrony between them should promote encounters, hence facilitating the completion of the parasite's life cycle and ultimately increase infection prevalence in the fish population.
2 Materials and Methods
2.1 eDNA Sampling
The study area is located at the foothills of the Pyrenean Mountains, including the French departments of Ariège, Haute-Garonne, and Hautes-Pyrénées (Figure 1). The presence of the disease in this region was confirmed in 2016, when the Departmental Federation of Fishing of Ariège observed a high mortality of juvenile trout (Lautraite 2017). We then confirmed the presence of the parasite in many sites throughout the French Pyrenees rivers, with prevalence reaching 90%–100% in many disturbed sites (Duval et al. 2022, 2024).
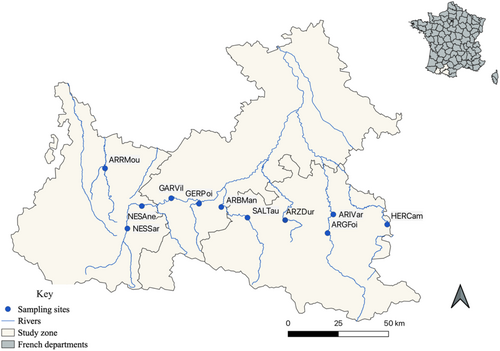
To investigate changes in parasite, brown trout, and bryozoan DNA concentrations over time, water samples were collected from eleven sites (Table 1, Figure 1) over a period of 1 year, from June 2019 to June 2020. Sampling sessions occurred in June, August, September, and November 2019, and in February, May, and June 2020, with six 1-L water samples collected in sterile bags (Whirl-Pak) at each site during each sampling session. A multiparameter probe (In Situ) was used to measure the physicochemical parameters of the water at each session, including pH, conductivity, dissolved oxygen concentration (DO), turbidity, and temperature. We also automatically recorded water temperature in each site every 4 h between June 2019 and June 2020 with a HOBO logger. Using this data, we measured the water temperature averaged across the five days preceding each water sampling (hereafter named T5). This allowed for precisely accounting for the thermal conditions before sampling given that T. bryosalmonae and F. sultana life cycles are expected to be strongly associated with water temperature (Tops et al. 2006). We also retrieved the average daily discharge (m3/s) from the Hydroportail (v3.1.4.3) data portal, using the closest gauging station from each study site. As the water flow strikingly varies over the course of the season, DNA concentrations may have been either diluted or concentrated during high-flow and low-flow periods, respectively, hence biasing eDNA concentration estimates. We therefore adjusted the raw eDNA concentration estimates to a constant water flow by dividing each eDNA concentration value by the discharge observed on the sampling day, and we expressed the eDNA concentration per m3.s−1.
| Code | River | Location | Latitudinal position (°) | Longitudinal position (°) | Parasite prevalence (%) | Mean temperature (°C) |
|---|---|---|---|---|---|---|
| ARBMan | L'Arbas | Mane | 43.0725 | 0.9506 | 5 | 13.60 |
| ARGFoi | L'Arget | Foix | 42.9654 | 1.6030 | 0 | 11.21 |
| ARIVar | L'Ariège | Varilhes | 43.1064 | 1.2061 | 75 | 11.66 |
| ARRMou | L'Arros | Moulédous | 43.2299 | 0.2317 | 91 | 12.99 |
| ARZDur | L'Arize | Durban-sur-Arize | 43.0215 | 1.3469 | 80 | 12.43 |
| GARVil | La Garonne | Villeneuve-de-Rivière | 43.1057 | 0.6431 | 25 | 13.13 |
| GERPoi | Le Ger | Pointis-Inard | 43.0845 | 0.8138 | 0 | 13.93 |
| HERCam | L'Hers | Camon | 43.0086 | 1.9667 | 80 | 12.73 |
| NESAne | La Neste | Anères | 43.0664 | 0.4643 | 30 | 10.68 |
| NESSar | La Neste | Sarrancolin | 42.9635 | 0.3809 | 5 | 9.80 |
| SALTau | Le Salat | Taurignan | 43.0276 | 1.1124 | 25 | 10.80 |
2.2 DNA Concentration Measurements
2.2.1 DNA Filtration and Extraction
Water filtration was performed back in the laboratory a few hours after sampling and using a Nalgene vacuum filtration system. Each water sample was filtered using nitrocellulose filters of 47 mm diameter and pore size of either 0.45 μm or 0.8 μm, depending on water turbidity. The filters (6 per site per session, with 1 filter per sample) were then pooled by pairs in 5 mL Eppendorf tubes to constitute three replicates per site and per session. DNA was then extracted from the filters using the DNeasy Power Soil Pro kit (Qiagen) in accordance with the supplier's protocol.
2.2.2 DNA Amplification and Quantification
DNA from T. bryosalmonae, F. sultana, and S. trutta was quantified by Digital PCR (dPCR). We chose dPCR because it has been shown to be more precise and repeatable than qPCR for aquatic eDNA samples (Brys et al. 2021; Mauvisseau et al. 2019; Wood et al. 2019). We used published species-specific primers and probes to amplify the DNA of each species, targeting mitochondrial DNA regions: the 16S RNA and the COI gene for the parasite and the bryozoan, respectively (Carraro et al. 2018), and the cytochrome B for the trout (Carim et al. 2016).
dPCRs were conducted in duplex (parasite-bryozoan and parasite-trout) using the Bio-Rad QX200 ddPCR (droplet dPCR) system in a 22 μL total volume. Each mix was composed of 2.4 μL of extracted DNA, 1.9 μL of primer per species, 0.5 μL of probe, and 11 μL of Digital PCR Supermix (Bio-Rad). This PCR preparation is then mixed with oil and emulsified using the QX200 Droplet Generator (Bio-Rad) to generate the droplets. Within each of these drops, a PCR takes place independently when target DNA is present. The QX200 Droplet Reader (Bio-Rad) was then used to estimate the absolute concentration of each species for each sample.
Three negative controls and one positive control were included in each 96-well plate. Amplification results were read using Quanta Soft (Quanta Soft Software 1.7, Regulatory Edition, Bio-Rad). The threshold for the separation of negative and positive drops was arbitrarily set for each 96-well plate in accordance with the distribution of positive and negative control drops. Finally, the DNA concentration in each sample was quantified in copy number/μl using the ratio of positive drops to the total number of drops formed (then divided by the daily water flow, see above).
2.3 Data Analyses
All statistical analyses were conducted using R software (R Core Team, RStudio 2021). Datasets and scripts are available online (Blanchet 2024).
To test whether the abundance of each species (DNA concentration in the water samples) varied among sampling sessions, we performed generalized linear mixed models (GLMMs) using the glmmTMB package (Magnusson et al. 2017; Bolker 2016). Sampling session was included as a fixed categorical effect, whereas sampling site was included as a random effect due to the temporal dependency of the data. As the parasite and bryozoan DNA concentrations were sporadically distributed in the rivers, we used a negative binomial distribution to account for overdispersion in the data. Each model was then tested with a type II analysis of variance (ANOVA). For testing associations between the abundance of each species and water temperature during sampling, we used linear models in which the DNA concentration of each species (log-transformed to ease linear modeling) were the response variable and T5 (the water temperature averaged across the five days preceding sampling) was the continuous dependent variable. For this analysis, we used only the subset of sites for which all the parasites have been found, either in the water or in the fish (Table 1). In each model (one per species), we included the quadratic and cubic terms for T5 (in top of the single term) to test for potential non-linear relationships (a cubic model allows fitting up to two incurvations). The significance of each term (T5, T52, T53) was tested using a t-test. We simplified models to conserve only significant terms, and we verified that these simplified models were the most likely for fitting the data using the Akaike Information Criteria. Note that running the models with raw data (not logged-transformed) and assuming a negative binomial distribution (as above) provided the same findings.
To test whether peaks of DNA concentration occur at the same timing among sites (spatial synchrony), we used Spearman correlation tests to assess whether the DNA concentration of each species measured along the year co-varied between pairs of sites. This approach resulted in a matrix of correlation coefficients for each species. These correlation coefficients were interpreted as a measure of synchrony between sites; if the DNA concentrations measured at a given site are associated (co-vary) positively with DNA concentrations measured at another site, this indicates a synchrony between these sites. To test whether—over all pairwise comparison—there was a significant signal of spatial synchrony, we estimated the mean coefficient of correlation from each matrix, as well as its 95% confidence intervals (95% CI).
We then ran multiple regression models on distance matrices (MRMs) to identify environmental factors that may influence the strength of the synchrony between pairs of sites for each species. The MRM function, available in the ecodist package (Goslee et al. 2020), was used to implement distance matrices between pairs of sites as explanatory variables in regression models. We used a matrix of mean water temperature distances over the year, a matrix of mean environmental distances (combining pH, conductivity, turbidity, dissolved oxygen concentration), a matrix of distances between geographic coordinates (latitude and longitude), and a matrix measuring the difference in parasite infection prevalence measured in fish (see Table 1) between pairs of sites (assuming that two sites with similar prevalence should display similar synchrony) as explanatory variables. Distances were estimated using the dist() function in R, and we used Euclidean distance for all matrices. The significance of each factor was assessed using the permutation test (1000 permutations) in the MRM function.
We then tested for temporal synchrony between pairs of species within each site by conducting Spearman correlation tests. As described above, correlation coefficients were interpreted as estimates of species synchrony; the higher the correlation coefficients between two species, the higher the synchrony. Mean synchrony across all sites (and its 95% CI) and for each species pair was estimated as above by averaging all values of correlation coefficients. We tested parasite/bryozoan and parasite/trout combinations but also the bryozoan/trout combination to test whether the two hosts were synchronous in time.
Finally, we estimated the association between parasite prevalence measured in the fish host (Table 1) and the level of trout-parasite synchrony and bryozoan-parasite synchrony, respectively, using a multiple linear regression (lm function in R). We predicted that parasite prevalence (an estimate of cycle efficiency) should be higher in sites with a strong synchrony between the parasite and the hosts.
3 Results
3.1 Seasonal Variation in DNA Concentrations and Association With Water Temperature
For all species, that is, T. bryosalmonae, F. sultana, and S. trutta, DNA concentrations varied between sessions (respectively χ2 = 14.469, df = 6, p = 0.025; χ2 = 9.281, df = 6, p = 0.158; χ2 = 164.21, df = 6, p < 0.001), although these variations were significant only for T. bryosalmonae and S. trutta. For all species, there was a trend toward a higher peak of DNA concentration during summer (August and September 2019, Figure 2). For both trout and bryozoans, we observed another peak (although less important in intensity) during winter (February, Figure 2b,c). As expected, we also observed significant associations between DNA concentrations and the water temperature for all three species (Figure 2a–c, Table S1a–c). However, the form of the relationship varied among species. For T. bryosalmonae (Figure 2a), the best-fit model (Table S1a) indicated a linear and positive association between DNA concentrations and temperature; the highest concentration in T. bryosalmonae was observed when the water was the warmest, corresponding to the summer months. For F. sultana (Figure 2b), the best-fit model (Table S1b) indicated a non-linear relationship between DNA concentrations and temperature characterized by two inflections; during winter months the DNA concentration of F. sultana slightly increased with water temperature until reaching a plateau at ~9°C–10°C (i.e., corresponding to the spring months), and then strongly increased substantially as soon as temperature reached ~15°C (during the summer months). This suggests that two phases of growth are observed for F. sultana. Finally, for S. trutta (Figure 2c), the best-fit model (Table S1c) described a U-shaped curve suggesting that brown trout DNA concentration was the highest when water temperature was the lowest and the highest, respectively, which deviates from our expectation.

3.2 Synchronism Between Sites for Each Species
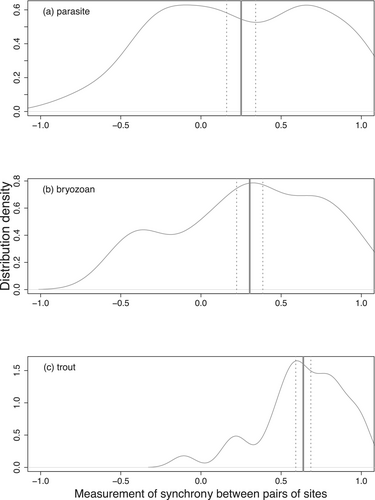
Overall, the DNA concentration of the parasite, the bryozoan, and the trout were positively correlated between pairs of sites (Figure 3). Bryozoan and parasite show significant but relatively weak synchrony between pairs of sites (mean [95% CI]; 0.304 [0.222–0.385]; 0.251 [0.160–0.341], respectively). The distributions of pairwise coefficient correlations for both the bryozoan and the parasite show both strong synchrony (positive values) and some levels of asynchrony (negative values) among the sites, and the range of synchrony estimates displays a strong variance among pairs of sites (Figure 3a,b). For trout, the mean synchrony between pairs of sites was high and strongly significant (mean [95% CI], 0.638 [0.591–0.685]). Most individual estimates of synchrony were higher than 0.3 (Figure 3c), which indicates that, over all sites, trout abundances are synchronized over time.
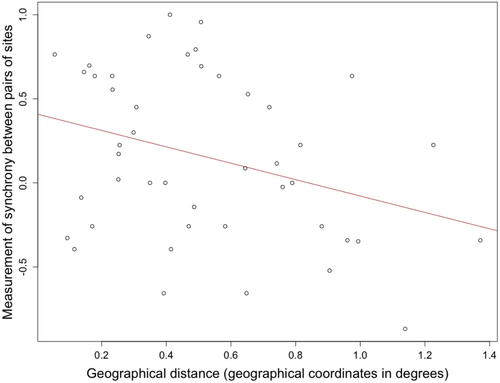
Regarding factors (water temperature, other measured environmental factors, geographic distance, infection prevalence), MRMs revealed that none of the factors we tested was significantly associated with variation in synchrony between pairs of sites. We only detected a tendency toward a negative relationship between the parasite synchrony between sites and their geographic distance (rM = −0.396, p = 0.057, Table 2). As expected, the closer the sampling sites geographically, the higher the temporal synchrony for the parasite eDNA concentration (Figure 4).
| Predictor variables | Correlation coefficient | p |
|---|---|---|
| a. Tetracapsuloides bryosalmonae | ||
| Physicochemical distance | −0.012 | 0.930 |
| Geographic distance | −0.396 | 0.057 |
| Temperature distance | 0.013 | 0.838 |
| Prevalence distance | 0.259 | 0.239 |
| b. Fredericella sultana | ||
| Physicochemical distance | −0.030 | 0.865 |
| Geographic distance | 0.278 | 0.200 |
| Temperature distance | 0.080 | 0.181 |
| Prevalence distance | −0.314 | 0.112 |
| c. Salmo trutta | ||
| Physicochemical distance | −0.157 | 0.100 |
| Geographic distance | 0.052 | 0.709 |
| Temperature distance | 0.022 | 0.444 |
| Prevalence distance | −0.132 | 0.287 |
3.3 Synchronism Between Hosts and Parasite
For all species pairs, we found that species abundances were synchronous over time within sites. The average measures of synchrony between parasite and bryozoan DNA concentration and between parasite and trout DNA concentration were both moderate in strength but significantly different from zero (mean [95% CI], 0.316 [0.079–0.553] and 0.344 [0.156–0.531], respectively, Figure 5a,b). Contrastingly, the synchrony between trout and bryozoan DNA concentration was strong (mean [95% CI], 0.589 [0.329–0.850], Figure 5c).

3.4 Association Between Parasite Prevalence and Hosts–Parasite Synchrony
We found a significant association between infection prevalence in the fish host measured at the site level and the strength of the temporal synchrony between the eDNA concentration of the parasite and that of the fish host (F1,8 = 6.099, p = 0.043, rPearson = 0.624). The higher the strength of the synchrony, the higher the parasite prevalence in the fish populations (Figure 6a). We did not detect a significant association neither between prevalence in fish populations and the synchrony between the eDNA concentration of the parasite and that of the bryozoan host (F1,9 = 1.706, p = 0.232), nor between parasite prevalence and the synchrony between the eDNA concentration of the fish and that of the bryozoan host (F1,7 = 1.865, p = 0.214). However, a visual inspection of biplots revealed a positive tendency between parasite prevalence in fish populations and the strength of the synchrony between bryozoan and the parasite (Figure 6b), and this tendency was strongly significant when an outlier site (HERCam, see Figure 6b) was not taken into account in the model (F1,8 = 13.306, p = 0.011, rPearson = 0.752). Hence, there is substantial evidence that parasite prevalence in fish populations is higher when the temporal synchrony between the parasite and the two hosts is high.

4 Discussion
The use of temporal environmental DNA monitoring has, for the first time, enabled the study of temporal synchrony between T. bryosalmonae and its two main hosts in a natural environment. Firstly, this approach enabled the detection of patterns of variation in the DNA concentrations of each species, avoiding the need for invasive or complex sampling strategies, and underlining the effectiveness of eDNA as a tool for monitoring host–parasite interactions over time. Indeed, by combining eDNA with dPCR techniques, which have been shown to provide reliable abundance estimates (Doi et al. 2015; Carraro et al. 2018), we were able to gain insights into the temporal dynamics of the three interacting species. Accordingly, Stelzer et al. (2023) have recently indicated that the use of dPCR together with precise sampling combinations—such as filtration methods and the number of replicates—allows for the optimal detection of Tetracapsuloides bryosalmonae.
Our results suggest that there is seasonal variation in the abundance of each species. As we expected, the highest levels of parasite eDNA were observed during the summer months (from June to September). This observation in multiple sites in nature is consistent with previous in vivo studies (Tops et al. 2009) suggesting that T. bryosalmonae multiply when water temperatures increase, that is, in spring and summer. The eDNA peak of the parasite observed from June to September may correspond to the overlap of two shedding events: one from the released spores from infected bryozoans in early summer and one from the released spores from infected trout later on (Bailey et al. 2017; Ros et al. 2022). Nevertheless, we may not see a peak as high as in 2019 in the early summer of 2020 (June) probably because the changing environmental conditions may prevent the parasite from being released, or only to a very small extent. Concerning the bryozoan, we detected the highest concentration in August and September. This probably corresponds to mechanisms of asexual propagation including fission of gelatinous colonies (Hill and Okamura 2007) and fragmentation of tubular, branching colonies (Tops et al. 2006). Conversely, in our study, the absence of the bryozoan peak expected in spring could be explained by the fact that overt infections generally reduce bryozoan colony growth rates (Tops et al. 2009), thus the statoblast production could be very low, and statoblasts are generally malformed and exhibit markedly reduced hatching success (Hartikainen and Okamura 2012). This hatching issue could explain the finding that the DNA concentration of bryozoan hosts remains low and plateaued during spring, that is, from ~9°C to ~15°C, whereas in the meantime (in particular in June 2019) the DNA concentration of the parasite in the water increases. Overall, the dynamics of DNA concentrations of both the bryozoan and the parasite over the year were remarkably similar, suggesting a potential synchrony between these two species. However, due to their small size and ability for dispersal in water, these organisms are likely to leave minimal DNA traces in the environment outside the periods of reproductive activity, resulting in low or near-zero concentrations during other periods. We also observed another peak of bryozoans (less important in intensity) during winter (February) probably corresponding to the production of numerous asexual, seed-like propagules called statoblasts which are highly resistant, dormant stages that persist during unfavorable conditions (Hartikainen and Okamura 2015). Our analysis linking water temperature to DNA concentration in the water confirms that two periods of growth may exist for bryozoans: a first period as soon as the water reaches 8°C–9°C and a second period of intense growth later in the season as soon as the water temperature reaches ~15°C. Where traditional methods often struggle to capture such fine temporal dynamics, especially in the case of low-density or cryptic organisms like bryozoans and their parasite, the use of eDNA enabled us to detect these very weak concentrations in a diluted environment.
Furthermore, our findings indicate the presence of concentration peaks in August–September for brown trout, and a secondary peak (smallest in intensity) in February. We also observed that brown trout DNA concentration was—contrary to expectation—significantly associated with water temperature, with DNA concentration being highest when water temperature was either maximal or minimal. The first peak observed in August and September may be explained by an increase in the metabolic rate of trout due to higher water temperature, which is coherent with previous findings (Lacoursière-Roussel et al. 2016). The second peak observed in February was smallest in intensity and may correspond to either eggs hatching or fry emerging, which typically occurs in February–March in brown trout (Baglinière et al. 1979). If confirmed, this secondary peak in trout eDNA that we associate with the arrival of young-of-the-year (YOY) trout is important as it temporally coincides with the first peak of bryozoan (first period of growth), and because YOY are very important hosts of the parasite. Although further detailed studies are needed, this synchrony between the eDNA abundances of trout and bryozoan in February might not be anecdotal for the parasite life cycle.
The second aim of our study was to analyze spatial synchrony between the different sites for the studied species. Our analyses revealed that all species showed significant synchrony between sites. Notably, brown trout exhibited a stronger and less variable synchrony compared to the other species, which was expected given that trout abundance does not vary much over the season, but when YOY are emerging from the gravel. In comparison, the synchrony of bryozoan abundance between sites was relatively weak, though significant. For both brown trout and bryozoans, none of the measured environmental parameters were found to explain the observed synchrony. The strength of spatial synchrony between sites was not affected by temperature, geographical distance, or environmental parameters. Yet, based on previous studies on the life cycle of bryozoans, we expected to find a significant effect of temperature on their abundance (Tops et al. 2006). For brown trout, our results are consistent with previous studies that did not find any effect of environmental parameters or temperature on their spatial synchrony at the adult life stage (Bret et al. 2016; Cattanéo et al. 2003). According to these studies, environmental synchrony or hydrological synchrony between sites only affects the degree of synchrony of juvenile trout. It can therefore be assumed that the dynamics of this species are governed by internal phenomena that are specific to its life cycle.
Parasite synchrony between sites was found to be moderate, with significant variation observed depending on the pairs of sites considered. This heterogeneity between sampled sites was explained by their geographical distance, with closer sampled sites showing greater synchrony in parasite abundance. There are several potential explanations for the observed synchronism of populations between geographically related sites. First, it could be due to trout dispersal (Solomon and Templeton 1976; Ranta et al. 1998; Cattadori et al. 2000), which could result in dispersal of T. bryosalmonae spores between nearby sites when shed into the environment. However, given the large distances among sites, it is unlikely that trout dispersal strongly contributes to observed patterns. Alternatively, previous studies testing the effects of climate on animal populations have shown that geographic location can be a good proxy for climate (Rieman et al. 2007), as sites closer to each other tend to have more similar climates. Our measure of geographical distance could therefore be viewed as a proxy for a measure of climatic difference, suggesting that patterns of T. bryosalmonae synchrony between closer sites could be determined by climatic factors other than those studied here (water temperature), such as rainfall, percentage of sunshine, or ambient temperature, illustrating a Moran effect. The Moran effect (Moran 1953) describes the fact that the correlation between two population dynamics is equal to the calculated correlation between the climatic factors of the two habitats. It is therefore possible that a significant effect of climate on patterns of T. bryosalmonae synchrony could be revealed by a more precise environmental characterization of the local climate.
The eDNA monitoring further allowed us to observe a clear temporal seasonal synchrony between the parasite T. bryosalmonae and its two hosts, the bryozoan and the trout. It is noteworthy that the seasonal synchronies we observed may not represent adaptive strategies per se, but rather similar responses to similar stimuli (e.g., temperature), which are difficult to decipher in the wild. Nonetheless, it is widely accepted that parasites have evolved strategies in response to both host-related and environmental stimuli to reduce the risk of desynchronization between their infectious stages and the suitable hosts (Krasnov 2008; Calero-Torralbo et al. 2013). Despite the importance of seasonal synchrony in host–parasite life cycles, well-documented cases remain relatively rare compared to the extensive work on circadian rhythms (but see Larimore 1987; Rolff 2000; Randolph 2004; Loot et al. 2006). Indeed, most studies have focused on daily biological cycles, including diel, tidal, and lunar rhythms (see e.g., Motta et al. 2023). Also, most studies on synchrony have mainly focused on determining key environmental factors such as photoperiod, temperature, or host life-history traits, including predictability, distribution, and density that may drive synchrony. In contrast, seasonal dynamics, such as how parasites adjust their life cycles to match the host's seasonal behaviors, remain poorly explored. Our study fills this gap by contributing to a better understanding of temporal coordination between the parasites and its two hosts. Alternatively, some researchers have found cases of asynchrony and suggest that a degree of stochasticity might benefit parasites—particularly generalist parasites—by helping them cope with host unpredictability or access alternative resources. Then, synchrony appears to be the most effective strategy for specialized parasites targeting predictable hosts. In our study, the parasite is a specialist, infecting the predictable and abundant hosts F. sultana and brown trout. We can then hypothesize that synchrony in this parasitic cycle would be advantageous. Accordingly, we showed that stronger synchrony was associated with higher parasite prevalence in fish populations. This provides compelling evidence that temporal synchrony between the parasite and its hosts facilitates the parasite's life cycle and significantly increases its prevalence in fish populations.
In conclusion, this study provides new insights into the emerging parasite T. bryosalmonae by demonstrating that the temporal dynamics of its life cycle might be optimized to fit that of its two hosts. Beyond demonstrating that the abundances of T. bryosalmonae and its two main hosts' abundances are synchronous in space and time, we provide evidence that synchronism favors the prevalence of T. bryosalmonae in fish populations, which has, up to our knowledge, rarely been shown in the wild. However, the study of T. bryosalmonae synchronism with its hosts (and of other host–parasite interactions) could be refined by using a finer sampling temporality, with, for instance, samples collected on a bi-weekly basis during periods identified as peaks of abundances. Also, new tools such as automated samplers (e.g., Formel et al. 2021) might be particularly relevant to ease the sampling process of dynamics occurring at a fine time scale.
Overall, beyond the specific case of T. bryosalmonae, our study also highlights the value of environmental DNA as a noninvasive and highly sensitive tool for studying microscopic host–parasite interactions at both temporal and spatial scales. Indeed, the study of synchronism traditionally necessitates the use of sophisticated sampling methods to track each organism separately. It is evident that eDNA offers the distinct advantage of enabling the capture of DNA traces from a multitude of species within a single sample. Even when organisms are present in very small quantities within the environment, their DNA traces can still be identified in a targeted way. Consequently, scientists can obtain a more precise temporal picture of the dynamic interactions between the hosts and the parasite by employing this approach. Future studies employing more frequent eDNA sampling are likely to reveal even more precise patterns, potentially reshaping our understanding and management of ecological interactions and emerging diseases in freshwater ecosystems.
Author Contributions
G.L., S.B., and E.D. designed and coordinated the study. E.D., C.V., and M.L. contributed to the fieldwork. G.L., C.V., and E.D. performed laboratory work. C.M. and G.L. ran the statistical analyses. C.M., S.B., G.L., and E.D. interpreted the data and wrote the first draft of the manuscript. C.V. and M.L. read the initial draft, and all authors gave final approval for publication.
Acknowledgments
We warmly thank Camille Poesy and Laura Fargeot for their help in collecting water samples during the field work. This work was funded by the French Office for Biodiversity (OFB). E.D. was supported by a PhD grant from the French Ministry of Higher Education, Research and Innovation (MESRI). This work was supported by the LABEX TULIP (ANR-10-LABX-41).
Conflicts of Interest
The authors declare no conflicts of interest.
Open Research
Data Availability Statement
Data and script are made available from: https://doi.org/10.6084/m9.figshare.27198549.v1.




