Environmental Gradients, Not Geographic Boundaries, Structure Meiofaunal Communities in Siberian Seas
Funding: This work was supported by the Russian Science Foundation (21-77-30001), Ministry of Science and Higher Education of the Russian Federation (121021500057-4), Vetenskapsrådet (2017-01601, 2018-05489), Svenska Forskningsrådet Formas (2017-01513, 2018-01547), Norges Forskningsråd (315317), European Research Council (695331 ERC-AdG CC-TOP), and Naturvårdsverket (NV-802-0151-18).
ABSTRACT
Meiofauna (all invertebrates smaller than 1 mm) are not only sensitive to environmental changes but also contribute significantly to nutrient cycling and energy transfer to higher trophic levels. Despite their importance, meiofauna distribution and ecology in the Siberian seas remain understudied. Here, we employ sediment environmental DNA metabarcoding to characterize meiofauna diversity across the unexplored Siberian seas. We show that meiofauna community structure is primarily driven by river discharge and coastal erosion, which are heavily influenced by climate change, rather than geographical distinctions between the seas. We observed higher meiofauna diversity in nearshore areas where river plumes promoted colonizer nematode communities that are resilient to disturbances. Yet, their dominance may lead to decreased ecosystem stability in the future. This study provides a valuable baseline for meiofauna diversity in remote Siberian seas undergoing rapid environmental change, which will be useful for assessing the future direction and pace of benthic ecological trajectories.
1 Introduction
The Arctic is warming four times as fast as the global average rate (Rantanen et al. 2022). Among the Arctic seas, the Kara, Laptev, and East Siberian seas are particularly vulnerable regions to climate-driven changes, primarily due to their exceptionally shallow depths. The water depth is less than 50 m in more than 40% of the Kara sea (Osadchiev et al. 2021), 53% of the Laptev sea (Xie et al. 2023), and even 72% of the East Siberian sea area (Zonn et al. 2016). Therefore, projected changes in surface water such as warming, desalination, acidification, and nutrient enrichment can rapidly propagate to the seabed, impacting benthic communities.
Despite occupying only 1% of the global ocean volume, the Arctic Ocean receives a disproportionate 11% of the world's total riverine runoff, making it more influenced by riverine input than any other ocean (März et al. 2022). Hydrological models indicate that freshwater discharge to the Laptev and East Siberian shelf will increase by 25%–50% by 2100 (Mann et al. 2022) due to ongoing global warming. Such massive river runoff negatively affects macrofaunal communities near Siberian River deltas by creating a stressful environment with high sediment loads and variable salinities, leading to lower species richness and biomass (Vedenin et al. 2018). In addition, shallow nearshore zones in the Arctic experience intense coastal erosion and may freeze all the way to the bottom, leading to benthic habitat destruction (Ellis et al. 2000). Other factors influencing benthos along river-sea gradients are depth, sediment structure, and bottom water chemical parameters (Vedenin et al. 2015). The seawater chemistry can be significantly impacted by the combined effects of accelerating permafrost thawing, coastal erosion, lowering of groundwater levels, and increased river runoff, which together result in the delivery of even more particulate matter, carbon, and nutrients into the seas that were previously trapped in permafrost (Frey and McClelland 2009; Martens et al. 2022; März et al. 2022; Vonk Vonk et al. 2012). In addition, oxidation of eroded carbon to carbon dioxide (CO2) causes extreme acidification of the shallow Laptev and East Siberian seas (Anderson et al. 2011; Semiletov et al. 2016).
As the benthos is impacted by these changes, shifts in composition and behavior of the benthic communities will in turn affect the fate of accumulation of organic and inorganic carbon at the seafloor. It is still unclear; however, if the fauna in the three Siberian seas will be affected in the same way, as the seas have different riverine and oceanic inputs, which may lead to varied responses to climate change. For example, the East Siberian Sea has been singled out as the most biogeochemically active, oligotrophic sea, and at the same time has the most impoverished and thus fragile benthic diversity (Kokarev et al. 2017). Moreover, it is largely unknown how meiofauna (invertebrates < 1 mm)—the most diverse and abundant faunal component in marine systems—will respond to the changing Arctic. Meiofauna is being recognized as an excellent tool for monitoring environmental changes due to its quick generation turnover, high diversity, abundance, and sensitivity to environmental parameters (Zeppilli et al. 2015). In addition, meiofauna is important for sediment biogeochemistry as there is evidence of meiofauna contributing significantly to benthic oxygen respiration (Maciute et al. 2023), stimulation of organic matter degradation (Nascimento et al. 2012), increase in denitrification rates (Bonaglia et al. 2014), oxygenation of the sediment, and toxic sulphide removal (Bonaglia et al. 2020). Recently, it has also been shown that methane fluxes can be slightly offset by high meiofauna abundance (Broman et al. 2024), which may be especially relevant to the Siberian seas. The effect on sediment biogeochemistry, however, depends on both the diversity and abundance of meiofauna.
There is a lack of comprehensive data on the distribution and diversity of meiofauna across the Kara, Laptev, and East Siberian seas. Dedicated studies are scarce and often focus on small areas or a single meiofauna group (foraminifera, ostracods, copepods, nematodes) (Chertoprud et al. 2018; Garlitska et al. 2019; Portnova et al. 2019, 2021; Vanaverbeke et al. 1997; Zenina et al. 2024). One reason behind this is that meiofauna studies in the Siberian Arctic rely exclusively on morphological identification, consisting of sediment sorting under a microscope. Manual morphological identification is time consuming and requires specific taxonomic expertise, making it costly and unpractical for large-scale surveys, such as the large Siberian seas. In such systems, addressing biodiversity changes requires monitoring strategies that account for the rapid pace of environmental change. High-throughput amplicon sequencing, so-called metabarcoding, of environmental DNA (eDNA) samples is a potential alternative or addition to the traditional morphology-based approach. Despite its potential, metabarcoding has not been applied in the Siberian Arctic to investigate macro- or meiofaunal diversity.
Our goal was to investigate how environmental parameters affect spatial diversity and vertical distribution within the sediment of meiofauna and nematode communities on the Siberian shelf. To achieve this, here we explore the potential of benthic metazoan 18S small subunit (SSU) rRNA metabarcoding across three sediment depth horizons sampled from latitudinal transects of 150 to 533 km length within the Kara, Laptev, and East Siberian seas. We then compared our molecular data to existing morphological identification-based studies and investigated the influence of environmental parameters on metazoan and nematode diversity. We used environmental parameters published in previous studies from the same expedition and additionally performed sediment porewater oxygen concentration measurements. The latter was not only used to explain diversity patterns but also to provide unique insight into sediment oxygen penetration, diffusion-mediated oxygen uptake, and volume-specific oxygen uptake within coastal Siberian sediments.
2 Materials and Methods
2.1 Sediment Sampling
Sediment samples were collected during the 82nd expedition aboard the Russian R/V Akademik Mstislav Keldysh (AMK82) as a part of the International Siberian Shelf Study 2020 (ISSS-2020) expedition. This expedition is a continuation of a two-decade study and was nominated by NASA as the best biogeochemical expedition of the International Polar Year (2007/2008). A notable characteristic of the East Siberian Arctic Shelf is an extremely large gradient of hydrological and biogeochemical parameters from Wrangel island to the New Siberian islands, that corresponds to geographically critical contrasts in the Arctic system where the Pacific and local shelf waters interact over the shelf (Semiletov et al. 2005, 2016). Based on the distribution of the chemical and hydrological data, two biogeochemical provinces have been identified in the shallow Siberian seas: a western province that is influenced strongly by the freshwater flux and particulate material transport of the coastal eroded material (Lena discharge signal is negligible), and an eastern province that is under the influence of Pacific-derived waters. From year to year, the longitude shift of the frontal zone (FZ) between western and eastern provinces may reach 10° and more. During the ISSS-2020 cruise, a narrow FZ located roughly near 160 E separated the local shelf waters and the Pacific-derived waters, which were not reached yet. Thus, the ISSS-2020 study area is fully located in the Western biogeochemical province characterized by low primary production (Rusanov et al. 2024).
The expedition took place from late September to early November 2020, covering three shelf seas: Kara, Laptev, and the western part of the East Siberian Sea (Figure 1). The stations on this expedition were designated as AMK82-6931 to AMK82-7012, following the sequential station numbering system used in R/V Keldysh expeditions (Table S1). However, in this study, we have assigned our own station labels, indicating the respective sampled seas (Table S1). Sediment sampling for this study was performed at 20 stations (Figure 1) using a Multicorer (MUC) sampler equipped with 8 acrylic liner tubes (10 cm diameter). At each station, one sediment core was sampled for sediment microprofiling and an additional core for bulk sediment DNA preservation. For sediment oxygen microprofiling, large MUC core liners were carefully subsampled using smaller (4.5 cm diameter) liners and were then immediately brought to the laboratory for microprofiling.

To minimize cross-contamination between sediment layers during later DNA sediment sampling, MUC liners were predrilled with holes (2.3 cm diameter). The holes were positioned on three sides of the liners and continued from the top to the bottom of the liner at 1.5 cm intervals. Before each MUC cast, the holes were tightly sealed using tape. After retrieval of the sediment cores, the tape was removed, and 5 mL cutoff sterile syringes were inserted horizontally into the sediment through the holes. Three sediment layers (0–1.5, 1.5–3, and 3–4.5 cm) were sampled. A 3 mL of sediment was collected from each layer. Temperature fluctuations can degrade DNA. Thus, to address potential temperature fluctuations during later transportation of sediment samples to Europe, we added 10 mL of RNAlater stabilization solution (Thermo Fisher Scientific, AM7021) to the 15 mL Falcon tubes containing the sediment, vortexed it, and immediately froze it at −80°C onboard. At the end of the expedition, frozen samples were transported from Russia to Sweden by refrigerated trucks (−20°C). In Sweden, the samples were placed back in a −80°C freezer until DNA extraction.
2.2 Ex Situ Porewater O2 Measurements and Calculations of O2 Uptake
High-resolution vertical oxygen concentration profiles were obtained to determine oxygen penetration depths, diffusion-mediated oxygen uptake (DOU), and depth distribution of the volume-specific oxygen uptake in the sediment. Oxygen microprofiles were recorded using oxygen Clark-type custom-made microsensors (tip diameter 20–40 μm, detection limit 0.3 μM). The sensors were mounted onto a motorized micromanipulator (MM33, Unisense, Denmark) and sensor signals were recorded on a four-channel multimeter (Unisense, Denmark) connected to a laptop. The profiling software was set to perform oxygen measurements at 100 μm depth intervals. The relative position of the sediment surface was derived from a distinct shift in the concentration gradients. During the measurements, sediment cores were in an aquarium filled with tap water, which was cooled to the lowest possible temperature (1.5°C) using a water circulator unit (Julabo GmbH, Seelbach, Germany). To recreate a realistic diffusive boundary layer of 500 μm above the sediment, a gentle airflow was introduced at a 45° angle, just above the water column.
At each station, two to five oxygen profiles were recorded, with oxygen sensors calibrated at each location. The O2 sensor was calibrated using a two-point calibration procedure in air-saturated bottom water (100% air saturation [or 21% O2]) and in bottom water after vigorous bubbling with nitrogen gas (0% O2). Oxygen penetration depth (OPD) was defined as the depth at which O2 porewater concentration became 0 μM. To calculate the diffusive oxygen uptake (DOU) and volume-specific oxygen uptake, each recorded oxygen concentration profile was analyzed using PROFILE software (Berg et al. 1998). The software provides the best fit to the measured oxygen concentration and estimates the O2 flux as a function of depth by assuming steady-state conditions (Berg et al. 1998).
2.3 DNA Extraction and Sequencing
Before DNA extraction, sediment samples were thawed and centrifuged at 3000 g for 5 min to remove RNAlater stabilization solution. DNA was then extracted from 2.9 ± 0.7 g of the sediment using the DNeasy PowerMax Soil DNA Isolation Kit (Cat# 12988-10; QIAGEN). After the DNA extraction, samples were frozen at −20°C in 5 mL of C6 elution buffer (10 mM Tris). Extracted DNA samples were then shipped to Novogene (Cambridge, UK) for PCR amplification, library preparation, and sequencing. The DNA concentration was normalized by Novogene according to their in-house company protocols. Amplification of metazoan 18S rRNA gene V4 region was conducted with the TAReuk454FWD1 (5′-CCAGCA(G/C)C(C/T)GCGGTAATTCC-3′) and TAReukREV3 (5′-ACTTTCGTTCTTGAT(C/T)(A/G)A-3′) primers (Stoeck et al. 2010). Library preparation was conducted using the TruSeq DNA PCR-Free Sample Preparation Kit (Illumina, USA) following manufacturer's recommendations. 19 samples (out of 60) did not pass the quality control after library preparation and could not be sequenced. Library of the remaining 41 samples was sequenced on the Illumina NovaSeq 6000 SP platform with a 250 bp paired-end setup.
2.4 Sediment and Bottom Water Properties
Surface sediment and bottom water properties were used to explain patterns in meiofauna diversity and to determine how different the three Siberian seas were. Bulk surface (0–1 cm) sediment data (TOC, TN, OC:TN molar ratio and δ13C) are shown in Table S1 and were obtained from a previously published study (Wild et al. 2023), which presents data from the same expedition. Briefly, upon sediment collection, the top layer was oven-dried at 60°C onboard, then acidified and analyzed using a Finnigan Delta Plus XP mass spectrometer coupled to a Thermo Fisher Scientific Flash 2000 Isotope Ratio Mass Spectrometer Element Analyzer via a Conflo II interface (Wild et al. 2023). Total organic carbon and nitrogen concentrations are given in weight percent (wt%). TOC shows the sum of both labile and refractory organic carbon. The organic carbon to nitrogen ratio (OC:TN) was calculated based on molar concentrations. Bottom water parameters, except bottom water oxygen concentration, were also obtained from the same previously published study (Wild et al. 2023). Depth, temperature, salinity, and turbidity were obtained using a Seabird SBE911 plus conductivity–temperature–depth (CTD) equipped with Niskin bottles, while bottom water oxygen was determined from oxygen concentrations in the sediment overlying water during sediment microprofiling. Bottom water nutrient (DON, NH4+, NO2−, NO3−) concentrations were analyzed using a QuAAtro-39 continuous segmented flow nutrient analyzer.
2.5 Bioinformatics
A total of 10.3 million sequences, averaging 251,871 pair-end reads per sample (41 samples in total) were analyzed according to the DADA2 (version 1.28) pipeline (Callahan et al. 2016) with default settings except for the filter commands: truncLen = c(0,0), maxEE = 2, truncQ = 2, trimLeft = c(0,0); error model: MAX_CONSIST = 30; merging of pair-ends: minOverlap = 10; and chimera removal: allowOneOff = TRUE, minFoldParentOverAbundance = 4. The DADA2 algorithm uses a parametric model of the errors (err) introduced by PCR amplification and sequencing. The error model is used to infer the true sample composition. Instead of creating operational taxonomic units (OTUs), DADA2 deduces sequence variants (ASVs), thereby offering a more precise approach that records the number of times each exact amplicon sequence variant was observed in each sample. After trimming, filtering, merging pair-ends, and chimera removal, a total of 36,121 amplicon sequence variants and 9.2 million sequences were retained, averaging 223,339 reads per sample (minimum 30,232, and maximum 310,926 reads per sample).
After building the ASV table, sequences were assigned to taxonomy using the SILVA database version 132 (Quast et al. 2012) using the assignTaxonomy function. This version was selected because it is the latest one that is compatible with the dada2classifier, has been extensively benchmarked, and all diversity metrics reported herein are calculated from ASVs and therefore remain unaffected by subsequent taxonomic annotation. Because SILVA taxonomy annotation has been previously found insufficient for microscopic metazoans (Broman et al. 2019), DADA2 sequence variants were additionally aligned and annotated against the NCBI NT database using blast 2.7.1+ (Altschul et al. 1991) on 25 October 2023 with a 0.001 e-value threshold and -max_target_seqs 1 to only report the top hits (Broman et al. 2019). The NCBI NT accession numbers for each sequence were imported into MEGAN6 and were linked to taxonomic labels with the MEGAN supplied database (megan-nucl-Feb2022.db). This made it possible to retrieve taxonomy names based on NCBI accession numbers and estimate more specific taxonomy with the use of the Lowest Common Ancestor (LCA) algorithm (Huson et al. 2016). The function “readName_to_taxonPathKPCOFGS” was used to extract all assigned DADA2 sequences with their affiliated taxonomy path. These results were then combined with the DADA2 sequence variant counts, and the results based on the NCBI NT database were used for taxonomy analyses. Sequences affiliated with Metazoa in the taxonomic description were extracted from the dataset and analyzed further as relative abundances (i.e., [counts/∑counts] × 100) in R software (version 4.3.0) (Team 2020). In the final dataset, Sipuncula phyla was included in the Annelida, as it is not considered a separate phylum anymore (Dordel et al. 2010).
Based on assigned nematode genera, the maturity index (MI) (Bongers 1999) was calculated to distinguish between habitat colonizers and persisters, using a 1–5 scale where values closer to 1 represent colonizers, and values closer to 5 indicate persisters. The index was calculated by summing the weighted c-p values for each nematode ASV, using their relative abundances in each sample (Broman et al. 2019).
2.6 Calculation of Terrestrial Carbon Contribution in Surface Sediments
The δ13C data were used for the relative contribution of terrestrial organic carbon to total sediment organic carbon in surface sediments estimation with a linear two-end-member carbon isotope model according to this formula: TerrC (%) = (δ13Caut − δ13Csample)/(δ13Caut − δ13Cterr) × 100, where TerrC is the fraction of terrestrial carbon while δ13Cterr and δ13Caut are the δ13C values of the terrestrial and autochthonous (marine) end-members, respectively. The δ13C sample is the measured value of the sample at each station. For the Kara Sea, we used the δ13C value of −27.8‰ as the terrestrial end-member, and −19.1‰ as a marine end-member (Fernandes and Sicre 2000). For the Laptev Sea, we used −23‰ and −28‰ as marine and terrestrial end-members, respectively (Brüchert et al. 2018). While for the East Siberian Sea, we used −19‰ and −28‰ (Brüchert et al. 2018).
2.7 Statistics
Statistical analyses were conducted in RStudio software (Team 2020). Bottom water and sediment physicochemical and environmental variables were used to determine optimal number of clusters ranging from k = 2 to k = 8, we performed hierarchical clustering using the base R function hclust() with Ward's method on the Euclidean distance matrix. Clustering results were visualized using the factoextra v1.0.7 R package to explore the similarities among sampling stations based on these variables (Kaufman and Rousseeuw 2009). Each parameter within each cluster was then tested to detect statistical differences using a nonparametric Wilcoxon signed-rank test. Spearman correlation coefficients were calculated to assess the relationships among all the variables. Subsequently, only the statistically significant variables that had correlation coefficients below 0.7 were selected for distance-based redundancy analyses (db-RDA). db-RDA was performed on ASV level in vegan package (Oksanen 2010), environmental variables were projected onto the ordination space using the envfit() function in vegan, with fitting performed on linear scores of the ordination axes. The variance explained by each variable was compared using permutation tests with the remaining variables included as covariates, using the anova.cca function in vegan (Oksanen 2010) with 999 permutations. To visualize the multivariate data in two-dimensional space and detect differences in metazoan and nematode community composition between the two clusters, nonmetric multidimensional scaling (NMDS) ordination was performed by uploading sequence variants (ASVs) into RStudio software. Bray–Curtis dissimilarity was calculated using raw relative abundance data. Statistical differences in centroid distances were investigated by running adonis2 function (within vegan package) with 999 permutations, and homogeneity variation in data dispersion was investigated using betadisper (vegan package) and anova functions. To investigate metazoan taxa responsible for changes between the nearshore (cluster 1) and offshore stations (cluster 2), SIMPER (SIMilarity PERcentages; vegan package) was carried out (default settings) on a distance-based matrix (distance = “bray-curtis,” using raw relative abundance data). Before any statistical tests comparing diversity indexes, species richness, MIs, environmental parameters, Shapiro–Wilk normality tests were performed. Subsequently, ANOVA was used to assess differences in community composition between groups when data were normally distributed, while Kruskal–Wallis tests were applied for non-normally distributed data.
3 Results and Discussion
3.1 Clustering of Sampling Stations Based on Abiotic Data Reveals Two Distinct Groups
The Kara, Laptev, and East Siberian seas are characterized by extensive shallow regions, yet they are differently affected by varying degrees of input from three major rivers and three different oceanic sources (Arctic, Atlantic, Pacific oceans) (Figure 1) (Anderson et al. 2011; Osadchiev et al. 2024). Consequently, it can be expected that the three seas exhibit differences in freshwater input and supply of particulate and dissolved materials. We, however, did not observe clear differences in bottom water and surface sediment chemical and hydrophysical data between the three seas. Instead, our cluster analysis based on environmental variables such as distance to shore, bottom water depth, temperature, salinity, turbidity, concentrations of DON, NH4,+ and NO3− as well as sediment composition (TN, TOC, δ13C, and OC:TN ratio) indicates that sampled stations fall into two main groups: cluster 1—nearshore stations and cluster 2—offshore stations (Figure 2A).
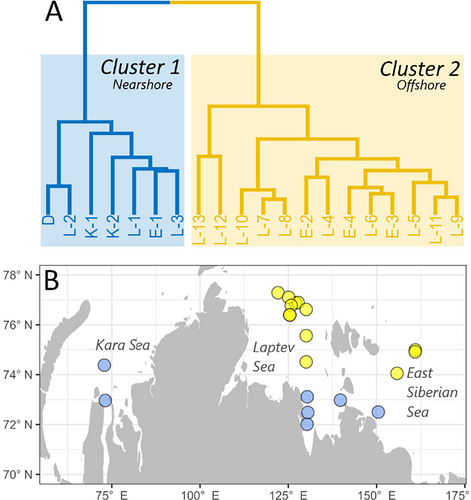
Remarkably, stations from different seas were part of the same cluster (Figure 2B). Cluster 1 (Figure 2, blue) grouped seven stations that were located at shallower depths (average depth 20 ± 6 m) compared to the 13 stations that were within cluster 2 (Figure 2, yellow), which were generally deeper (average depth 50 ± 12 m). Cluster 1 stations were characterized by significantly (Wilcoxon–Mann–Whitney, p < 0.05) closer distances to shore, shallower depth, higher temperature, lower salinity, higher turbidity, concentration of DON, NH4+, lower NO3−, lower sediment δ13C, and higher sediment molar OC:TN ratio (Figure 3).
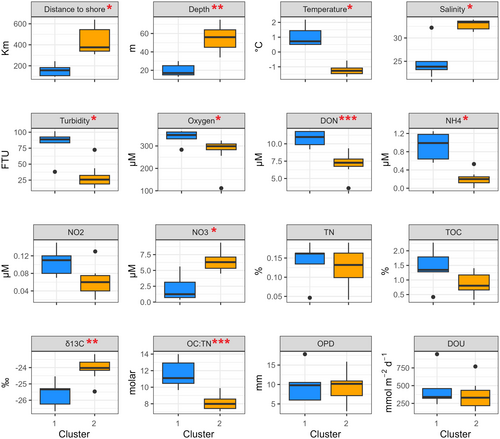
Significant differences in δ13C values, salinity, turbidity, and temperature between the two clusters suggest that the primary factor driving environmental differences is the coastal input via river discharge and coastal erosion, regardless of the seas' geographic positions. The size of an area impacted by river plume and coastal erosion (nearshore stations in Figure 2), however, is far from being static. Instead, it is constantly reshaped by environmental factors such as wind forcing, large-scale circulation, and runoff intensity (Xie et al. 2023). River plume in the Laptev Sea can spread 600 km northward, making all our sampling stations appear in the plume area (Xie et al. 2023). This implies that although offshore stations (Cluster 2) were not in the plume area during our sampling, they may still be periodically exposed to the plume. In addition, although the river plume is usually confined to the top 0–15 m, in October, when the water mass shifts from highly stratified to mixed, we could detect the river signal down to the bottom water and surface sediments potentially affecting benthic communities.
We observed substantial heterogeneity in sediment volume-specific oxygen uptake even at millimeter scale, horizontally, which consequently translated to a high variation across the stations (Figure 4). Such horizontal heterogeneity (i.e., variation across horizontal plane) can be seen in, for example, stations L-6, E-3 (Figure 4). Thus, due to the high variation, we recommend increasing sampling efforts to more than one core per station when looking for patterns across such large areas. Nonetheless, the high-resolution porewater oxygen profiles presented here represent the first investigation of the coastal sediment oxygen conditions in the Siberian seas. We show that diffusion-mediated O2 uptake (DOU) is independent of the TOC concentration (Spearman correlation, ρ = 0.4, p = 0.09), most likely due to the high percentage of refractory terrigenous and marine carbon (Magen et al. 2010; Figure S1). Indeed, based on the end-member binary model, the contribution of terrigenous organic carbon was high: 71%–96% in Kara Sea, 7%–49% in Laptev Sea, 46%–80% in East Siberian Sea, and 39% in Dimitry-Laptev strait (Table S2).
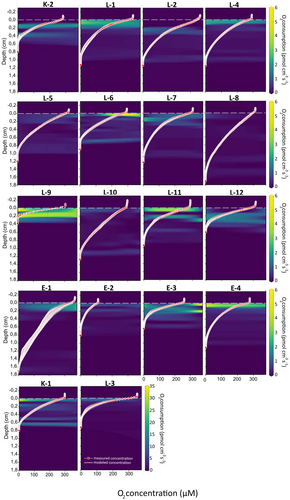
The obtained 53 oxygen microprofiles provide valuable information about sediment microbial activity in this sampling area. Despite bottom water being well oxygenated (256–366 μM O2, equivalent to 67%–98% air saturation, with an outlier station E-2 at 111 μM O2) at all stations and experiencing low temperatures, the oxygen gradients were comparable to those in more temperate coastal muddy marine systems, where oxygen is consumed within the top few millimeters or a centimeter (Glud 2008), compared to our observed average 0.9 ± 0.3 cm oxygen penetration depth (Figure 4). This confirms that coastal Siberian seas are very biochemically active marine environments (Anderson et al. 2011; Rusanov et al. 2024). The above-discussed environmental parameters such as oxygen availability, salinity, temperature, and sediment properties play a major role in shaping meiofauna structure and distribution, as shown for other systems (Broman et al. 2020; Giere and Schratzberger 2023). Changes in these parameters may therefore affect benthic microscopic organisms that play a key role in sediment biogeochemistry.
3.2 Metabarcoding Expands Previously Known Geographical Ranges of Certain Metazoans
We extracted bulk sediment DNA from 41 samples across 20 stations in total, collected at 3 sediment depths (0–1.5, 1.5–3, and 3–4.5 cm) making this the first study utilizing molecular analysis to describe benthic metazoan diversity in the Siberian Arctic. Metazoan diversity resolved in the 18S rRNA data was composed of 14 phyla, 21 classes, 58 orders, and 154 families. Even though no macrofauna were visually observed in the DNA samples, we obtained some macrofaunal sequences in our 18S rRNA metabarcoding data. Despite that, metabarcoding of macrofauna from small bulk sediment samples fails to represent true macrofaunal communities, whereas this technique effectively captures meiofaunal diversity (Mazurkiewicz et al. 2024).
Only 4% of all meiofaunal DNA metabarcoding studies analyzed meiofauna diversity across multiple sediment depth layers (Gielings et al. 2021). In our study, we did not observe any differences in community diversity between the 0–1.5, 1.5–3, and 3–4.5 layers (Metazoan Shannon index ANOVA, p = 0.93, Nematoda diversity Kruskal–Wallis, p = 0.2383, Figures 5 and 6). This is unexpected because meiofauna communities have been shown to be structured vertically (Giere 2008) and all oxygen was consumed within 1.8 cm depth at all stations (Figure 4), which should have resulted in markedly distinct community composition compared with the oxygenated 0–1.5 cm sediment layer. It is important to note that sedimentation rates in Siberian seas can be substantial (0.075–1.6 cm year−1) with higher rates closer to river mouths or in proximity to straits (Rusakov and Borisov 2023). Therefore, over time, some DNA of dead organisms from surface layers can be buried in deeper layers, diminishing differences between sediment layers. This is especially true for low-temperature environments. Because of these reasons, we decided to use the samples from different sediment depths as replicates per station for statistics and interpretation, rather than considering them as distinct samples.


At the phylum level, segmented worms—Annelida (average 34% ± 22%) and Arthropoda (average 33% ± 15%) had the highest overall relative abundance at most stations (Figure 5). Dominance of the two metazoan phyla (Annelida and Arthropoda) has also been observed in other studies across the Siberian Arctic shelf seas, particularly at shallow depths (Gerasimova et al. 2021; Payer et al. 2013; Saeedi et al. 2019, 2022). Annelida had the highest relative abundance with the presence of both Lysippe and Cirrophorus genera (Figure S2). Because there are no studies utilizing DNA-based methods to investigate the diversity of sediment metazoan communities in the Siberian Arctic, we can only compare it with results obtained from microscopy, based on which Lysippe is one of the polychaete genera that has been previously found in the Kara Sea (Gagaev 2021). We found that namely the Ampharetidae family (Lysippe genus) was the most abundant in our dataset. Interestingly, Ampharetidae is a commonly occurring taxon at hydrothermal vents, cold seeps, and organic falls (Eilertsen et al. 2017). The Siberian Arctic is known for the occurrence of cold seeps and thus it might provide favorable habitats for the Ampharetidae taxon, even more so in the future if the occurrence of the cold seeps increases (Shakhova et al. 2019). Paraonidae (Cirrophorus genus) was the second most abundant family, which has been reported as a macrofauna before in Siberian seas (Drumm et al. 2016), but it does have meiofaunal representatives as well (Schmidt-Rhaesa 2020).
The most abundant Arthropoda genera were copepod Itunella (family Canthocamptidae) and ostracod Cyprideis (family Cyprididae) (Figure S3). Itunella copepods have remarkable adaptability and wide geographical distribution. The same species of Itunella found in Iceland thrive equally well in brackish (salinity 8) and fully marine (salinity 32) waters and can even survive for months in freshwater (Steinarsdóttir and Ingólfsson 2004). Despite Itunella's presence at high latitudes and its wide tolerance of salinities, it has not been reported in the Siberian Arctic before. However, members of its family (Canthocamptidae) have been found in the Kara (Garlitska et al. 2019) and Laptev sea bays (Abramova and Tuschling 2005), and new species of Canthocamptidae have been reported in the Lena river delta at about 1 m depth (Novikov and Sharafutdinova 2022). Based on our data, this family was detected at all our sampling stations. Regarding ostracod Cyprideis, they are well adapted to unstable brackish environments (Ligios and Gliozzi 2012), yet there are no previous records of the genus being found in the Kara, Laptev, or East Siberian seas. However, when utilizing molecular methods, new occurrences of individuals or their expanded distribution ranges should be confirmed with visual data through traditional microscopy methods.
Nematoda (i.e., roundworms) were the third most abundant metazoan phyla (average 7% ± 4%) (Figure 5). In the majority of molecular and microscopy studies, meiofaunal Nematoda are the most abundant metazoan phyla (Schmidt-Rhaesa 2020). Yet, given that 90% of our stations were shallower than 100 m depth and our sediment samples were not sieved, this may have led to a lower relative abundance and biomass of strictly meiofaunal phyla. Thus, larger specimens with higher biomass can overshadow smaller specimens in the metabarcoding datasets (Elbrecht et al. 2017). Among Nematoda phyla, the majority of genera were unclassified (average 16% ± 18%), which could be due to a combination of factors, including limitations of the 18S rRNA marker, gaps in reference databases used for taxonomical assignments, and the presence of novel or undescribed genera (Gielings et al. 2021). Among nematodes, the most abundant genera were Sabatieria (average 16% ± 15%) and Oxystomina (average 15% ± 14%) (Figure 6), indicating that nematode communities were dominated by nonselective and selective deposit feeders, which play an important role in carbon recycling in benthic food webs. Throughout the samples, Comesomatidae (with genus Sabatieria) was the dominant family. This family has also been previously shown to be dominant and most widespread in the East Siberian Sea, at the shallow slope of the Kara and Chukchi seas, and on the Laptev Sea shelf (Portnova et al. 2021). Sabatieria is considered one of the most common taxa in shallow waters, at shelf breaks, and upper slopes (Platt 1985). Sabatieria nematodes are overall well adapted to extreme conditions and are therefore often selected as indicators of poor or bad ecological quality status (Moreno et al. 2011). In addition, Sabatieria nematodes are particularly known for their high tolerance to low oxygen (hypoxic) conditions (Broman et al. 2020). At the time of sampling, all stations were well oxygenated, with oxygen penetration depths comparable to temperate marine sediment. Thus, the dominance of Sabatieria in our samples may be more related to other stressors such as elevated turbidity, sediment organic matter content, and low temperatures.
The most different station was D, with the lowest nematode diversity and a strong dominance of the Molgolaimus genus at the top sediment layer (Figure 6). Molgolaimus nematodes have been previously associated with deep cold waters of the shelf (Portnova et al. 2021), whereas station D was shallow (14 m depth) and relatively warm (2°C). This suggests that Molgolaimus nematodes may exhibit a broader environmental tolerance than previously assumed, though further morphological confirmation is needed to fully support this hypothesis. This may prove useful for future studies aiming to assess how meiofaunal communities are responding to rapid environmental changes.
3.3 Nearshore Habitats Promote Colonizer-Dominated Communities
We found fewer taxa in the Kara, Laptev, and East Siberian seas compared to all taxonomic results in already published morphology-based investigations. It is important to note, however, that published studies cover a broader range of environments, which might have contributed to the higher observed diversity. For example, 38 nematode families were previously reported from 38 sampled stations (Portnova et al. 2021; Vanaverbeke et al. 1997), while we observed 24 nematode families from 20 sampled stations. Similarly, 22 harpacticoid families have been reported from 23 stations (Chertoprud et al. 2018; Garlitska et al. 2019), whereas we observed 18. And 28 ostracod genera were reported across 32 stations (Zenina et al. 2024), while we observed 9. Although the number of identified taxa differs between the literature and this study, direct comparisons are challenging mainly due to differing sampling efforts, stations, and sampling techniques (Van Veen grab, light dredge, multicorer, and Niemistö corer). It is important to note, however, that detailed species lists currently exist only for meiofaunal ostracods, nematodes, and harpacticoids, while in this study, we report representatives of all meiofauna groups simultaneously, often identified to a low taxonomic level. Overall, there is a strong agreement of dominant metazoan phyla and nematode families between our and literature results (Chertoprud et al. 2018; Garlitska et al. 2019; Portnova et al. 2019, 2017, 2021; Vanaverbeke et al. 1997; Zenina et al. 2024). We were now able not only to go to a lower taxonomic level but also to cover a larger spatial area, compared to most of the previous morphology-based studies. This approach revealed organisms that may have been missed using traditional sampling and isolation methods, enabling us to show that previously described metazoans are widely distributed in the Arctic.
One of the main theories regarding Siberian Arctic diversity is that macrofaunal communities in the East Siberian Sea are both taxonomically and functionally impoverished, resulting in the lowest species numbers compared to other seas in the region (Kokarev et al. 2017). For example, the Kara Sea has been shown to have about 1184 species, Laptev sea—1,105, and East Siberian Sea—780 (Kokarev et al. 2017; Vedenin et al. 2021). The much lower species richness in the East Siberian Sea is suggested to be due to considerable river runoff and low primary productivity (Kokarev et al. 2017) or due to simply much smaller study efforts in the East Siberian Sea. Although we sampled only four stations in the East Siberian Sea, we observed that Shannon diversity for metazoans and nematodes did not differ between the three seas (ANOVA type II, p = 0.9861, Kruskal–Wallis p = 0.1295, respectively) (Figure S4). In the case of the species richness indicated by the Chao1 index, only for metazoans did we detect a slightly significant effect of the sea (ANOVA, p = 0.044), but no specific pairwise differences were detected (TukeyHD, p = 0.0510) (Figure S4).
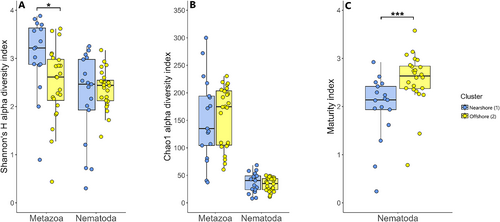
When comparing nearshore to offshore stations, however, the Shannon diversity index was significantly higher at nearshore stations (t-test, t = 2.6221, p = 0.013) for metazoan meiofauna, but not for nematodes (Wilxon rank sum test, W = 204, p = 0.989) (Figure 7A). Species richness (Chao1) was also not statistically different between the nearshore and offshore stations (Figure 7B). This suggests that the whole metazoan community diversity was more sensitive to coastal input via river runoff and coastal erosion compared to nematodes alone. Although we did not observe a coastal input effect on nematode diversity, their abundance may be affected (Portnova et al. 2021).
Previous studies have yielded inconsistent results: Some report significant changes in both diversity and abundance, while other studies have reported shifts in abundance only, highlighting the complex nature of these ecological transitions. For example, it has been shown that close to the shore of the East Siberian Sea, meiofaunal diversity and abundance are low due to shallow depths, desalination, high turbidity, and the abrasive action of ice (Portnova et al. 2021), compared to offshore areas. Similarly, in the Kara Sea, meiofauna and nematode diversity increased in the transition area from the freshwater zone of the Yenisei Bay to open sea areas (Portnova et al. 2017). However, specific estuarine nematode communities were not observed; instead, the community consisted of species characteristic of the nematode community both in the freshwater and marine zones (Portnova et al. 2017). In another study, it has been shown that while the nematode community composition remained similar with increasing water depth and distance from shore, the population density decreased from the Indigirka River mouth to the offshore stations (Portnova et al. 2021). Similarly, ostracod number of species and diversity maxima were observed at remote locations away from the river discharge area (Zenina et al. 2024).
The maturity index (MI) of nematode community can be used as an ecological measure of environmental disturbance based on nematode species composition, with smaller values of MI indicating a more disturbed system (Bongers 1999). In practice, MI varies from ~1, under extremely disturbed conditions, to between 3 and 4 under undisturbed conditions (Bongers 1999). In this study, nematode community indexes varied highly: between 0.2 and 3.6 (Figure 7C). Observed lower MI at nearshore stations indicates the presence of more colonizer-dominated communities closer to the shore, compared to offshore stations, which had more persister-dominated communities. Generally, severe disturbances lead to the dominance of opportunistic species (Schratzberger and Somerfield 2020). The absolute lowest values (0.2, 0.8) were at station K-1, which, as discussed earlier, was potentially under the highest riverine and coastal erosion influence, indicated by the highest terrigenous organic matter contribution.
We demonstrate that while coastal erosion and river discharge did not impact nematode diversity or species richness, it significantly influenced the nematode MI. This indicates that the near-river environment is more disturbed or unstable, with only offshore stations supporting communities with longer life spans, lower reproduction rates, and potentially overall larger individuals (Bongers and Bongers 1998). As riverine runoff and coastal erosion increase, we anticipate an expansion of colonizer-dominated communities, extending the area of low nematode MI. Although these colonizer nematodes are more resilient to disturbances, their dominance may lead to decreased ecosystem stability. Their smaller sizes and explosive population growth only under food-rich conditions (e.g., settlement of bloom) could potentially lead to unstable bottom-up effects. In this study, the observed effect on metazoan diversity indicates that metazoans as a whole are more sensitive to these disturbances compared to nematodes alone.
3.4 Abiotic Variables Drive Meiofauna Community Structure Across Different Seas
To look closer into factors affecting metazoan and nematode community composition, we performed distance-based redundancy analysis (db-RDA), using variables that did not correlate with each other (sediment TOC, OPD, OC:TN molar ratio and bottom water concentrations of NO3− and NH4+) (Figure 8). The environmental variables that were significant to community structure explained 30% (Metazoa) and 34% (Nematoda) of the total variation in community. For the Metazoa, the most significant variables were NH4+, TOC, and OC:TN ratio (ANOVA, p = 0.001, p = 0.001, p = 0.002, respectively) distinguishing nearshore from offshore stations. However, it is important to note that these variables were strongly correlated with several others that were not included in the db-RDA analysis to avoid multicollinearity. For instance, TOC showed a strong positive correlation with TN, the OC:TN ratio had a strong negative correlation with d13C, and NH4 exhibited a strong negative correlation with both depth and salinity. While these variables were not incorporated into the db-RDA, they may still contribute to the overall patterns observed between nearshore and offshore communities. By contrast, NO3− was slightly less significant (ANOVA, p = 0.029), and OPD was not significant (ANOVA, p = 0.051). Although all the environmental variables had the same effect (direction) on Metazoa and Nematoda communities, all those variables were slightly more significant for Nematoda (ANOVA, NH4+ p = 0.001, OPD p = 0.019, NO3− p = 0.003, TOC p = 0.001, OC:TN p = 0.003). db-RDA did not show a large distance between the two clusters (Figure 8A,B). Thus, to investigate the community difference between the two clusters, we performed a nonmetric multidimensional scaling (NMDS) ordination (Figure S5).
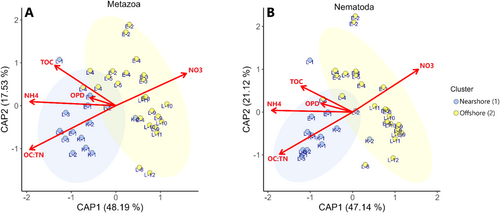
NMDS showed that there was a significant difference between the centroids for Metazoa and Nematoda (PERMANOVA, Metazoa p = 0.001, Nematoda p = 0.001). However, in the case of Nematoda, we also observed that variability within cluster groups is not homogeneous (betadisper test, p = 0.001), which could lead to a higher likelihood of falsely detecting differences in community composition between the two clusters. Thus, the structure of only metazoan communities as a whole primarily reflects differences between clusters defined by environmental variables, such as distance to shore, bottom water depth, temperature, and sediment characteristics, rather than distinctions between the Kara, Laptev, and East Siberian seas. Similar results have also been found when comparing meiobenthos assemblages in two estuaries, where natural stressors (salinity, particle size, and water nutrients) were found to be major factors controlling the meiobenthos community structure, rather than the variability between the two estuaries that were located > 270 km apart (Alves et al. 2009). Numerous studies have shown the importance of sediment organic matter content. An increase in organic matter often enhances meiofaunal abundance but also changes the community composition (Giere 2008), while in some studies C:N ratio (Tachibana et al. 2023) or, more specifically, the terrestrial versus marine source of carbon (McMahon et al. 2021), rather than the total amount of organic matter in sediments, was the main factor associated with meiofaunal community structure. Salinity has also been recognized as a potential main driver influencing meiofaunal diversity in the Baltic Sea, where low diversity is associated with low salinities (Broman et al. 2019). As all of these variables change along the river–sea transect, some studies identified the overall vicinity of the river mouth as an important factor influencing nematode abundance (Portnova et al. 2021). A similar pattern has been observed with another major world river, the Amazon, where meiofauna richness increased with distance from the river (Pereira et al. 2022). This is due to massive input of terrigenous debris and organic matter, which are extensively reworked and remobilized along the amazon continental shelf, leading to poor benthic communities near the river compared to adjacent deep-sea meiofauna communities (Pereira et al. 2022).
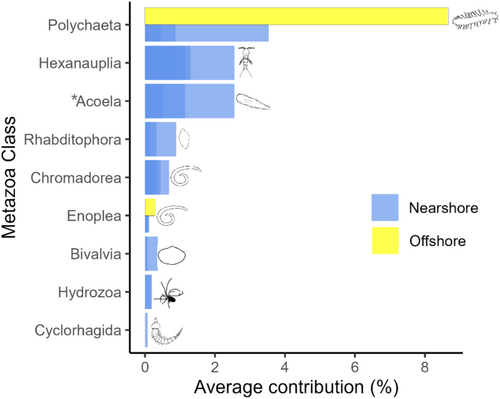
A SIMPER analysis was used to detect the main metazoan classes that drove the compositional shifts of metazoan communities nearshore and offshore. The results showed that among metazoans, Polychaeta, Hexanauplia, and Acoela contributed the most to the dissimilarities between nearshore and offshore metazoan communities (Figure 9). Most of the classes were present at nearshore stations, whereas some polychaetes and Enoplea nematodes that are known to be predators/omnivores preferred more stable offshore environments. Interestingly, soft-bodied meiofauna—Acoela was the third most important contributor to the dissimilarity. This highlights the importance of including soft-bodied meiofauna in benthic investigations. Traditional meiofauna isolation techniques, such as sieving and flotation, are often inadequate for identifying soft-bodied organisms, potentially leading to their underrepresentation in studies. However, the use of molecular methods can help to overcome this limitation. This is important because while our understanding of Acoela ecology remains limited, it has been shown that Acoela plays a role in sediment stabilization through mucus secretion (Klauser 1986) and their importance in the Siberian seas might have been overlooked.
4 Conclusions
This study, for the first time, reports on benthic metazoan diversity using molecular tools in the Arctic Siberian seas. Our findings reveal that meiofauna diversity across the three seas was comparable to some extent. However, diversity at nearshore stations was significantly influenced by river discharge and coastal erosion, whereas offshore stations were characterized by nematode communities with extended lifespans, reduced reproductive rates, and potentially larger individuals. Global warming is predicted to increase riverine runoff (Mann et al. 2022) and it will likely have significant impacts on at least metazoan community alpha and beta diversities, and the maturity of nematode communities. Thus, more studies on the interaction between river plumes and benthic fauna are needed to understand the interplay between changing riverine runoff and benthic fauna dynamics. In addition, the outflow of Siberian rivers shows significant seasonal and interannual variability (Semiletov et al. 2000; Xie et al. 2023). Therefore, future studies investigating benthic communities should consider these seasonal differences. Overall, with the expected intensification of river discharge and coastal erosion driven by ramping climate change, we anticipate a future shift towards colonizer-dominated communities. Such a transition could destabilize ecosystems across all three seas, posing significant risks to their ecological balance.
Author Contributions
A.M., E.B., F.J.A.N., T.T., E.Y., B.W., I.S., Ö.G., and S.B. planned the expedition and actively contributed to the research. A.M. led manuscript writing, analyzed samples and data with substantial contribution from E.B. A.M. and T.T. sampled sediment. S.B. and F.J.A.N. supervised and administered the project. S.B. and F.J.A.N. conceived the overarching idea. All authors gave comments/approved the manuscript.
Acknowledgments
We thank the crew and researchers of 82nd cruise of R/V “Akademik Mstislav Keldysh” involved in planning and implementation of the ISSS-2020 expedition. We also thank Geoffrey Cairola for assisting with DNA extractions. We are especially grateful to Ronnie N. Glud for the valuable comments on the manuscript and input when planning the study. We acknowledge Anni Glud for constructing the microelectrodes. Molecular data collection, analyses and geochemical work was supported by the Swedish Research Council Formas (2017-01513 to S.B.). The expedition was supported by the Russian Science Foundation (21-77-30001 to I.S.), Ministry of Science and Higher Education of the Russian Federation (grant “Priority-2030” to Tomsk State University, grant 121021500057-4 to POI, “Priority-2030” to Sakhalin State University-SakhalinTECH), the Swedish Research Council VR (2017-01601 to Ö.G. and 2018-05489 to B.W.) and the European Research Council (695331 ERC-AdG CC-TOP to Ö.G.), Research council of Norway (315317 and contract 342628/L10 to E.Y.). Additional funding was provided by the Swedish Research Council Formas (2018-01547 to B.W.), The Swedish Environmental Protection Agency's Research (NV-802-0151-18 to F.J.A.N.) in collaboration with the Swedish Agency for Marine and Water Management.
Conflicts of Interest
The authors declare no conflicts of interest.
Open Research
Data Availability Statement
All data from this study are available as Supporting Information S1. 18S rRNA sequences with assigned taxonomy and station metadata are available under the NCBI BioProject PRJNA1264051.




