Environmental DNA Methods for Detection of Varroa destructor in Honey Bee (Apis mellifera) Hives
Funding: This work was supported by Department of Agriculture, Forestry and Fisheries, Biosecurity Innovation Program (182020) and PSNAP Professional Development grant.
ABSTRACT
The parasitic mite, Varroa destructor, is a worldwide problem for honey bees (Apis mellifera). The recent spread of this pest to Australia, which was one of the few varroa-free honey bee populations remaining, highlights the importance of effective biosecurity methods for managing incursions. Detection of varroa mite environmental DNA (eDNA) could be a valuable complementary tool to increase the sensitivity and cost-effectiveness of varroa surveillance as it spreads to new areas. Using a newly developed V. destructor quantitative PCR assay, we deployed eDNA testing on honey bee hives in New Zealand and Australia with a range of mite infestation levels. The detection sensitivity of eDNA methods using honey and hive surface swabs was comparable with a conventional alcohol wash method used by beekeepers, with the advantage of not harming bees. However, we observed greater variability in eDNA detection, particularly for hive entrance swabs, when estimated mite infestation levels were below 1%. Using varroa-free hives introduced from the Chatham Islands to Wellington, New Zealand, we were also able to show that V. destructor eDNA was detectable during the initial invasion of hives. Our data highlight the potential for incorporating eDNA methods into Australia's biosecurity surveillance programs to help limit the spread of varroa mites to new areas and detect future incursions.
1 Introduction
Varroa destructor Anderson and Trueman is a major pest of the honey bee (Apis mellifera) with a significant impact on global apiculture and the provision of managed pollination services (Anderson and Trueman 2000; Traynor et al. 2020; Warner et al. 2024). This obligate ectoparasitic mite weakens honey bee colonies by feeding and reproducing on the developing brood as well as vectoring and exacerbating pathogenic viruses (Noël et al. 2020; Rosenkranz et al. 2010). Varroa mites also feed on adult bees, providing a key mechanism for intercolony dispersal through bee behaviors like foraging, robbing, and swarming (DeGrandi-Hoffman et al. 2017; Rosenkranz et al. 2010). However, the global spread of V. destructor has been largely human mediated through the movement of managed colonies and queen bees and increasingly from incursions of bee swarms through international trade networks (Boncristiani et al. 2021; Heersink et al. 2016). The result has been V. destructor becoming a key biological driver of colony losses worldwide (Warner et al. 2024) with only a handful of varroa-free honey bee populations remaining (Roberts et al. 2017).
Until very recently, Australia was one of the last few countries still free of V. destructor. This was in part due to its geographic isolation but also stringent biosecurity measures helping to prevent the introduction of mites for decades. This includes a surveillance network of sentinel hives positioned at ports of entry that are frequently monitored for exotic bee pests and pathogens (Clifford et al. 2011). In June 2022, V. destructor was detected in biosecurity sentinel hives at the port of Newcastle on Australia's east coast (Chapman et al. 2023). While this gave relatively early detection of the incursion, epidemiological analysis indicated that mites had been in the local area undetected for at least 12 months (McFarlane et al. 2024). Despite early success in suppressing the incursion, eradication efforts were ultimately unsuccessful, and beekeepers will need to manage this new pest. However, effective tools for early detection of V. destructor are still needed as varroa-free areas remain that are separated by large geographical barriers both in Australia and abroad, such as Western Australia, Tasmania, and many island populations. Monitoring the spread to new areas will enable earlier intervention. Current methods can be effective for surveillance but rely on physical collection and observation of mites, which is time-consuming and has variable sensitivity (Jack and Ellis 2021). Such methods are often more suited to countries that already have varroa mites, where these detection techniques are used to monitor mite burden within a honey bee colony and thus assist beekeepers in their treatment decisions. The physical effort required to deploy such techniques highlights monitoring and surveillance challenges both in the recent Australian incursion and for routine varroa management in countries where the mite is endemic.
In biosecurity and surveillance, molecular methods utilizing environmental DNA (eDNA) have emerged as a valuable pest and pathogen detection approach (Clarke et al. 2023; Collins et al. 2013; Pochon et al. 2017; Scriver et al. 2023; Trujillo-González et al. 2020; Zaiko et al. 2018). Recent methodological advances permit surveys for terrestrial insects from the direct collection of eDNA deposited on vegetation or other surfaces (Batchelor et al. 2023; Bell et al. 2024; Kestel et al. 2022; Liu et al. 2024; Milián-García et al. 2023; Pirtle et al. 2021; Valentin et al. 2018). While applications of eDNA analysis to aquatic environments and organisms have been well-studied, little is known about how detection rates for terrestrial eDNA surveys compare with conventional survey methods (Allen et al. 2021). The ability to detect traces of DNA from pest organisms makes eDNA a highly sensitive method that can be implemented more efficiently at scale. For example, multiple studies have demonstrated the potential of hive products, such as honey and pollen, and hive surfaces to provide insights into a hive's pest and disease status (Boardman et al. 2023; Bovo et al. 2020; Mackay et al. 2024; Ribani et al. 2020; Ribani et al. 2022). However, this has mainly focused on microbial pathogens while the detection of V. destructor still relies upon visual methods to determine mite infestation. Gene targets for varroa mite detection and characterization have been explored (e.g., mtDNA cox1 gene), but there has yet to be a broader application of these tools for eDNA surveillance (Utzeri et al. 2019). Methods for the effective collection of trace levels of varroa DNA from beehives have also not been published.
To address this knowledge gap, we developed a species-specific, hydrolysis probe qPCR assay for the detection of V. destructor eDNA. We assessed the sensitivity of the assay with two eDNA-based sampling methods using beehives in New Zealand where V. destructor is established. We also conducted longitudinal eDNA sampling on varroa-free hives relocated from the Chatham Islands to Wellington, New Zealand. Finally, we used this assay to investigate varroa eDNA detection in newly infested beehives in Australia.
2 Materials and Methods
2.1 Varroa destructor Quantitative PCR Assay
Quantitative PCR assay design and assessment were carried out at the National eDNA Reference Centre (University of Canberra, ACT). Varroa destructor–specific primers and probe were designed based on sequences accessioned for the mitochondrial cytochrome oxidase (cox1) gene of V. destructor and Varroa jacobsoni (Traynor et al. 2020) using Geneious (10.4.0) (Table S1). Primers and probe were designed to amplify a 284-nucleotide fragment spanning the 784–1068 nucleotide region of V. destructor cox1, wherein three mismatches were present within primers and probe binding sites compared with V. jacobsoni to avoid possible cross-reactivity with this closely related species (Figure S1, Table S2). Lastly, the selected assay was tested for specificity in silico using NCBI Primer BLAST.
The V. destructor qPCR was optimized on an Applied Biosystems QuantStudio ProFlex 7 Real-Time System (Thermo Fisher Scientific, Massachusetts, USA). Each reaction consisted of 5 μL TaqMan Environmental Master Mix 2.0 (Thermo Fisher Scientific), 0.4 μL of forward (5’-AGAGGGAAGAAGCAGCCTTT-3′) (10 μM) and reverse (5’-ACACCAGTAATACCCCCTAAAGT-3′) (10 μM) primer, 0.1 μL of probe (FAM-5’-ACTCGAGCATATTTTACTGCAGCT-3’-MGB) (10 μM), 2.1 μL of ultrapurified deionized water, and 2 μL of DNA extract for a final volume of 10 μL. Cycling conditions were 95°C (10 min), followed by 50 cycles of 95°C (15 s) and 61°C (1 min) ramping at 1.6°C/s, followed by a final holding stage at 4°C.
Specificity tests were completed to test cross-reactivity against the closely related mite species (V. jacobsoni), three known bee hosts for V. destructor (Apis mellifera, Apis cerana, and Apis florea), and three co-occurring mite species that parasitize honey bees (Tropilaelaps clareae, Tropilaelaps mercedesae, and Acarapis woodi). Specimens for V. destructor were collected from infested Apis colonies in England and provided by James Cook University, A. cerana specimens were provided by James Cook University, and A. florea specimens were provided by the Australian Government Department of Agriculture, Fisheries and Forestry. DNA from these specimens was extracted using a Qiagen DNeasy Blood and Tissue kit (Qiagen, Germany) as per the manufacturer's instructions. Genomic DNA for A. mellifera, T. clareae, T. mercedesae, and A. woodi were provided by CSIRO for testing.
Sensitivity tests were performed to obtain the limit of quantification (LOQ) and limit of detection (LOD) (Klymus et al. 2020) using genomic DNA and synthetic standards designed for V. destructor. Standard curves were established using dilution series ranging from 107 copies/μL and decreasing 10-fold down to 1 copy/μL. Six PCR replicates were used in each dilution step to assess the LOD and LOQ of the assay (Figure S2).
2.2 Collection of eDNA Samples From New Zealand Honey Bee Hives
Samples were collected from n = 42 managed hives in New Zealand in February 2023 across six apiaries in the geographic region around Wellington. Colonies of A. mellifera had varied levels of V. destructor infestations, as determined for each hive using an alcohol wash method, where 300 adult bees were immersed in 70% ethanol to remove mites and estimate the mites per bee infestation level (Taylor and Goodwin 2021).
A total of n = 344 samples were collected, including stored honey from capped comb and swabs from hive entrances and the surface of brood frames for eDNA testing (Figure 1). Honey samples were collected from a single frame per hive by drawing a 50-mL tube across the surface of capped honeycomb to fill the tube. Triplicate swab samples were collected from hive entrances by inserting and twisting a dry FLOQSwab swab (552C, Copan Diagnostics, Italy) into the hive to sweep across the length of the hive floor and entrance. Triplicate swab samples were collected from brood frames with a mix of sealed and unsealed broods by sweeping a dry swab across one comb surface. Negative control samples were also collected in the field at each sampling location by exposing a swab to the open air for 30 s. Swabs were placed into eNAT preservation medium (Copan Diagnostics) for transport to the laboratory and then stored at 4°C.

2.3 Collection of eDNA Samples From Varroa-Free Honey Bee Colonies During Initial Invasion
A total of n = 10 five-frame nucleus colonies (prepared within five hive boxes using division boards) were transported from the Chatham Islands, a remote varroa-free archipelago situated ~800 km east of mainland New Zealand, to the Wellington Beekeepers Association apiary. The nucleus colonies were left to acclimatize for 24 h upon arrival. Duplicate swab samples of the hive entrances and both sides of a brood frame were collected daily from each colony during the first week (Days 1–8) and then sampled on Days 14, 30, and 60. Negative control samples were collected at each sampling event by exposing a swab to the open air for 30 s. Varroa infestation levels were estimated using an alcohol wash on Days 8, 14, 30, and 60. Alcohol wash testing was not possible at earlier time points as it risked compromising the survival of the small colonies.
2.4 Collection of eDNA Samples From Newly Varroa-Infested Australian Honey Bee Hives
Single wetted swab samples were collected from the hive entrances of n = 140 managed hives at two apiary locations (hereafter, Apiary 1 and Apiary 2) within the varroa-infested zone in Kempsey, New South Wales. Sampling was repeated three times on April 2–3, 2024, April 22–23, 2024, and May 18–30, 2024. Hives at Apiary 1 were untreated during the experiment, whereas 25 hives at Apiary 2 received a miticide (amitraz) treatment after the first sampling point on April 7, 2024 (Supplementary 2). Swabs were placed into eNAT preservation medium for transport to the laboratory and then stored at 4°C. Negative control samples were collected at each sampling event by exposing a swab to the open air for 30 s. Varroa infestation levels were estimated using an alcohol wash at each time point.
2.5 DNA Extraction
DNA was extracted from swab heads and the preservative buffer separately, followed by pooling of extracts. Extraction was achieved using a modified version of the Qiagen DNeasy Blood and Tissue kit (Qiagen, Germany). Briefly, swab tips were placed into a 2-mL tube containing 360 μL of ATL buffer and 40 μL of proteinase K, then incubated at 56°C overnight on a laboratory shaker at an agitation speed of 60 rpm. For the preservative buffer, 200 μL of proteinase K was added directly before overnight incubation. Then, 400 μL of ethanol and AL buffer were added to the tubes and homogenized before the mixture was transferred into a spin column and centrifuged for 1 min at 8000 × g. This step was repeated until all the mixture had been filtered through the column. All wash steps were completed as per Qiagen instructions, and DNA was eluted in 100 μL of milli-Q water. All swab DNA extracts were processed at the University of Canberra using three qPCR replicates per sample; each qPCR run included corresponding extraction negative controls, field controls, positive controls (synthetic oligonucleotide standardized to 1000 copies/μL), and nontemplate controls.
DNA was extracted from honey using the Maxwell RSC PureFood GMO and Authentication kit (Promega, Wisconsin, USA) by first dividing each sample equally into four 50 mL centrifuge tubes and diluting each to a 50 mL volume with milli-Q water. After incubating the diluted honey at 40°C for 30 min, tubes were centrifuged at 11,000 × g for 10 min, and the supernatant was discarded. Pellets were resuspended in 5 mL milli-Q water and combined into a single tube with 30 mL milli-Q water before being centrifuged at 11,000 × g for 10 min, and the supernatant was discarded again. Pellets were resuspended in 1 mL CTAB buffer (Promega) and transferred to a 2-mL BeadBug tube with 0.5 mm zirconium beads and homogenized at 7 m/s for 1 min in a BeadBug 6 Microtube Homogenizer (Benchmark Scientific, New Jersey, USA). Each sample had 20 μL RNase, and 40 μL proteinase K was added, then incubated at 65°C for 30 min. Lysates were then cleared by centrifuging at 16,000 × g for 10 min, and 300 μL used for kit DNA extraction. All honey DNA extracts were prepared and analyzed at the CSIRO laboratory using two qPCR replicates; each qPCR run included corresponding extraction negative controls, positive controls (synthetic DNA standardized to 1000 DNA copies/μL), and nontemplate controls.
2.6 Comparison of Alcohol Wash and eDNA Detection of V. destructor
We compared the performance of alcohol wash and eDNA-based detection using the diagnostic test evaluation and comparison for two tests of the EpiTools epidemiological calculator (Ausvet, epitools.ausvet.com.au; Sergeant 2018). We calculated McNemar's Chi-square test and proportions of positive and negative agreement to quantify the level of agreement between both detection methods at a 95% confidence level. For this purpose, we grouped qualitative outcomes (positive/negative) and compared method sensitivity between apiaries for each visit. Given that apiaries were located within varroa-infested areas (New Zealand and Kempsey, New South Wales), performance comparisons were made with the conservative assumption that all populations were infected. In the same way, and to provide a measure of positive and negative agreement at a 95% confidence level, all eDNA putative positive detections were confirmed by Sanger sequencing without implementing an assay cut-off value informed by the limit of detection to minimize false-negative detection rates.
3 Results and Outcomes
3.1 Sensitivity and Specificity of qPCR Assay for V. destructor
The qPCR assay we developed for V. destructor showed high reproducibility and detection sensitivity with R2 = 0.99 and an efficiency of 100.5% at a limit of quantification and detection of 3.6 DNA copies/μL (Figure S2). Assessment of specificity showed no amplification from DNA of other mite species that are known pests of bees (Varroa jacobsoni, Tropilaelaps clareae, T. mercedesae, and Acarapis woodi) or their associated bee hosts (Apis mellifera, A. cerana, and A. florea).
3.2 Environmental DNA Yield and Quality From New Zealand Hives
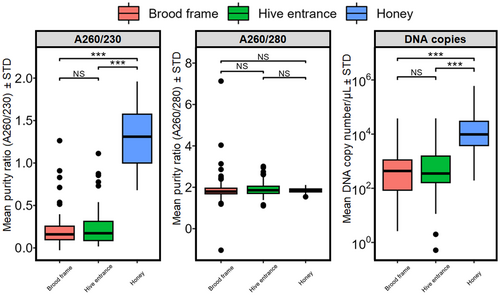
DNA purity in the 260:230 ratio was significantly higher in honey DNA extracts compared to swab DNA extracts taken from hive entrances and brood chambers (Figure 2). Similarly, the amplified number of DNA copies in honey samples was significantly higher than in swab samples. There were no significant differences in purity for the 260:280 ratio.
3.3 Detection of V. destructor eDNA in New Zealand Hives
Honey and swabs from hive entrances and brood frames were collected from n = 42 managed A. mellifera colonies from around the Wellington region of New Zealand with a mean parasite load of 6.95 ± 14.05 mites/300 bees ± STD (Table 1). Alcohol wash sensitivity was 0.55 (Table 1). Detection of varroa eDNA had a significantly higher sensitivity of 0.91 in honey (McNemar's Chi-square = 11.52, p < 0.001), compared to 0.571 for swabs from brood frames, which was comparable to alcohol washes, and 0.31 for hive entrance swabs, which had significantly lower sensitivity than alcohol wash (McNemar's Chi-square = 8.1, p < 0.001, Table 1).
| Estimate | Honey | Brood chamber | Hive entrance |
|---|---|---|---|
| McNemar's Chi sq | 11.53 | 0 | 8.10 |
| p (Chi sq) | 0.0007 | 1 | 0.004 |
| Proportion positive agreement | 0.72 | 0.72 | 0.72 |
| Proportion negative agreement | 0.26 | 0.65 | 0.79 |
| Overall proportion agreement | 0.60 | 0.69 | 0.76 |
| Kappa lower 95% limit | −0.075 | 0.090 | 0.32 |
| Kappa upper 95% limit | 0.32 | 0.65 | 0.76 |
| eDNA sensitivity (95% CI) | 0.91 (0.78–0.96) | 0.57 (0.42–0.71) | 0.31 (0.19–0.46) |
| Ethanol sensitivity (95% CI) | 0.55 (0.40–0.69) | ||
| Mean mite load ± STD | 6.95 ± 14.05 | ||
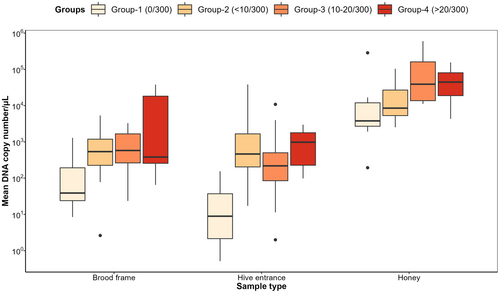
There were significant differences in V. destructor DNA copies/μL between varroa mite infestation categories (ANOVA, F291,3 = 21.07, p < 0.001) and sampling methods (ANOVA, F291,2 = 19.79, p < 0.001), wherein detected DNA copies/μL significantly increased with increasing varroa mite infestation categories (Figure 3). Of note, DNA copies/μL detected from honey samples were significantly higher than swab samples from hive entrances and brood frames across all mite infestation categories (Figure 3).
3.4 Detection of V. destructor eDNA During the Initial Invasion of Varroa-Free Hives
Varroa-free hives were introduced into a varroa-infested apiary and monitored over the initial invasion period of 2 months by eDNA and alcohol wash detection methods. The first V. destructor eDNA detections were apparent on Days 5 and 6 in Hive 5A and 5B, respectively, and by Day 8, n = 3 colonies (1B, 3A, and 5A) had V. destructor eDNA detected (Figure 4). The earliest alcohol wash to be performed was on Day 8 (to avoid overt harm to the nascent colony), with varroa mites only observed in Hive 5A and only a single mite (0.33% mite infestation).
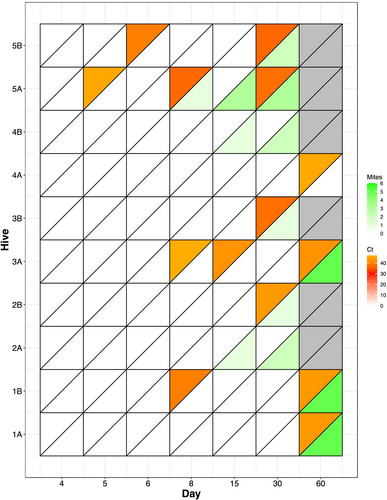
All 10 colonies had mites present by Day 60, and this was corroborated by eDNA detection in all but two colonies (2A and 4B), which had no eDNA detection during the experiment despite mites observed from Day 15. Amplification was late in the reaction (Cq > 35) for all detections, indicative of only trace amounts of V. destructor DNA in the swab samples and consistent with the observed low (below 1.6%) mite loads in the alcohol washes.
3.5 Detection of V. destructor eDNA in Newly Infested Australian Hives
Hive entrance swabs were collected at three time points from n = 140 managed Australian hives newly infested by varroa mites. Twenty hive samples from Apiary 1 collected during Visit 2 failed DNA extraction and could not be used to assess detection sensitivity; for this reason, these hives were excluded from the study across all three visits (See Supplementary 2). Similarly, samples taken from hives 71–92, 120–121, and 129 in Apiary 2 were treated with pesticides during the study and were excluded from this study (Supplementary 2).
Mean parasite loads significantly increased over time across both apiaries (two-way ANOVA, F3,263 = 129.63, p < 0.001). Mite loads in hives from Apiary 1 increased from a mean of mites/300 bees ± STD = 2.8 ± 2.24 during Visit 1 to 18.72 ± 14.5 in Visit 3, while Apiary 2 hives showed mites/300 bees ± STD = 3.5 ± 4.44 during Visit 1 and 9.55 ± 15.99 during Visit 3 (Figure 5A). Consistent with the New Zealand hives, there was a significant increase in mean V. destructor DNA copies/μL detected across both apiaries with increasing parasite loads (two-way ANOVA, F3,263 = 2.55, p < 0.05), wherein detections in Visits 2 and 3 showed significantly higher detections compared to Visit 1 (Figure 5B).
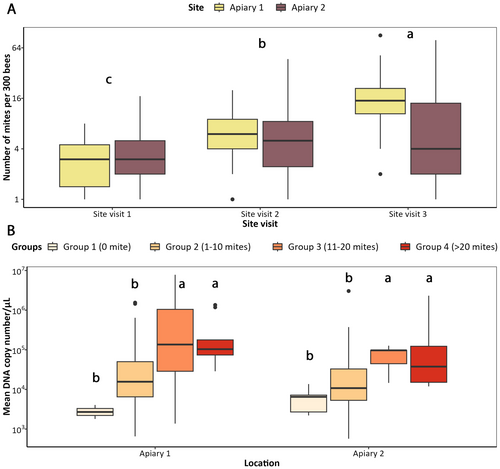
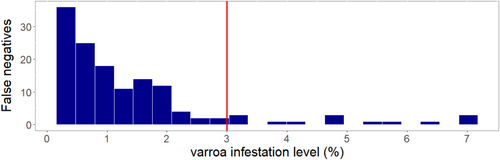
Environmental DNA detection of swabs from hive entrances in Visit 1 across both Apiaries had a significantly poor performance compared to alcohol wash (McNemar's Chi-square = 18.89Apiary 1 and 19.36Apiary 2, p < 0.001). Specifically, eDNA and alcohol wash methods showed a sensitivity of 0.25 and 0.73, respectively, for Apiary 1, and 0.14 and 0.69 for Apiary 2 (Table 2). During this visit, hives in Apiary 1 had an average of 2.80 ± 2.24 mites/300 bees ± STD, while Apiary 2 had 3.50 ± 4.44. There were no differences in detection sensitivity between alcohol wash and eDNA detections during the next two visits. During Visit 2, eDNA and alcohol wash methods showed a sensitivity of 0.96 and 0.92, respectively, for Apiary 1, and 0.86 and 0.91 for Apiary 2. This same trend was observed in Visit 3, where both methods showed comparable detection sensitivities. Lastly, the majority (57%) of nondetections of eDNA methods were from hives with infestations under 1% (Figure 6).
| Estimate | Visit 1 (Apr 2–3, 2024) | Visit 2 (Apr 22–23, 2024) | Visit 3 (May 18–30, 2024) | |||
|---|---|---|---|---|---|---|
| Apiary 1 (n = 44) | Apiary 2 (n = 41) | Apiary 1 (n = 50) | Apiary 2 (n = 43) | Apiary 1 (n = 49) | Apiary 2 (n = 45) | |
| McNemar's Chi sq | 18.89 | 19.36 | 0.25 | 0.125 | 0 | 1.7857 |
| p (Chi sq) | 0 | 0 | 0.6171 | 0.7237 | 1 | 0.1814 |
| Proportion positive agreement | 0.440 | 0.286 | 0.959 | 0.895 | 0.969 | 0.794 |
| Proportion negative agreement | 0.46 | 0.490 | 0.333 | 0.2 | 0.4 | 0.364 |
| Overall proportion agreement | 0.451 | 0.405 | 0.923 | 0.814 | 0.941 | 0.689 |
| eDNA sensitivity ± 95% CI | 0.25 (0.16–0.39) | 0.14 (0.07–0.28) | 0.96 (0.87–0.99) | 0.86 (0.72–0.93) | 0.96 (0.87–0.99) | 0.69 (0.54–0.81) |
| Ethanol sensitivity ± 95% CI | 0.73 (0.59–0.83) | 0.69 (0.54–0.81) | 0.92 (0.82–0.97) | 0.91 (0.78–0.96) | 0.94 (0.69–0.91) | 0.82 (0.69–0.91) |
| Mean mite load ± STD | 2.80 ± 2.24 | 3.50 ± 4.44 | 6.78 ± 4.30 | 6.65 ± 9.77 | 18.72 ± 14.51 | 9.55 ± 15.99 |
4 Discussion
Detection and monitoring of varroa mites have become essential for managing the impacts of this harmful pest on honey bee populations worldwide. In varroa-free areas, like in Australia, early detection is key for authorities to effectively implement eradication responses and strategies for limiting the spread of new incursions. Conventional mite surveillance methods, such as alcohol washes and sticky boards, continue to be valuable tools for monitoring mite levels but can lack the sensitivity and efficiency needed for truly early detection. We have shown here that varroa eDNA can be sensitively detected in beehives with our new species-specific qPCR assay. Using field data collected from New Zealand and Australian hives, we demonstrated how low V. destructor parasite loads can affect the detection of alcohol wash and eDNA methods and indicate at which parasite loads both methods achieve a high overall sensitivity. We also showed that varroa eDNA can be detected in hives as they first become invaded by mites. These results are an important step toward implementing eDNA methods for bee biosecurity.
While all sampling methods were effective for varroa eDNA detection in this study, our results highlight several advantages and disadvantages for each approach. Based on the estimated detection sensitivity, honey samples offer greater sensitivity for detecting varroa eDNA than surface swabs from brood frames and hive entrances. It is important to highlight, however, that hive surface swabs are simpler collection techniques and less disruptive than collecting honey from hives. Within this context, swabs taken from brood chambers offer greater sensitivity at low mite parasite loads, which is expected as there is higher mite activity around the brood, but this will vary across the brood nest. Our method was to swab both sides of a single brood frame. Collecting swabs from all brood frames would reduce this variability. However, this method is more invasive than hive entrance swabbing and requires more technical skill, as hives must be opened down to the brood nest. The convenience and low technical skill needed for hive entrance swabbing are major advantages of this method that would enable its implementation at scale and by nonexperts.
DNA purity and PCR amplification were both higher for honey than for the swab methods. Using dry swabs in New Zealand hives rather than prewetted swabs likely influenced the collection of varroa eDNA (Van Oorschot et al. 2003), and greater concentrations may be present on hive surfaces than estimated here. In fact, prewetted swabs were used for the Australian hives and resulted in PCR amplification equivalent to that found with honey samples. However, honey may be a more reliable sample type for detecting varroa eDNA due to greater accumulation of varroa eDNA in honey and less degradation of eDNA compared to hive surfaces. Unfortunately, routine collection of sealed honey from hives is not a convenient method, as it requires opening hives and damaging combs. Honey stores are also not always available in hives depending on the season and size of the colony. A solution to this could be coordinating with commercial beekeepers and honey packers to subsample extracted honey, helping simplify this process and enabling broad-scale surveillance. This type of surveillance would be particularly beneficial for areas still varroa-free, and with further development, it could also be used to monitor the effectiveness of varroa management and alert beekeepers to local outbreaks.
There was considerable variation in detection when mite infestations were very low. Many honey samples from hives with no observed mites had varroa eDNA detected, which we cautiously interpret as trace-level DNA detection rather than false positives. Since the persistence of varroa eDNA on hive surfaces and in honey is unknown, these detections could reflect past mite infestations. However, the influence of eDNA persistence is not supported by the data, with amplification and DNA concentration significantly different between infestation levels. There were also many nondetections with eDNA-based methods, particularly for hive entrance swab samples. These were predominantly when mite infestations were below 1%. The variability in detection was greatly reduced as mite infestations reached common treatment thresholds of 3%. Many biotic and abiotic factors will influence eDNA detection variability at low mite levels, and some will be common challenges for terrestrial eDNA applications (Newton et al. 2025; Valentin et al. 2020). For instance, the relatively warm and humid hive environment may reduce eDNA persistence on hive surfaces. The biology and behavior of varroa, which spend much of their time inside brood cells, will certainly influence the deposition and dispersion of eDNA throughout the hive. As early detection is essential for biosecurity, further optimization of eDNA recovery is needed for these low infestation levels. However, the ability of these eDNA methods to provide more efficient varroa mite surveillance at scale should not be overlooked.
Varroa eDNA was detected during the first few days of mites invading the varroa-free hives from Chatham Islands, but across the experiment, only 57% (8/14) of mite observations were confirmed by eDNA swabs. This may be associated with the use of dry swabs rather than prewetted swabs, which could have improved detection as noted above. An interesting observation was the lack of eDNA detection on Day 15 when mites were observed in several colonies despite positive eDNA detections on earlier days. Variability in eDNA detection will likely be high during initial hive invasions, as factors related to varroa biology and eDNA shedding rates affect eDNA dispersion in the hive (Furlan et al. 2016). For example, varroa mites enter hives on adult bees but quickly move into brood cells to reproduce (Rosenkranz et al. 2010). Very little eDNA will be left from these first invading mites, but as developing bees emerge, there is likely a spike in varroa eDNA released. Indeed, mite population growth in hives can increase exponentially (Rosenkranz et al. 2010), meaning that varroa eDNA will likely be at low concentrations and highly clustered for several reproductive cycles. As a result, increased sample replication and frequency will likely be needed to ensure a high probability of detection and consistency in varroa-free areas.
There are few terrestrial examples of eDNA detection methods being used in biosecurity (Bell et al. 2024). However, they have helped demonstrate the value of eDNA over conventional methods (Allen et al. 2021; Valentin et al. 2018) and where cryptic species complicate surveillance (Pirtle et al. 2021; Trujillo-González et al. 2022). Our study is the first to directly compare eDNA detection with conventional methods for varroa mite surveillance, making this an excellent model case for eDNA biosecurity surveillance. While other studies used honey eDNA to monitor varroa mitochondrial lineages (Utzeri et al. 2019), we highlighted here how this method could be extended to broad-scale monitoring of varroa-free areas. Equally, this method could also be applied to detecting incursions of other bee mites such as the morphologically cryptic pest V. jacobsoni (Roberts et al. 2015), which becomes extremely challenging amid a background of V. destructor. While a recent study used eDNA metabarcoding on various sample types to characterize the local hive and foraging environment (Boardman et al. 2023), they only detected varroa eDNA from debris collected beneath hives and nearby pond water. In contrast, we reliably detected varroa eDNA from hive surface swabs. This may be attributed to our species-specific qPCR primers offering greater sensitivity than general metabarcoding primers.
The simplicity and noninvasiveness of hive surface swabs are particularly advantageous by enabling more frequent sampling and surveillance during winter months without disrupting hives. This approach has recently been developed by Mackay et al. (2024) for qPCR detection of the bee bacterial pathogen Paenibacillus larvae, the causative agent of American foulbrood disease. This method could similarly be used for routine monitoring of infestation levels in areas where varroa is established. However, this would require a better understanding of how eDNA levels correlate with mite population levels. In this study, there was a trend for higher varroa eDNA concentrations with increasing mite infestation levels, but there was insufficient resolution to differentiate around current treatment thresholds of 3%. Ultimately, understanding how eDNA accumulates and persists in the dynamic hive environment will be important for further developing this approach and broadening the use of eDNA for bee biosecurity and hive health management.
Author Contributions
J.M.K.R., R.J.H., F.E.V., L.M., A.W., D.G., and A.T.G. contributed to the conception and design of the study. J.M.K.R., R.J.H., F.E.V., F.S., F.B., J.S., N.S., F.M., and A.T.G. contributed to the acquisition, analysis, or interpretation of the data. J.M.K.R., R.J.H., F.E.V., L.M., F.M., and A.T.G. contributed to the writing of the manuscript.
Acknowledgments
This research was funded by the Australian Government Department of Agriculture, Forestry and Fisheries Biosecurity Innovation Program (Project 182020) and by the Catalyzing Australia's Biosecurity Initiative. F.M was funded through a Plant Surveillance Network Australasia-Pacific (PSNAP) residential program delivered under the National Plant Biosecurity Surveillance Professional Development and Protocols Project coordinated by Plant Health Australia and funded by the Australian Government Department of Agriculture, Fisheries and Forestry. We thank Dr. Mark Johnston and Dr. John Carr for facilitating the collection and provision of Varroa destructor specimens from infested hives in England. Dr. John Carr also provided Apis Florea specimens for DNA extraction. We thank Dr. Luke Watson from the Department of Agriculture, Fisheries and Forestry for providing Apis cerana specimens. We thank Frank Lindsay, Richard Braczek, the Wellington Beekeeper's Association, Manuka Health, and Matt Bryan for providing access to their apiaries in New Zealand, and Toni Croon for providing varroa-free hives from the Chatham Islands and the Ministry for Primary Industries for coordinating the transfer of those hives. We also thank staff from the Department of Agriculture, Forestry and Fisheries and Queensland Department of Agriculture and Fisheries who collected swab samples from Australian hives.
Conflicts of Interest
The authors declare no conflicts of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.




