The role of cultivated versus wild seeds in the diet of European turtle doves (Streptopelia turtur) across European breeding and African wintering grounds
Abstract
Agricultural intensification is a major driver in species declines, with changes in land use resulting in widespread alteration of resource availability. An increase in anthropogenic food resources, alongside decreasing natural resources, has resulted in species undergoing dietary changes that can have important ecological consequences, particularly for declining species. Here we use high-throughput sequencing to analyze the diet of the migrant European turtle dove (Streptopelia turtur), a species that has experienced significant population decline throughout its European range. We analyze the diet of this species on both breeding and wintering grounds to gain an understanding of resource use throughout the annual cycle and compare areas of more and less intensive agriculture in western and eastern Europe, respectively. We examine associations with body condition, spatiotemporal variation and the source of food (wild or cultivated). We identified 121 taxonomic units in the diet, with significant variation across sampling seasons, and very little overlap between the breeding and wintering seasons, as well as high levels of cultivated food resources in the diet of turtle doves in both breeding and wintering grounds, with the highest proportion of wild seeds in the diet occurring in birds caught in Hungary, where agricultural intensity was lowest. We detected no association between body condition and the consumption of cultivated food resources. We demonstrate the importance of wild resources in birds on the wintering grounds as they approach migration, where body condition increased as the season progressed, concurrent with an increased consumption of wild seeds. These findings indicate the importance of habitats rich in wild seeds and the need to consider food availability on the wintering grounds, as well as the breeding grounds in turtle dove conservation strategies.
1 INTRODUCTION
Diet is a central aspect of an animal's ecology, with an appropriate diet key to ensuring they receive the required nutrients to maintain a healthy body condition for survival, breeding and migration (Martin, 1987; Newton, 2004a). However, there are increasing instances of species changing diet and consuming novel food resources, driven by two anthropogenic influences: an increased availability of novel food resources or a decrease in natural food resource availability (Dunn et al., 2018). Some common factors resulting in an increase in novel foods are supplementary feeding, human food waste and an increase in farmed crops (Browne & Aebischer, 2003a; Newsome & Van Eeden, 2017; Robb et al., 2008). Farmed crops not only increase the availability of novel foods in the environment but may also, depending on farming practices, remove many of the natural food resources from the environment. The removal of natural flora and invertebrates heavily impacts farmland biodiversity, affecting species feeding on a range of organisms and resulting in widespread species declines (Benton et al., 2003; Donald et al., 2001; Potts, 1991).
Dietary switches do not necessarily have a detrimental effect. Across the world, species such as Red Foxes (Vulpes vulpes) and Black-headed Gulls (Chroicocephalus ridibundus) thrive in urban environments (Contesse et al., 2004; Scott et al., 2015), thanks to an abundance of calorifically rich food (McCleery et al., 2014). However, species' declines are commonly associated with dietary switches and nutritional stress, as alternative food resources are often of poorer quality than optimal natural resources (Moorhouse-Gann et al., 2020; Will et al., 2015), potentially resulting in diminished survival rate and reproductive success (Litzow et al., 2002; Wanless et al., 2005).
Agricultural intensification is a leading cause of dietary shifts, with changing farming practices dramatically increasing crop yields, but depleting natural food resources (Chamberlain et al., 2000). For example, in the UK, removal of traditional hedgerows and weedy habitats in favor of cultivated crops, extensive use of herbicides reducing wild plant cover and seed abundance, and a shift to autumn-sown cereals decreasing the fallow period between seasons all contribute to a depletion in food resources (Browne et al., 2005; Gillings et al., 2005; Marshall et al., 2003). Intensity of agriculture varies geographically, for example between western and eastern European countries, with a greater extent of traditional, less intense farming methods used in eastern Europe (Donald et al., 2001).
The European turtle dove Streptopelia turtur (hereafter turtle dove) has experienced long-term population declines across its European breeding range (Burns et al., 2020), with a decline of 33% since 1998 leading to the species being classified as “Vulnerable” to extinction (BirdLife International, 2019; Lormée et al., 2020). Various studies in the UK have shown that, in that country, the main cause of these declines is agricultural intensification, which has led to shifts in diet, fewer nesting attempts and lower annual productivity (Browne & Aebischer, 2003a, 2004), but agricultural intensification has occurred on both breeding and wintering grounds (Binswanger-Mkhize & Savastano, 2017; Brink & Eva, 2009; Browne et al., 2004; Chamberlain et al., 2000; Zwarts et al., 2018). As the only obligate granivorous Afro-Palearctic migrant in Europe, the turtle dove is ecologically unique and is particularly vulnerable, due to a lack of mitigation for depleted natural food resources, with most agri-environment schemes targeting invertebrate food. Being a migratory species, female turtle doves require a good food source at a very specific time, early in the breeding season, to rapidly increase body condition after migration in preparation for egg-laying; however, most agri-environment schemes focus on providing habitat for invertebrates rather than early-seeding plants (Robinson & Sutherland, 1999). Other granivorous species are similarly experiencing severe declines. In Africa, dramatic declines have been observed in seed-eating species, including the loss of the three most abundant granivorous species, the Sudan golden sparrow (Passer luteus), the black-crowned (Eremopterix nigriceps) and chestnut-backed (Eremopterix leucotis) sparrow larks from parts of North West Senegal (Zwarts et al., 2018). Furthermore, Zwarts et al. (2018) found an average decline of 80% in seed-eating resident birds between the early 1960s and 2014/2015, estimating a loss of 24 million African granivorous birds. An even larger relative decline was observed in the European turtle dove, attributed to a combination of adverse effects on wintering and breeding grounds (Zwarts et al., 2009, 2018).
There has been a shift in the diet of turtle doves in the UK since the 1960s. The prevalence of seeds of non-cultivated arable plants declined from 95% in the 1960s (Murton et al., 1964), to just 40% in the 2000s (Browne & Aebischer, 2003a, 2003b), replaced by the seeds of cultivated crops, principally wheat (Triticum sp.) and rape (Brassica). Furthermore, seeds such as niger (Guizotia abyssinica), sorghum (Sorghum bicolor) and hemp (Cannabis sativa), common components in garden bird food, have only recently become prevalent in turtle doves' diet in the UK (Dunn et al., 2018).
In the UK, a higher proportion of cultivated seeds in adult turtle doves' diet was associated with better body condition, but the opposite was true for nestlings, which were in better condition when fed a higher proportion of wild seed species (Dunn et al., 2018), when they were subsequently more likely to survive 30 days post-fledging (Dunn et al., 2017). Together, these findings indicate that, whilst anthropogenic food resources may benefit adult birds early in the breeding season in food-limited areas like the UK, the availability of wild food resources is important in increasing the likelihood of successfully fledging chicks. It would also be important to assess whether these patterns are maintained in other areas. The diet of turtle doves on the wintering grounds also consists of a mixture of wild and cultivated seeds (Jarry & Baillon, 1991), but little is known of the relative effects of each on body condition and survival. A better understanding of turtle dove diet across its range, and their use of wild and cultivated resources, will help inform the development of appropriate conservation measures for this species (Newton, 2004a).
Unlike other dietary studies focussed solely on the breeding grounds (Browne & Aebischer, 2003a; Dunn et al., 2018; Gutiérrez-Galán & Alonso, 2016; Mansouri et al., 2019), this study includes sites on both the breeding and wintering grounds. We used high-throughput sequencing (HTS) to reveal the food resources used by turtle doves across a range of environments, testing the following hypotheses: (i) dietary resources used will differ between breeding and wintering grounds; (ii) turtle doves will feed on a higher proportion of wild seeds in a country with less intensive agriculture and (iii) birds feeding on a higher proportion of cultivated seeds will be in better condition than those feeding on more wild seeds.
2 METHODS
2.1 Sample collection
Fecal samples were collected in three countries, across both wintering and breeding grounds (Figure 1). A wintering population of turtle doves was sampled at the Beer Sheba Project (Beer Sheba Project, 2002), an agricultural resource center encompassing a 100 ha Acacia woodland near Sandiara (14°22′N, 16°48′W), Senegal between November and March over four winters (2014/2015 to 2017/2018). Mist nets were used to catch birds when they returned to the site to roost in the evening. Birds were caught at two different European breeding locations, in western Europe (France) and eastern Europe (Hungary) in order to sample geographically distinct populations using different migratory flyways (Marx et al., 2016), early in the breeding season. In France, samples were collected in spring 2017 (15th – 30th May) from two mainland sites, comprising large areas of deciduous forest: Chizé Forest (46°07′N, 0°25′W) and Aulnay Forest (46°02′N, 0°14′W), and a more open and varied habitat on Ile d'Oléron (45°93′N, 01°28′W), an island just off the west coast. In Hungary birds were sampled in spring 2018 (20th May – 7th June) from Balotaszállás-Öttömos (46°16′N, 19°35′W), consisting of a mix of plantations and native forest (Appendix S1: sampling location information). Whilst there was variation in the surrounding environment, all sites were located within a landscape containing a mix of agricultural land and small towns or villages, often interspersed with natural grassland. As we aim to compare two breeding grounds with varying levels of agricultural intensity (between more intense agriculture observed in western Europe and less intense in eastern Europe) the extent of agriculture around the breeding grounds field sites was determined using land use maps (CLC, 2018) by calculating the proportion of land within a 10 km radius accounted for by different land use types. In France, potter traps were baited with bird-seed mix (species composition identified by DNA sequencing; see Appendix S2: bait composition), and in Hungary, the capture site, where mist nets were used, was baited with ground maize. Baiting frequency and regimes varied between sites, with bait being provided daily in Chizé, every other day in Aulnay and on Ile d'Oléron, and every day for the week of 26th May – 2nd June, then every other day after this date in Hungary. Further information of baiting methods are provided in Appendix S2: delivery of bait.
Fecal samples were collected from sterile bird bags within which birds were temporarily held after capture, and dried to preserve plant DNA (Rayé et al., 2011). In the first three sampling seasons in Senegal (2014/15–2016/17), fecal samples were air-dried; in all other sampling seasons, fecal samples were dried using self-indicating silica gel beads (2.5-6 mm, Fisher Scientific). Silica gel was added to samples, which were stored at room temperature. Samples were checked after 12–24 h and if all silica gel had turned from orange to clear, indicating moisture absorption, silica gel was poured out and replaced. This process was repeated until silica stopped changing color. Samples were frozen as soon as possible following arrival in the UK (Appendix S3: Sample storage). Due to field conditions and time between sample collection and import into the UK, the time to freezing varied, and was a maximum of 38 days for the majority of samples. However, for samples collected between November 2015 and March 2016, this time was considerably longer (241–346 days), due to import restrictions.
Each bird was fitted with a standard metal ornithological ring and a range of morphometric measurements taken, including weight, and maximum flattened wing chord (Svensson, 1992). In Senegal, birds were aged (Baker, 1993) as either adult first winter or unknown. Birds caught in Europe were not aged, but all were at least one year old. All bird handling was conducted by licensed ringers.
2.2 DNA extraction and PCR amplification
2.2.1 Fecal samples for dietary analysis
DNA was extracted using either a QIAamp DNA Stool Mini Kit or QIAamp Fast Stool Mini Kit (Qiagen, Hilden, Germany), following manufacturers protocols with minor modifications, as detailed in Dunn et al. (2016). Two extraction negatives, containing nuclease free water instead of sample DNA, were included in every extraction batch to detect potential contamination.
PCR amplification of the ITS2 region was carried out using the UniPlant primer pair (F: 5′-TGTGAATTGCARRATYCMG-3′; R: 5′-CCCGHYTGAYYTGRGGTCDC-3′) (Moorhouse-Gann et al., 2018), specifically designed for short amplicons (187–387 bp) to maximize amplification of degraded plant DNA from fecal samples. Both forward and reverse primers were labeled with MID-tags (Multiple Identifiers) to provide a unique combination of tags for each DNA sample. PCRs were carried out in 25 μL reaction volumes, consisting of 12.5 μL Multiplex PCR Master Mix (Qiagen), 2.5 μL nuclease free water, 2.5 μL each of 0.01 M UniPlantF and UniPlantR MID-tagged primers (Eurofins, Ebersberg, Germany), and 5 μL template DNA. Within each 96-well plate, 11 extraction negatives, one PCR negative, two unused MID-tag combinations and two PCR positives (DNA extracted from plants endemic to Mauritius, thus not occurring within our study system) were included as controls (Taberlet et al., 2018). Replicates were included for a total of 16 samples to assess the consistency of sequencing.
Reaction conditions were initial denaturation at 95°C (15 min); 40 cycles of 95°C (30 s), 58°C (90 s), 72°C (90 s); final extension at 72°C (10 min). Concentration of amplified DNA was determined using a DNA fast analysis cartridge with QIAxcel (Qiagen). Each plate of MID-tagged PCR product was combined into a single pool of uniquely identifiable samples, with approximately equal concentration per sample. Plates were pooled individually due to unavoidable variation between QIAxcel runs. Following pooling of samples, the quality and size of amplicons in each pool was checked via TapeStation2200 (Agilent, Santa Clara, USA) and pools cleaned using SPRIselect beads (Beckman Coulter, Brea, USA) following manufacturer's instructions, at a ratio of 1:1.1, to retain fragments of the desired size range (207–400 bp). Once cleaned, the concentration of DNA was measured for each pool using a Qubit dsDNA High-sensitivity Assay (ThermoFisher Scientific) and pools were combined into a single equimolar pool.
The library preparation for Illumina sequencing was carried out on this pool using NEXTflex Rapid DNA-seq Kit (Bioo Scientific, Austin, USA), following the manufacturer's protocols. The library was sequenced using a V2 chip, with 2 × 250bp paired-end reads on an Illumina MiSeq sequencer (Illumina, San Diego, CA).
2.3 Bioinformatic analysis
Bioinformatic analyses were carried out using a custom pipeline designed for analysis of diet metabarcoding data from Illumina MiSeq (Drake et al., 2021). Briefly, sequences were trimmed, aligned and quality checked using FastP (Chen et al., 2018), using a minimum sequence length of 170 bp and quality threshold (Q) of 33. Sequences were demultiplexed and assigned to their sample ID, prior to removal of MID-tag and primer, using Mothur v1.39.5 (Schloss et al., 2009). Following demultiplexing, samples were dereplicated and error-corrected via Unoise3, in Usearch 11, to remove sequencing and PCR errors and chimeras, producing sequence outputs of zero-radius taxonomic units (zOTUs) (Edgar, 2016). BLASTn v2.7.1 (Camacho et al., 2009) was used to assign sequences to taxa using the GenBank BLAST database (Altschul et al., 1990), with the minimum percent identity of 95%. BLAST results were filtered using the “dplyr” package in R to retain only the top BIT score for each zOTU, calculated by combining the percent ID and e-value. These BLAST outputs were analyzed using MEGAN v6.15.2 (Huson et al., 2016) to assign taxonomic names to each zOTU.
Following taxonomic assignment, results were manually checked to identify zOTUs not assigned to species level, generally resulting from variations in nomenclature or erroneous sequences on GenBank. Where possible, sequences were assigned to species level; however, where there were multiple top hits on GenBank, they were assigned to the highest taxonomic rank in common. The geographic range of all taxa identified was checked using Kew Science “plants of the world online” (POWO, 2019). We identified 15 species assignments which did not occur within our sampling range, these were thus assigned the most appropriate taxonomic level occurring within our study region. The final step was aggregation, whereby all zOTUs assigned to the same taxon were aggregated in R using the “aggregate” function.
Data cleaning was carried out as per Drake et al. (2021), using complementary filtering methods of removing maximum contamination (based on negative controls and unused MID-tag combinations) and removal of uncommon sequences, to remove both exogenous contaminants, resulting from lab procedures, and internal contamination, resulting from sequencing errors, respectively.
For the removal of uncommon sequences, the filtering threshold was based on frequency of occurrence of taxa per sample; accounting for variation in read depth between samples (in this case, read depth/sample ranges from 4276 to 172,171), facilitating a standardized clean up across all samples. Different filtering thresholds, determined based on the frequency of non-target DNA occurring in positive controls, were tested to assess the efficiency of removal of false positives from positive controls, as per Drake et al. (2021), with a threshold of 4% being selected. (Appendix S4: Filtering threshold selection).
Following data filtering, fungal and bacterial taxa were removed, as were any taxa which were not identified to at least family level.
2.4 Statistical analysis
All statistical analyses were carried out in R, version 4.0.1 (R Core Team, 2020). Due to differences in the amplification efficiency of DNA from different plant species, as demonstrated during development and testing of the primer pair used in this analysis (Moorhouse-Gann et al., 2018), presence or absence of each taxonomic unit within each sampling unit, rather than read count, was used in all statistical analyses (frequency of occurrence) (Lamb et al., 2018).
As sample size varied across countries, with a relatively small number of birds being caught in Hungary, we assessed sampling completeness using nonparametric Chao estimators using the package vegan (Oksanen et al., 2020). The “specpool” function was used for to extrapolate the species richness in each country sampled, using incidence-based estimates, using the default Chao equation. The extrapolated species richness was used to estimate the proportion of total taxonomic units of the diet likely to have been detected in each sampling site. Repeatability of sequencing was assessed using Jaccard distance, to assess the dissimilarity between data sets of the repeated sampling runs, using the “vegdist” function in the package vegan.
2.4.1 Dietary variation in turtle doves
Two GLMs with Poisson errors and logarithmic link functions were fitted to assess differences in dietary species richness (number of taxa found within a fecal sample) between: (i) sampling seasons and (ii) years. The latter just used data for Senegal, as this was the only site sampled more than once (four consecutive winters). An additional independent variable of the number of days between sample collection and freezing, and the interaction between this and sampling season was included to account for potential effects of the variation in time that samples were stored in the field. For all GLMs, models were fitted using the “glm” function in base R, and goodness of fit assessed by comparing the residual and fitted values, and with Q–Q plots. Significant pairwise differences were determined using Tukey's post-hoc test, using the multcomp package in R (Hothorn et al., 2008). Most analyses carried out included the supplementary seed provided to birds as bait, as this was considered an important aspect of the diet of birds sampled in France. However, some additional analyses were conducted to assess the effect of removing bait seed from the analysis.
An index of bird body condition (hereafter referred to as “condition”) was calculated using linear regression, and model assumptions of normality were checked as previously described. Bird weight was the dependent variable, and wing length and time of day were independent variables, the latter to account for within-day variation in weight as there was an approximate linear increase in weight through the daily sampling period. Whilst date of capture may be a factor in body condition, in particular for birds returning from migration, this was not included as a variable in calculating body condition because birds returning from migration land on the breeding grounds over a period of approximately one month, and there was no way for us to know how soon after migration birds were captured. Residuals from the model were used as a proxy for condition of individual birds, as this residual variation in weight not accounted for by wing length is associated with relative fat mass in adults, a commonly used estimate of body condition in birds (Labocha & Hayes, 2012). Body weight can be also influenced by the amount of seed in a bird's crop. This would have a greater effect on the weight of birds captured in France, as they were caught in baited potter traps, rather than mist nets as in other sites. As this variation could not be corrected for, birds sampled in France were not included in assessment of body condition. Variation in condition between the five remaining sampling seasons was assessed using a one-way ANOVA, modeling condition as the dependent and sampling season as the independent variables.
Multivariate Generalized Linear Models (MGLMs) were fitted using the mvabund package (Wang et al., 2012) to investigate variation in the diet of turtle doves. As not all dietary items were differentiated to species, the presence of taxa at genus level was used for this analysis, including 85 different genera. Senegal data had unique attributes of multiple years of sampling, a mix of identifiable adult and first year birds, and sampling over a five-month period, therefore two MGLMs were fitted to address different questions. All models including birds captured in France were run twice, once using the full dietary data set and one with the bait seeds omitted, in order to assess the effect of bait seed on the analysis. In all instances, the “manyglm” function was used to create an MGLM using the binomial family and complementary log–log link function. An “mvabund” object was created using the presence-absence matrix of dietary items, which was the dependent variable in the models. Using a subset containing only data from Senegal, the following terms and interactions were included as independent variables: year, age, condition, Julian date (calculated as number of days from November 1st), and the following interactions: year: age, year: condition, year: Julian date, condition: age and condition: Julian date. As there was no evidence of a linear trend in Julian date across winter, Julian date was fitted as a quadratic term to allow for basic non-linearity. Using the full dataset, containing all birds, the only independent variable was sampling season, as the use of bait in France meant that body condition was not comparable across all samples, thus it was not included as a variable in this model. Because different study sites were sampled in different years, year and country could not be distinguished in the model: instead, “sampling season”, comprising the six combinations of year and country, were used. The fit of the model was checked using the “plot.manyglm” function, modeling residuals against fitted values and generating Q–Q plots.
Model selection was carried out based on Akaike's Information Criterion (AIC), using R's “step” command. The significance of terms in the final models was assessed using the “anova” function. To examine which taxa contributed to dietary variation, univariate tests were run on each dietary item separately using the “p.uni = ‘adjusted’” argument in the “anova.manyglm” function. Two methods were used to visualize distribution of consumed genera: (i) genus level bipartite plots were created using the bipartite package (Dormann, 2019) to illustrate the overlap of genera consumed between countries; and (ii) differences in genera consumed between sampling seasons were visualized using non-metric multidimensional scaling (NMDS). For NMDS analysis, genera occurring in a single dietary sample were removed, as this analysis is liable to outliers skewing results. NMDS used the “metaMDS” command from the vegan package (Oksanen et al., 2020) based on using Jaccard dissimilarities. “Ordispider” was used to visualize results from nMDS via plotting with ggplot2 (Wickamn, 2011).
2.4.2 The effect of cultivated vs. wild seeds on bird condition
To test if variation in food types consumed in the different sampling countries influenced turtle dove condition, plants were categorized into “cultivated” (34 taxonomic units), consisting of crop plants, those cultivated for animal fodder and seeds commonly provided in supplementary feed, and “wild” (95 taxonomic units). For the most part, wild/cultivated status did not differ across sampling sites, with the exception of Panicum sp., which occur naturally in Senegal, but primarily as a constituent of bird feed in Europe. The genus Brassica occurred in one instance where it could not be identified to species level. Due to the diversity of this genus including both wild and cultivated species within our study area, this taxonomic unit was excluded from the analysis of food type.
These classifications were used to analyze the effect of the type of food consumed on body condition. Condition and the proportion of wild seed taxa consumed were compared between sampling seasons by generating an analysis of covariance, with Gaussian family and identity link function, using body condition as the dependent variable and sampling season, proportion of dietary items accounted for by wild seed and their interaction as predictor variables. Model selection was carried out using “step.” In order to assess whether body condition changed as winter progressed, the same analysis was carried out on a subset of data consisting of only birds sampled in Senegal, including Julian date (with quadratic transformation), and interactions between Julian date and year as additional dependent variables. The distribution of sampling dates in each year was visualized in order to demonstrate variation between years, to consider if this could be driving between-year trends (Appendix S5: Bird capture date across sampling seasons).
All mean values are reported ± standard error.
3 RESULTS
3.1 DNA amplification, sequencing and taxon identification
DNA was successfully amplified from 203 turtle dove fecal samples across three countries, including four winter sampling seasons and two spring sampling seasons (Table 1). The Illumina sequencing run yielded 17.1 million paired-end reads, which was reduced to 9.7 million reads following data processing (Appendix S6: processing of Illumina MiSeq output) The average read count per sequenced fecal sample was 43,586 ± 1322 reads (min = 323, max = 136,843). A total of 121 taxonomic units were present in the diet of turtle doves in this study (N = 203). Of these, 57.9% (n = 70) were identified to species level, another 38.8% (n = 47) to genus, 2.5% (n = 3) to tribe or subtribe and the remaining 0.8% (n = 1) to family (Appendix S7: taxonomic units identified).
| Country | Year | Season | Sample size | SR range | Mean SR ± SE | Significant pairwise differences |
|---|---|---|---|---|---|---|
| Senegal | 2014/15 | Winter 1 (W1) | 25 | 1–5 | 2.920 ± 0.237 | AB |
| Senegal | 2015/16 | Winter 2 (W2) | 14 | 1–6 | 3.214 ± 0.482 | A |
| Senegal | 2016/17 | Winter 3 (W3) | 28 | 1–5 | 2.536 ± 0.260 | AB |
| Senegal | 2017/18 | Winter 4 (W4) | 61 | 1–6 | 1.918 ± 0.147 | B |
| Senegal | Total | 128 | 1–6 | 2.391 ± 0.121 | ||
| France | 2018 | Spring 1 (S1) | 63 | 1–7 | 3.381 ± 0.350 | A |
| Hungary | 2019 | Spring 2 (S2) | 12 | 1–6 | 3.583 ± 0.288 | A |
| Overall | 203 | 1–7 | 2.768 ± 0.100 | |||
| France (bait removed) | 2018 | Spring 1 (S1) | 38 | 1–4 | 1.711 ± 0.155 |
- Note: Mean SR differed between years sampled in Senegal, (GLM; Dev3,124 = 12.676, p = 0.005, N = 128), and between sampling seasons, (GLM; Dev5,197 = 30.199, p < 0.001, N = 203). Letters (A-B) show significant difference according to Tukey's post-hoc test: species richness was lower in W4 than in S1, S2 and W2. Species richness for France was also included following removal of taxa found in bait.
When assessing sampling completeness, species richness of estimated available sampling units varied considerably between the three countries sampled, with the lowest richness predicted in Hungary (67.365 ± 28.80) and the highest in France (143.575 ± 55.11) (Appendix S8, Table 1). This analysis suggests that we detected 46% of available taxonomic units in Senegal, 34% in France and 39% in Hungary. Neither the number of days samples were stored prior to freezing nor the interaction between days to freezing and sampling year had a significant effect on species richness (GLM: Dev3,120 = 0.100, p = 0.751; GLM: Dev3,120 = 1.489, p = 0.685, respectively).
3.2 Land use around field sites
A 10 km radius was plotted around each capture site to determine the land use in the surrounding areas (Appendix S9: European land use). In France, 50% of the land use was attributed to agriculture, including non-irrigated arable land, vineyards and fruit tree plantations and 21% was accounted for by suitable natural habitat, including broad-leaved forest, coniferous forest and transitional woodland and shrub. A further 2% was accounted for by pastures, which often have appropriate foraging habitat for turtle doves. The remaining 27% was accounted for by water, marshes and intertidal flats and urban areas, which are not suitable foraging habitats for turtle doves. In Hungary, 39% of land was cultivated, and the majority of the remaining land was also suitable for turtle dove foraging, with 45% being accounted for by forest and woodland, 12% being pasture and only 4% being accounted for by marshes, water bodies and urban areas.
3.3 Dietary variation
Mean species richness was 2.827 ± 0.288 taxa consumed per individual (range 1–7; Table 1). Species richness in samples from Senegal differed between years (GLM: Dev3,124 = 12.676, p = 0.005); and across all data, species richness varied between sampling seasons (GLM: Dev5,197 = 30.199, p < 0.001; Table 1). Birds sampled from Senegal in the winter of 2017/18 had the lowest mean species richness and birds in Hungary had the highest mean species richness (Table 1).
When supplementary food from bait was excluded in France, feces from birds caught in potter traps had the lowest average species richness, and lowest maximum species richness within a single individual, indicating a less varied use of natural resources in the diet of birds visiting potter traps supplemented with bait.
The most frequently occurring taxa in the diet of turtle doves differed across countries sampled. In Senegal, the taxa consumed by the greatest proportion of birds sampled were Cenchrus americanus (pearl millet) and Sorghum sp., each being detected in 32.0% (n = 41) of birds sampled. Cenchrinae, the subtribe to which C. americanus belongs, was detected in a further 14.1% (n = 18) of birds sampled. In France, the most prevalent taxa were Triticum sp. (74.6%, n = 47), Helianthus sp. (41.3%, n = 26), Triticeae (the subtribe within which Triticum falls) (36.5%, n = 23), and Sorghum sp. (33.3%, n = 21), all of which were present in the seed-mix used to bait traps. Triticum monococcum (einkorn wheat) was detected in 22.2% of birds (n = 14). The species of Triticum present in the seed mix could not be differentiated between three top hits on GenBank (T. turgidum, T. aestivum and T. dicoccoides), but as T. monococcum is grown in France, this seed may have come from the environment or from garden bird feeders. Excluding items known, or likely, to be fed to birds, the most frequently detected food resource was Geranium molle (dove's foot cranesbill), an arable weed commonly occurring across Europe. In Hungary, the three most frequently detected taxa identified were Euphorbia sp. (50%, n = 6), Euphorbia pseudoesula (Figert's spurge) (50%, n = 6), and Euphorbia cyparissias (Cypress spurge) (33.3%, n = 4), all of which are naturally occurring species.
At the family level, Poaceae was the most frequently consumed taxon in Senegal and France, occurring in the diet of 86.7% (n = 111) and 90.5% (n = 57) of turtle doves, respectively. When bait seeds were removed from the diet of birds sampled in France, Poaceae was detected in the diet of a considerably smaller proportion of birds sampled (24%, n = 9), but this was still comparatively common, being the second most detected taxa in birds sampled. Of the 14 taxonomic units within this family detected in Senegal, 28% were cultivated, including C. americanus and Sorghum sp., Panicum sp. and Echinochloa sp. were consumed by 22.6% (n = 29) and 18.0% (n = 23) of birds sampled respectively; both are widespread genera in the grass family, native to Africa. In France, 50% of taxonomic units within the Poaceae family were cultivated (n = 6), including Sorghum sp., Triticum sp. and Panicum miliaceum. This frequent occurrence of cultivated seed is expected due to the trapping method, and when bait taxa were excluded from analysis, the wild genera in this family (Argrotis sp., Lolium sp., Poa sp. and the subtribe Loliinae) appeared in the diet of a quarter of birds sampled. Poaceae was the second most commonly consumed family in Hungary, appearing in the diet of 41.7% of birds sampled (n = 5). Fewer taxonomic units from this family were identified in Hungary (n = 5), but of those present 60% were cultivated.
In initial analysis, Hungary was the only country where the dominant family consisted solely of wild food resources. Euphorbiaceae were detected in the diet of 58.3% (n = 7) of birds, and consisted of five different taxonomic units: Euphorbia sp. and four distinct species of Euphorbia, all of which are naturally occurring. Despite being used to bait capture sites in Hungary, maize was not detected in the diet of birds in this location. When bait seed was excluded from samples collected in France, Euphorbiaceae was the second most frequently consumed taxa, being detected in the diet of 24% (n = 9) of birds sampled, equal to the proportion of birds consuming wild Poaceae. When analyzed with bait seed excluded, the diet of birds in France was also dominated by wild resources, with the dominant family being consumed by individuals retained in the sample being Geraniaceae, predominantly Geranium molle, which was present in 39% (n = 15) of samples.
Asteraceae occurred in the top three families consumed by individuals in all countries sampled, being the third most commonly detected in Senegal (10.9%) and Hungary (25.0%) and second in France (41.3%). In France, this was dominated by Helianthus sp., a component of the bait used and common element in bird-seed, which was detected in 41.3% of all birds sampled. When bait was removed from analysis, Asteraceae occurred in the diet of 8% (n = 3) of birds retained in analysis, with Carduus sp. (true thistles) accounting for the wild genera consumed from this family. In contrast, in Hungary, Helianthus sp. was only identified in one individual, and the other two, naturally occurring genera: Ambrosia sp. (ragweeds) and Carduus sp., accounted for most of the occurrence of the Asteraceae family. In Senegal, the most commonly occurring member of the Asteraceae family was Blainvillea, another wild genus.
Following model selection using step, the only variable retained in the model assessing dietary variation between years in Senegal was Julian date (MGLM: LRT2 = 344.6, p < 0.001). In the full data set, sampling season was significantly associated with genera consumed (MGLM: LRT5 = 647.4, p < 0.001; Figure 2). There was little overlap of consumed taxa between countries sampled (Figure 3). After adjusting for multiple testing, sampling season had a significant effect on 13 dietary items (N = 85 genera), indicating that these genera were responsible for a large proportion of the seasonal variation (Appendix S10: Univariate test results). Of these, eight occurred only in Senegal (Cenchrus, Echinochloa, Dactyloctenium, Senna, Balinvillea, Melochia, Nymphaea and Urochloa) and two occurred only in France (Triticum and Geranium), supporting the idea that country drove the observed dietary variation.
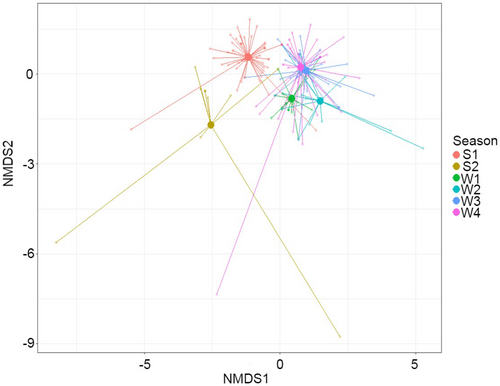

When investigating the effect of removal of bait genera from analysis of the genera consumed by turtle doves, the significant terms in the model did not change, with both the full data set and that with bait excluded producing the same level of significance for the association between sampling season and dietary items consumed by turtle doves (Appendix S11: Effects of removing bait, Table 1). There was a small amount of variation between the two data sets when adjusting for multiple testing, with differences in the genera consumed responsible for the largest portion of dietary variation (Appendix S12: Effects of removing bait, Table 2). Two items found in bait (Triticum sp. and Helianthus sp.) significantly contributed to dietary variation when bait was included, but these were no longer significant following the exclusion of bait. One genus which did not significantly drive variation when bait was included did have an effect when bait was excluded, this was a wild seed found only in France (Mercurialis sp.).
| % individuals sampled with wild/cultivated taxa detected in diet | % individuals sampled with only wild/cultivated taxa detected in diet | % dietary items accounted for by wild/cultivated taxa | ||||
|---|---|---|---|---|---|---|
| Wild | Cultivated | Only wild | Only cultivated | Wild | Cultivated | |
| W1 | 88.0% | 68.0% | 32.0% | 12.0% | 67.2 ± 6.6 | 32.8 ± 6.6 |
| W2 | 85.7% | 78.6% | 21.4% | 14.3% | 58.9 ± 8.6 | 41.1 ± 8.6 |
| W3 | 67.9% | 96.4% | 3.6% | 32.1% | 36.3 ± 5.6 | 63.7 ± 5.6 |
| W4 | 45.9% | 86.9% | 13.1% | 54.1% | 30.7 ± 4.7 | 69.3 ± 4.8 |
| S1 (full data set) | 91.3% | 95.2% | 4.8% | 39.7% | 28.0 ± 3.6 | 72.0 ± 3.7 |
| S1 (bait removed) | 100.0% | 10.5% | 89.5% | 0.0% | 95.4 ± 2.1 | 3.9 ± 2.0 |
| S2 | 91.7% | 33.3% | 66.7% | 8.3% | 80.6 ± 9.9 | 19.4 ± 9.9 |
- Note: First two columns represent the percentage of individual birds in which wild or cultivated taxonomic units were detected. Middle two columns show the percentage of individual birds in which the diet consisted solely of either wild or cultivated seeds. Last two columns show mean percentage of diet (±SE) accounted for by wild and cultivated plants.
3.4 Food type and its effect on body condition
Hungary had the greatest proportion of wild taxonomic units recorded in the diet (80.6%; Table 2), whilst France had the highest proportion of cultivated seeds recorded in the diet (72.0% of taxonomic units detected), with cultivated taxa present in the diet of 95.2% of birds sampled (Table 2). This is unsurprising due to the use of bait to capture birds. When excluding seeds present in the bird seed mix, 25 individuals (39.7%) sampled in France had no taxonomic units recorded in their diet, indicating that this feed mix is an important part of the recent diet of birds caught in potter traps, as expected.
Only sampling season (GLM: F4,130 = 10.154, p < 0.001) affected turtle dove body condition. Proportion of wild taxa consumed (GLM: F1,134 = 0.003, p < 0.955) (Figure 4) and the interaction between these two terms (GLM: F4,126 = 1.557, p = 0.190) were not significant. Birds sampled in Senegal in winters 1 and 3 (2014/15 and 2016/17, respectively) had the lowest body condition scores, both of which were significantly less than the body condition score for birds sampled in Hungary and in Senegal in winter 4 (2017/18).
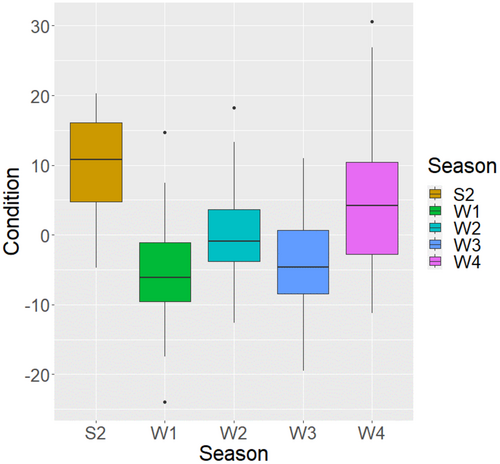
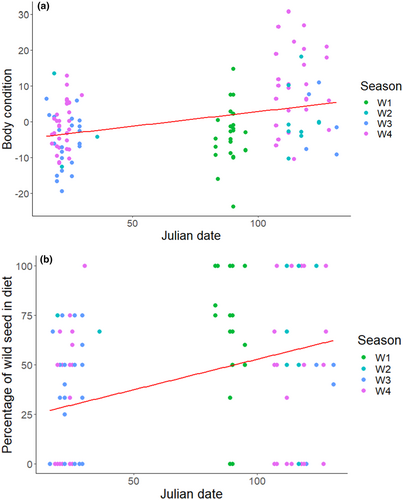
In Senegal, year (GLM: F3,120 = 8.048, p < 0.001) and Julian date (GLM: F2,118 = 14.354, p < 0.001) both influenced body condition, but the proportion of the diet comprised of wild genera did not have a significant effect on body condition (GLM: F1,123 = 2.045, p = 0.155). Body condition increased as the winter progressed (Figure 5a), as did the proportion of wild taxa consumed (Figure 5b), indicating increased use of wild seeds later in the season. There were two periods of sampling in W2, W3 and W4 (November and March) but only one in W1 (January).
3.5 Repeatability
Sixteen repeated fecal DNA samples were included to assess the consistency of sequencing within a single run. The average Jaccard distance was 0.390, and dissimilarity scores ranged from 0.019 to 0.785 (Table 4). The range of species richness and average species richness detected was slightly higher in repeat 2, but the proportion of taxonomic units accounted for by wild and cultivated seeds, and two of the most abundant families (Poaceae and Asteraceae) were relatively consistent between repeats (Table 3). The number of taxa recorded for repeated samples, as well as the number of taxa in common between the two repeats is presented in Table 4. Seven of the 16 samples had identical results between repeats. All taxa occurring in only one of the two repeats after cleaning occurred at relatively low read counts (Appendix S12: Sequencing repeats raw data). Variation between repeats could not be mitigated by altering threshold for data cleaning.
| SR range | Mean SR ± SE | Proportion wild taxa | Proportion cultivated taxa | Proportion Asteraceae | Proportion Poaceae | |
|---|---|---|---|---|---|---|
| Repeat 1 | 1–5 | 2.583 ± 0.417 | 0.581 | 0.419 | 0.194 | 0.419 |
| Repeat 2 | 1–6 | 3.083 ± 0.379 | 0.541 | 0.459 | 0.194 | 0.581 |
| Jaccard distance | N. Taxa recorded | N. Taxa in common | ||
|---|---|---|---|---|
| B02 | 0.288 | Repeat 1 | 4 | 4 |
| Repeat 2 | 5 | |||
| B24 | 0.426 | Repeat 1 | 3 | 3 |
| Repeat 2 | 3 | |||
| EG82510 | 0.215 | Repeat 1 | 4 | 4 |
| Repeat 2 | 4 | |||
| EP38521 | 0.164 | Repeat 1 | 1 | 1 |
| Repeat 2 | 1 | |||
| EP38582 | 0.276 | Repeat 1 | 3 | 2 |
| Repeat 2 | 2 | |||
| EY46975 | 0.785 | Repeat 1 | 1 | 1 |
| Repeat 2 | 2 | |||
| EZ11354 | 0.786 | Repeat 1 | 1 | 1 |
| Repeat 2 | 2 | |||
| EZ11358 | 0.490 | Repeat 1 | 5 | 4 |
| Repeat 2 | 4 | |||
| EZ11376 | 0.413 | Repeat 1 | 2 | 2 |
| Repeat 2 | 3 | |||
| GY149801 | 0.130 | Repeat 1 | 1 | 1 |
| Repeat 2 | 1 | |||
| GY149826 | 0.504 | Repeat 1 | 2 | 2 |
| Repeat 2 | 3 | |||
| GY154243 | 0.461 | Repeat 1 | 4 | 4 |
| Repeat 2 | 6 | |||
| GY154862 | 0.494 | Repeat 1 | 4 | 4 |
| Repeat 2 | 4 | |||
| GY161023 | 0.274 | Repeat 1 | 4 | 4 |
| Repeat 2 | 4 | |||
| GY161034 | 0.591 | Repeat 1 | 1 | 1 |
| Repeat 2 | 3 | |||
| HA70752 | 0.019 | Repeat 1 | 3 | 3 |
| Repeat 2 | 3 |
4 DISCUSSION
We detected a wide range of dietary items consumed by turtle doves across breeding and wintering grounds, with 121 taxonomic units identified, although only a small number accounted for the majority of the diet, with most observed infrequently. The mean number of different taxonomic units detected in the diet of individuals (2.827 ± 0.288) was lower than previously described when analyzing turtle dove diet using HTS (Dunn et al., 2018). There was little overlap between the genera consumed in different countries. The only genera found in all three regions were Panicum sp., Sorghum sp., Cannabis sp. and Helianthus sp. With the exception of Panicum sp., which is native to West Africa (National Research Council, 1996), these genera are not naturally occurring within the regions, rather they are cultivated crops or common components of bird seed.
4.1 Turtle dove diet in the wintering grounds
A mix of cultivated and wild seeds were found in individuals sampled in Senegal, although the former were more frequent (between 60.8% and 96.4%). The most common seeds consumed in this study were Cenchrus americanus (pearl millet) and Sorghum sp., common crops in the landscape surrounding the roost site where the birds were caught (personal observation). Field observations of a high proportion of caught birds having Sorghum, millet or both in their crop support these findings (unpublished data). Both of these species have previously been shown to be important constituents of turtle doves' diets (Morel & Morel, 1979; Morel & Roux, 1966), and the annual production of these crops (along with rice Oryza sp.) in west Africa has been correlated with the annual survival of turtle doves (Eraud et al., 2009). However, cultivated rice itself, commonly regarded as important to turtle doves (Jarry & Baillon, 1991), was absent in the present study. This probably reflects the geographical variation in the different crops grown, as very few areas of rice cultivation were present in our study area (personal observation).
Wild grass seeds were also an important constituent of turtle doves' diet in Senegal, especially Panicum and Echinochloa sp. which, although often harvested for food (National Research Council, 1996), are not cultivated crops. As there is currently no ITS2 region sequence for P. laetum on Genbank, this species could not be confirmed in the present study; however, it is commonly found in Senegal, and has been shown to be an important food source for turtle doves on the wintering grounds (Jarry & Baillon, 1991; Morel, 1987). P. laetum was present in previously wet areas around Beer Sheba, but it was not abundant, and was more abundant at sites along the Senegal river than at our study site (unpublished data). Two more wild grasses, Dactyloctenium aegyptium and Urochloa mosambicensis were also detected relatively frequently in the diet, further suggesting that wild grass seeds are an important element of the diet of turtle doves in their wintering grounds. The abundance of wild grass seeds in the diet of turtle doves is consistent with the findings of Morel and Morel (1974), that resident doves studied in the Sahelian region, including African collared dove (Streptopelia roseogrisea), Senegal dove (Streptopelia senegalensis) and vinaceous dove (Streptopelia vinacea) fed largely on the seeds of wild grasses, including Panicum laetum, Brachiaria hagerupii and Dactyloctenium aegyptium.
The proportion of cultivated seed species in the diet increased with each winter sampled, potentially a result of variation in the sampling between years. Average Julian date was earlier in W3 and W4 than W1 and W2, as a result of varied capture success and DNA extraction or PCR failure. The two most prevalent cultivated crops, millet and sorghum, are harvested September–November in Senegal (GIEWS, 2020), resulting in an abundance of spilt grain earlier in the season, which decreases as these resources are consumed post-harvest. As more birds were sampled during this earlier period in W3 and W4, this may, at least in part, explain the higher prevalence of cultivated seed in the diet in these years. Furthermore, as the wintering season progressed, it appears birds became more heavily reliant on wild seeds than cultivated, as the proportion of wild seeds in the diet increased with Julian date.
4.2 Turtle dove diet in the breeding grounds
The relative contributions of cultivated and wild seeds to the diet differed between the countries surveyed, although variation in methods of trapping may account for those differences. While turtle doves sampled in France consumed the highest proportion of cultivated seeds, much of this would have consisted of the bait used to attract birds as, pre-harvest, when trapping occurred, these seeds would not have been readily available in the surrounding farmland. Due to the necessity of using bait to capture birds in this region, and the repeated provisioning of sites each year for long-term monitoring purposes, it is difficult to discern the extent to which birds would utilize alternative cultivated versus wild resources if this feed were not provided. Whilst significant quantities of bait are provided in France (Appendix S2: delivery of bait), other species, including wild boar (Sus scrofa) and roe deer (Capreolus capreolus), are known to eat large quantities of this. Although bait (maize) was used in Hungary, it was not detected in their diet, despite evidence that UniPlant primers detect this species, both in in vitro primer testing, and in HTS studies (Dunn et al., 2018; Moorhouse-Gann, 2017). Unlike in France, the site in Hungary was not baited to attract birds every year, and the bait was not put out every day during sampling. In addition, birds trapped in mist-nests are not necessarily attracted to bait, and maize is a less attractive bait for turtle doves than alternatives such as sunflower seeds.
In addition to the cultivated seeds present, a diverse range of wild seeds was also detected in the diet, with 33 wild taxonomic units identified in France and 20 wild taxonomic units identified in Hungary. This may reflect the higher floral diversity in the forest habitat where they were caught, compared to heavily managed agricultural land. Turtle doves captured in forests in Spain also consumed a greater number of wild species than previously recorded from farmland habitats (Gutiérrez-Galán & Alonso, 2016). However, turtle doves forage over distances of several kilometres (Browne & Aebischer, 2003a), thus would likely also feed in non-forest habitats, such as agricultural fields.
The most commonly detected wild species in France was Geranium molle, which was present in 20.6% of birds sampled. This annual herbaceous plant occurs across Europe, and has previously been detected in the diet of turtle doves in Spain (Gutiérrez-Galán & Alonso, 2016) and the UK (Dunn et al., 2018). Gutiérrez-Galán and Alonso (2016) found G. molle to be among the most frequently consumed species in their study, and recognized it as a potential species of importance due to its availability early in the breeding season. Other wild seeds which were previously reported as important in the diet of turtle doves in a UK study were Fumaria officinalis (common fumitory) and Stellaria media (common chickweed) (Murton et al., 1964). In contrast F. officinalis was not detected in this study, and S. media only in the diet of two birds in France (3.2%), similar to findings of recent studies in the UK (Dunn et al., 2018). These plant species, once common in arable land across Europe, are declining as farming practices reduce arable plant biodiversity across Europe (Andreasen et al., 1996; Dunn et al., 2016; Storkey et al., 2012). For example, Stellaria media, previously one of the most abundant arable weeds, declined significantly between 1979 and 2009 in one study in France (Fried et al., 2009).
The proportion of the diet accounted for by wild seeds was highest in Hungary (80.6%), with wild seeds being detected in the diet of over 90% of birds sampled, as opposed to cultivated seeds, which only occurred in the diet of one third of birds sampled. This is supported by a previous study in Hungary (Haraszthy, 1998). When investigating the gut contents of 36 turtle doves, wild seeds such as Sinapsis arvensis, Chenopodium sp., Stellaria sp. and Fumaria sp. dominated the diet, accounting for 83% of seeds identified, whilst common crops and animal fodder such as Alfalfa sp. and wheat accounted for just 13% of seeds present (Haraszthy, 1998). Birds in Hungary also had, on average, the greatest richness of taxonomic units per individual (3.583 ± 0.288), indicating a more varied diet, although this was still lower than previously observed in turtle doves in the UK (10.400 ± 0.61) (Dunn et al., 2018). This may reflect a higher diversity of food available in the UK than our study sites, or variation caused by different bioinformatic pipelines and methods of data cleaning used in the two studies, illustrating the need for a more standardized approach to data cleaning (Drake et al., 2021).
Euphorbia species were the most abundant taxonomic units identified in Hungary, including E. pseudoesula, E. cyparissias, E. seguieriana and E. stepposa. Taxa from the Euphorbia genus and Euphorbiaceae family have previously been detected in the diet of turtle doves in small amounts in the UK and France (Dubois, 2002; Dunn et al., 2018; Murton et al., 1964), but to our knowledge, this is the first study that has identified this genus as a dominant food resource. Despite a relatively small sample size in Hungary in this study, sampling completion analysis predicted relatively consistent level of available dietary items being detected across all three study sites (between 34% and 46%), with 39% of estimated available taxonomic units being detected in samples from Hungary.
The frequency of wild seeds in the diet in Hungary is consistent with the hypothesis that birds would consume a higher proportion of wild food in areas of lower agricultural intensity. Agricultural intensity tends to be lower in central and eastern European countries, which are either not members or new members of the EU (Donald et al., 2001). Traditional farming methods, such as smaller field sizes, lower use of chemical herbicides, and retention of semi-natural field margins (Tryjanowski et al., 2011), may have contributed to smaller declines in farmland bird populations than observed in western Europe. However, intensification is increasing across Europe (Verhulst et al., 2004), so it is important that appropriate agri-environment and other land management measures are implemented to ensure the declines observed across western Europe do not spread across the whole continent.
4.3 Body condition
When considering just birds caught without the use of bait in Hungary and Senegal, there was no correlation between the proportion of dietary items accounted for by cultivated seeds and body condition, therefore not supporting the hypothesis that a higher proportion of cultivated seed in the diet would result in better body condition.
A study in the UK indicated that an important contributor to the decline of turtle doves is a reduction in breeding attempts, with a 61% decline in the number of young successfully fledged per pair observed between the 1960s and 2000 (Browne & Aebischer, 2004). This has been suggested to be linked to reduced food availability, making it harder for adults to reach and maintain adequate body condition for breeding, resulting in a shorter breeding season (Browne & Aebischer, 2001). Whilst this has not been proven in turtle doves (Browne & Aebischer, 2003b), a reduction in wild food availability early in the breeding season, when nutritional demands to reach breeding condition are highest, occurred concurrently with a dietary switch to a greater proportion of cultivated seed (Browne & Aebischer, 2003a), making this a plausible cause for reduced breeding attempts in the UK. As crop plants do not provide seeds until later in the breeding season, restoration of early-flowering wild plants in the environment is essential for the species to provide seed-rich habitats. This is supported by a recent review of turtle dove habitat use (Carboneras et al., 2022), and the findings of Dunn et al. (2018) that nestlings in their study were in better condition when the diet included a higher proportion of naturally occurring weed seeds. In areas where it may be difficult to restore natural weed seed resources, such as the UK, provision of supplemental feed may be a potential strategy for species management, to help make up for the shortfall of wild food availability early in spring, although the latter should be considered as an “emergency action” rather than a continued strategy (Fischer et al., 2018).
During the breeding season, when there are energy demands for producing and laying eggs (Robb et al., 2008), and pre-migration when birds need to fuel their migratory journeys (Newton, 2006), it benefits birds to have higher fat reserves. However, in the non-breeding season, birds often carry less weight, as they only need to obtain enough food to satisfy their own subsistence needs to survive, and lower weight can enable more effective predator avoidance (Kullber et al., 1996). We observed birds with lower condition scores in winter, however, in Senegal, body condition increased with Julian date, suggesting that birds were investing more in improving body condition later in the season in preparation for migration, one of the most energetically demanding periods of the annual cycle (Cooper et al., 2015). This increase was concurrent with an increase in consumption of wild resources. This may be a result of a lack of cultivated seeds in the environment following harvest earlier in the season. Alternatively, turtle doves may be utilizing wild seeds for their nutritional value to increase fat reserves in preparation for migration. The latter argument is in concordance with what has been observed in turtle dove nestlings in the UK (Dunn et al., 2018).
Annual variation in environmental factors may influence the availability of wild and cultivated resources, potentially affecting body condition as a result. Whilst investigation of this is outside the scope of this work, it would merit future research to understand how changing weather conditions globally may impact resource availability and wildlife health.
4.4 The inclusion of bait in dietary analysis
This study uses metabarcoding to provide a more detailed insight into the variation in the diet of a migratory species, and demonstrates the effectiveness of using recently developed, plant-specific primers to identify a wide range of taxa consumed within a granivorous diet (Moorhouse-Gann et al., 2018). In addition, a key limitation of diet analysis is addressed in this study.
The need to use bait to attract rare species for capture in order to analyze diet comes with the inherent problem of dealing with bait items occurring in the diet. In some studies, bait items are simply removed from analysis as a known item that was fed to individuals (da Silva et al., 2020). Whilst we acknowledge that this may be the most appropriate response in some study systems, this is not always the case. The abundance of these bait seeds in the birds sampled in France highlight that this is an important component of the diet of birds caught in potter traps, although it is not possible to conclude how important it is for the population as a whole. A previous dietary study of turtle doves in the UK found that 89% of birds sampled had consumed seed commonly occurring in garden bird feed (Dunn et al., 2018), showing that turtle doves are commonly consuming this type of resource, even when it is not used as bait, at least in certain areas. It is arguable that turtle doves would not need to use such resources if an abundance of wild resources were available in their environment, although it cannot be excluded either that such food may be favored when available, given the usually higher fat contents of seeds used as bait, and the predictability of its occurrence.
Whilst we discuss the drawbacks of the need to use bait to attract birds to capture sites in this study, we acknowledge that supplementary food is a potentially important aspect of the diet in situations where wild food resources are scarce, particularly regarding the management of declining species, as reflected in several other studies related to supplementary feeding. In New Zealand, supplementary food is provided for the critically endangered Kakapo (Strigops habroptilus) to increase productivity, and the Mauritian pink pigeon (Columba mayeri) is highly reliant on the provision of supplementary food due to severe habitat degradation as a result of extensive deforestation (Edmunds et al., 2008; Elliott et al., 2001). Furthermore, in a study by Newton (2004b), a lack of food resources resulting from the widespread switch to autumn-sown crops and increased use of herbicides was determined to be a key driver in the decline of 10 seed-eating farmland birds in the UK. As demonstrated by these, and many other examples, there is an argument for supplementary feeding in the management of declining species, when habitat conversion and degradation lead to a lack of natural resources. Such is the case for many farmland birds in the UK, including turtle doves, resulting in a number of agri-environmental schemes being put in place (Newton, 2004b). These are designed to combat the loss of natural food resources resulting from extensive agricultural intensification, and includes a supplementary food scheme specifically targeting turtle doves in England (UK Government, 2021).
We conducted analysis both with and without the bait seed included to assess the impacts this provisioned food may have on ecological inferences made in dietary studies. Overall, the inclusion of bait did not affect the level of significance of terms in the full model. Some variation was observed in specific dietary items driving the majority of the variation in sampling season, however, the majority of the seeds determined to be driving the dietary variation remained the same. Triticum and Helianthus were two components of bait which significantly drove dietary variation. When bait was excluded, Triticum was absent from analysis as this was only consumed from bait. Helianthus was consumed in Hungary and Senegal as well as from bait, but when bait was excluded from analysis, the consumption of this genus no longer significantly contributed to dietary variation. The biggest ecological difference observed when removing bait was that of the similarity between diets, as represented by NMDS. When bait was removed, there was greater difference evident from plots between the diets of birds sampled in Europe and Africa, which will be driven by the use of seeds which are widespread in the environment in Senegal (Sorghum and Panicum) in bait used in France.
Whilst there is some variation in the results, the comparison of data with and without bait indicates that the dietary variation between sites was not heavily driven by the provision of bait, and taxonomic groups driving variation between the sites were primarily wild resources. Due to the abundance of bait in the diet detected in this study, and that of Dunn et al. (2018), as well as the known switch to an increase in consumption of cultivated seed in absence of wild resources observed in turtle doves (Browne & Aebischer, 2003a), we suggest that ecological inferences of diet would be less representative of the true nature of the foraging behavior of turtle doves if bait were excluded. The prevalence of cultivated seed in the diet of turtle doves in these studies suggests that this is a favorable food source when available, making it a useful short-term management tool in areas, such as the UK, where wild food resources are heavily depleted.
Another important consideration in using metabarcoding for diet analysis is the repeatability of the sequencing process, which we assessed by the inclusion of 16 pairs of replicate samples. The mean Jaccard dissimilarity between paired replicates was 0.309. We did find some inconsistencies in the absence/present of taxa between repeats. This is consistent with the findings of previous studies assessing the repeatability of diet metabarcoding (Jedlicka et al., 2017; Shutt et al., 2020), indicating it would be advisable to conduct repeat extraction and sequencing runs for avian dietary analysis where possible. However, we do show a reasonable level of repeatability in sequencing runs, suggesting that reliable results can still be obtained where this level of replication is not possible or practical.
We have shown significant variation in the diet of turtle doves not only between the breeding and wintering grounds but also between two different breeding sites within Europe with differing levels of agricultural intensity, with a greater abundance of wild seeds in the diet of birds sampled from the eastern European site, where agricultural intensity is lower. It would be beneficial to further investigate the diet of turtle doves in a wider range of countries subject to less intense agriculture. We did not find support for our hypothesis that birds feeding on a high proportion of cultivated seeds would be in better condition, as there was no significant effect of the proportion of wild or cultivated seeds in the diet on the body condition. The concurrent increase in the consumption of wild seed and body condition prior to migration in birds sampled in Senegal suggests that wild seeds may play a role in increasing body condition in preparation for spring migration. Further investigation into the importance of dietary diversity in the context of body condition would be useful in informing management plans to deliver optimal food resources to support his declining species.
AUTHOR CONTRIBUTIONS
WOCS: the conception or design of the study. JCD: the acquisition, analysis or interpretation of the data. REY: field work, lab work, analysis and interpretation. WOCS: JCD, IPV, JWM: analysis and interpretation. CJO, MK, MBD, MS: acquisition of data in – field sampling in Senegal. HL, CE: acquisition of data – field sampling in France. OK: acquisition of data – field sampling in Hungary. LED: analysis of data – designed bioinformatic pipeline. AM: acquisition of data – high throughout sequencing. REY: Writing of the manuscript.
ACKNOWLEDGMENTS
For the purpose of open access, the author has applied a CC BY public copyright license (where permitted by UKRI, “Open Government Licence” or “CC BY-ND public copyright licence” may be stated instead) to any Author Accepted Manuscript version arising. Natural Environment Research Council GW4+ Studentship NE/L002434/1; Royal Society for the Protection of Birds project CASE partner, Genetics Society Heredity Fieldwork Grant; British Society for Parasitology International Training and Fieldwork Award.
CONFLICT OF INTEREST STATEMENT
We confirm that no authors have any conflicts of interest to disclose.
Open Research
DATA AVAILABILITY STATEMENT
Data will be made available via Dryad repository following acceptance of the manuscript.




