Design and partial validation of novel eDNA qPCR assays for three common North American tick (Arachnida: Ixodida) species
Abstract
The range expansion of ticks to higher latitudes poses a severe threat to human health exposing human populations who had no prior contact with ticks to several harmful tick-borne diseases. Early detection of ticks in new areas is critical to help inform the public and medical professionals of the dangers associated with tick encounters. Environmental DNA represents a novel survey method that could provide reliable records of tick occurrences and timely warnings of their range expansions. In this study, we designed novel eDNA qPCR assays for three common North American tick species (Dermacentor variabilis, Amblyomma americanum, and Ixodes scapularis) and tested them on 51 samples of grasses and leaf litter collected from 12 grassland and forest sites in central and southern Illinois. We provide in silico and in vitro validation of all three assays; however, we were unable to generate any positive detections from field samples. Our lack of eDNA detections likely stems from low eDNA deposition rates coupled with rapid degradation in grasslands and forests, a problem exacerbated by terrestrial eDNA sampling methods limited by volume of substrate. We provide recommendations for improving sample collection methods to increase detection probability in future efforts. Continued research should focus on the viability of eDNA to detect small terrestrial invertebrates, like ticks, and it potential as early warning indicator of the spread of vector-borne diseases.
1 INTRODUCTION
Ticks are among the most clinically important arthropods, responsible for approximately 95% of reported cases of vector-borne diseases (Paddock et al., 2016). In recent decades, reported cases of tick-borne diseases have consistently increased, and new pathogens continue to be identified (Herrmann et al., 2014; Paddock et al., 2016). Moreover, a changing global climate may be hastening the spread of tick-borne disease, as research has revealed a correlation between warmer temperatures and occurrences of tick-borne diseases (Gatewood et al., 2009; Parola et al., 2008). Higher temperatures have led to accelerations and extensions of tick developmental cycles, causing increased egg production, longer active seasons, and concomitant range expansions (Gilbert et al., 2014; Süss et al., 2008). As a result, climatic changes will likely continue to promote range expansions of several tick species into areas where they were previously absent or of minor medical importance (Greer et al., 2008; Süss et al., 2008), putting humans and animals at higher risk of exposure to tick-borne pathogens.
As disease-carrying ticks extend into higher latitudes, early detection of these species is increasingly critical. Early detections allow health organizations to begin developing and implementing awareness campaigns, preventative messaging, and control measures (Gasmi et al., 2017; Ogden et al., 2009). Knowing that disease-carrying ticks may be in the area aids doctors in providing accurate diagnoses of tick-borne diseases (Aguero-Rosenfeld et al., 2005). Generally, there have been three main avenues for early detection of ticks: human reporting of tick encounters, passive surveillance, and active surveillance. Reports based on human encounters are often delayed indicators of range expansion, as these reports tend to take place long after species establishment. Moreover, healthcare providers have been shown to often misidentify even common tick species (Butler et al., 2017; Laga et al., 2022), so human reporting rarely leads to accurate locations of tick expansions until tick-borne diseases have already emerged from those areas (Ripoche et al., 2018). Passive surveillance typically involves soliciting members of the public to submit captured ticks to a central facility where they are identified to species and sometimes tested for disease-causing bacteria and viruses (Ballman et al., 2023; Ogden, Maarouf, et al., 2006; Ogden, Trudel, et al., 2006). Although useful, this kind of surveillance generally reveals only broad zones of emerging disease (Koffi et al., 2012; Ogden et al., 2010) and is ineffective in areas where human and/or tick populations are very low (Eisen & Paddock, 2021; Ripoche et al., 2018). Active surveillance is generally considered the most effective method at identifying fine-scale geographic areas where disease-carrying tick populations are emerging (Clow et al., 2018; Eisen & Eisen, 2021a; Koffi et al., 2012; Ripoche et al., 2018). This method involves targeted surveys for collection of ticks, and then testing for disease-causing microorganisms. Although often considered the “gold-standard” (Koffi et al., 2012; Ripoche et al., 2018), this method is prone to false negatives and/or is very expensive and time-intensive to carry out with high sampling density (Koffi et al., 2012; Ripoche et al., 2018). Manual identification of individual ticks is particularly time-consuming process involving stereoscopes or scanning electron microscopes (Abdullah et al., 2016; Keirans & Clifford, 1978; Keirans & Durden, 1998) that is also subject to human error in the case of specimen damage or slight morphological differences among species within the same genus. Misidentification of similar looking tick species can present serious economic and public and veterinary health consequences, as was seen when long-horned ticks (Haemaphysalis longicornis) were incorrectly identified as rabbit ticks (H. leporispalustris) in U.S. records ahead of the larger invasion event (Thompson et al., 2020). Novel methods of surveillance are needed that provide rapid and yet reliable detections of ticks earlier in their range expansion sequence, while remaining cost effective and deployable at scale.
One method that has gained substantial traction in recent years for early detection of nonnative species is environmental DNA (eDNA). This technique involves harnessing DNA shed by organisms from a natural substrate such as water, soil, or plant material (Taberlet et al., 2012). These samples can then be analyzed via qPCR to provide sensitive estimates of whether a species of interest is present in an area. Environmental DNA could yield a more sensitive, efficient, and cost-effective method for detection of new tick populations as it could eliminate the need to manually identify individuals. Environmental DNA has been less studied in terrestrial systems, but other studies have shown that eDNA can be successful in elucidating pollinator and arthropod communities on the surface of flowers (Johnson et al., 2023), identifying animal vectors of disease transmission including mosquitos (Amarasiri et al., 2021; Schneider et al., 2016), and monitoring for invasive arthropods (Allen et al., 2021; Valentin et al., 2020). Broad eDNA metabarcoding of flower heads and petals has revealed whole arthropod communities including extremely small arthropods like thrips (order: Thysanoptera; Harper et al., 2022; Johnson et al., 2023). Gamonal Gomez et al. (2023) further validated arthropod eDNA detections using camera traps to confirm species visitations. Meanwhile targeted assays using qPCR have proven more sensitive at detecting the invasive Lycorma delicatula (spotted lanternfly) via sampling eDNA on the surface of trees and grape vines (Allen et al., 2021; Valentin et al., 2020). Given these successful usages of eDNA for terrestrial arthropod detection from plant matter, this same method could yield a more sensitive, efficient, and cost-effective strategy for detecting new tick populations, more readily identifying invasion events and highlighting areas for more focused surveillance.
Here we report the first attempt to leverage eDNA methodologies as an early detection method for ticks expanding outside their documented ranges. Specifically, we developed and partially validated three novel qPCR assays to detect three disease-carrying ticks in Illinois: Dermacentor variabilis (American dog tick), Amblyomma americanum (lone star tick), and Ixodes scapularis (black-legged tick). All three of these species are widely distributed throughout central and eastern North America and are responsible for the majority of tick-borne diseases including tularemia and Rocky Mountain spotted fever, transmitted by D. variabilis (Burgdorfer, 1975), ehrlichiosis and alpha-gal syndrome, transmitted by A. americanum (Childs & Paddock, 2003), and a variety of diseases carried by I. scapularis, but most famously, Lyme Disease (Nelder et al., 2016). Not only are these species notorious vectors of harmful disease to humans and livestock (Eisen & Eisen, 2021b), but they are also particular concern to public health as their ranges have been rapidly expanding due to a changing global climate (Kopsco et al., 2022). By 2070, the suitable climate in North America for D. variablis is predicted to increase by 50% extending well into Canada and along the west coast (Minigan et al., 2018). Similar range expansions have also been observed with I. scapularis and A. americanum, and both species are expected to expand into additional regions in New England, the northwest United States, and Canada (Clow et al., 2017; Gabriele-Rivet et al., 2017; Monzón et al., 2016).
Our objectives were to provide in silico and in vitro validation of novel single-species qPCR assays that can reliably detect eDNA from Illinois ticks and also provide in situ validation of these primer sets on grass and leaf litter samples taken from local Illinois prairies and deciduous forests. The biology of each of our three target species requires them to host seek by either using a sit-and-wait strategy during part of the day from the tops of vegetation (D. variabilis and I. scapularis) or by moving along the ground toward heat and carbon dioxide signals (A. americanum; Nielebeck et al., 2023; Tietjen et al., 2020). Each of these strategies requires individuals to come in contact with leaf litter either as the primary host-seeking strategy or rehydrate and seek refuge when not actively questing. Previous research has shown that eDNA collected from the surface of plant material can be a more sensitive method to detect arthropods that interact with these plants than visual observation (Harper et al., 2022; Johnson et al., 2023; Valentin et al., 2020). Our study seeks to provide proof-of-concept of how next-generation genetics tools like eDNA can improve active surveillance of disease-transmitting organisms.
2 MATERIALS AND METHODS
2.1 Assay design and in silico testing
Primer and probe assay development took place in Geneious Prime v.2021 (https://www.geneious.com). All sequences including the cytochrome oxidase-1 (COI) region belonging to Dermacentor variabilis (24 sequences), Amblyomma americanum (23 sequences), and Ixodes scapularis (534 sequences) were downloaded from Genbank (NCBI) and aligned together using the plugin for the Clustal Omega aligner (Table S1). We searched the sequences for areas of the COI region that showed almost no intraspecific variation among individuals of the same species but with interspecific variation among different tick species. Our GC ratio was limited to between 35% and 65% and our maximum amplicon length was 200 bp. After identifying candidate regions we generated two different primers and probes for each species. We performed in silico testing, using the native PCR test function in Geneious Prime, on our candidate primers and probes to ensure that they would amplify every sequence from the target species and would not amplify the other two nontarget species. Our maximum primer mismatches were set at 3 with no allowable mismatches within the last 3 bp. Then, we tested the specificity of the primers on all COI sequences from every species within Parasitiformes (mites and ticks) on GenBank (Table S2). This group, consisting of 51 different families, 114 genera, and 423 species, included all North American tick species, multiple congener species, and several co-occurring mite species. We tested primers and probes against all nontarget sequences to ensure that primers were specific to their target species. Any primer/probe set that showed evidence of both the forward and reverse primer binding to a nontarget species was removed from consideration. The primer/probe set that resulted in amplification of all target sequences and the fewest amplification of nontarget sequences in the in silico testing was selected for in vitro testing.
2.2 In vitro testing and validation
We tested the sensitivity and specificity of just the primers by testing them against DNA obtained from the target species as well as each of the other two tick species. We wanted to test the specificity and sensitivity of the primers first to ensure that we had consistent and specific amplification of our primers as nontarget amplification of the primer sets could reduce qPCR efficiency and copy number estimates. Reference tick DNA was obtained by extracting DNA using the Qiagen DNeasy® Blood & Tissue Kit (Venlo, Netherlands) from 4 specimens collected from the state of Illinois. We ran seven qPCR plates containing 12 replicates and four non-target controls for each tick species (Figure S1). Each replicate contained 1 μL of reference DNA from all three tick species, with the four nontarget controls containing 1 μL of DNA from only the nontarget species. For each species, we used DNA from four different individuals to verify that there was no individual varation. All qPCR reactions contained 12.5 μL of SyberGreen Master Mix, 1 μL of each 10 μM primer, 7.5 μL molecular grade water, and 3 μL of tick DNA diluted to 0.1 ng/μL. The primers tested were the best performing from the in silico testing, as above (Table 1). Annealing temperatures increased for each plate, beginning at 55°C and increasing by 1°C increments up to 62°C. Reaction conditions included a 5 min denaturation at 95°C followed by 45 cycles of 30 s at 95°C, 30 s at annealing temperature, and 1 min at 72°C, followed by a melt curve. The melt curve was used to assess nontarget amplification. Optimal annealing temperatures were recorded based on the lowest Cq values and presence of only one peak in the melt curve. Assessing the annealing temperature with just the primers would provide a general estimate of the optimal annealing temperature so that we could then move on to testing the optimal concentrations of primers before finally fine-tuning the optimal annealing temperature once the probe was included.
| Target species | Assay name | Forward primer (5′–3′) | Reverse primer (5′–3′) | Probe (5′–3′) | Amplicon length (bp) |
|---|---|---|---|---|---|
| Dermacentor variabilis | DVCO1 | CCTTTATCATCAAATTTGTCTCACTATGG | GGTATTCGTTCAAGAGTTAAACCAATTG | /6-FAM/AGCCTTCAT/ZEN/CTAGCAGGTGCATCCTCT/IABKFQ/ | 155 |
| Amblyomma americanum | AACO1 | ACCAATTATAATCGGAGGATTCGG | CCACAAGAGAAGAGTTGATTAAAAGAC | /6-FAM/TGGCTTGTA/ZEN/CCCATTATGCTTGGAGC/IABkFQ/ | 143 |
| Ixodes scapularis | ISCO1 | GATCTCCTGGAATATCTCTTGAACG | TATAAAATAGGGTCACCTCCTCCAG | /6-FAM/TCTATCTCT/ZEN/TCCTGTTCTTGCTGGCGCA/IABkFQ/ | 178 |
- Note: These are the primers and probes that best performed during the in silico testing of the qPCR assays and were used for all of the in vitro and field testing.
Primers and probes were tested at four volumes: 1.5, 1.0, 0.5, and 0.1 μL. All primer and probe concentrations throughout the study were 10 μM. We ran one qPCR plate for each species. Each plate also contained 12 replicates and four nontarget controls, similar to the primer tests. All reactions contained 12.5 μL of TaqMan Environmental Master Mix 2.0, 3 μL of tick DNA, the designated volume of primers, and molecular grade water to bring the total volume up to 25 μL. Plates were run with the same reaction conditions as the temperature optimization, using the optimal annealing temperature recorded in the previous step. The optimal primer and probe concentration based on the concentration that maximized sensitivity by providing the lowest Cq value and consistency of amplification by reducing variation of Cq values between replicates.
Synthetic gBlocks for each species were used to fine-tune annealing temperatures and determine the limit of detection (LOD) and limit of quantification (LOQ) for each assay (Table S3). We made 1:10 serial dilutions of each gBlock, creating 7 standards for each species. Concentration of the seven standards was 106, 105, 104, 103, 102, 10, and 1 copies/μL. Three replicates of each standard were run at 56, 56.5, and 57°C for the D. variabilis and A. americanum assays and at 56.5, 57, and 57.5°C for the I. scapularis assay. Reaction conditions for all three assays were the same as the previous test plates. All reactions contained 12.5 μL of TaqMan Environmental Master Mix 2.0, 1 μL of primers, 1 μL of probe, 6.5 μL of molecular grade water, and 3 μL of gBlock standard. We calculated qPCR efficiency for each annealing temperature. The optimal annealing temperature was the one that provided the highest efficiency and the most detections from the 1 copy/μL standard. To calculate LOD and LOQ, we ran a full plate of gBlock standards with 12 replicates of seven standards. Concentration of the seven standards was 105, 104, 103, 102, 10, 1, and 0.33 copies/μL for the I. scapularis and D. variabilis assay. For the A. americanum assay, the lack of detections from the 1 copy/μL standard led us to replace the 0.33 copies/μL standard with a 25 copies/μL standard. We defined LOD as the lowest concentration of DNA in which we are 95% confident would be detected in a single replicate. We also calculated an effective LOD which was the concentration in which we were 95% confident would detected in at least 1 out of 3 replicates, as this was our number of replicates used in field samples. LOQ is defined as the lowest concentration of DNA for which the coefficient of variation of the Cq values was less than 0.35. LOD and quantification was calculated following the procedure outlined in Klymus et al. (2020).
2.3 Field sample collection
Tick and plant matter (fresh grasses/vegetation and leaf litter) were collected from 12 total sites from June 2, 2021, through November 5, 2021, throughout central (three sites) and southern (nine sites) Illinois (Figure 1a) by researchers at the University of Illinois College of Veterinary Medicine. Collection sites were characterized by a mix of three habitat types: open prairie, mowed prairie or forest edge, and mixed deciduous understory. These habitats included preferential environments within the state for each of the three targeted tick species and have documented tick presence (Bacon et al., 2022; James et al., 2015; Kopsco et al., 2023). Three 250 m linear transects were established at each site (one transect per habitat type) and sampled for both ticks and plant matter via a block random design (without replacement) to reduce bias (i.e., one of the three transects was selected at random for each site per visit, for a total of three visits per site on average throughout the field season; Figure 1b).
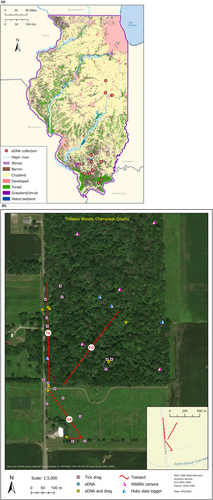
Along each transect, three samplings of plant matter were collected, and a field blank was included. Using a fresh pair of nitrile gloves for each of the three samples taken, a single 50 mL Falcon tube was filled halfway with three unpacked plant matter samples collected at approximately 0, 150, and 250 m along the transect to ensure adequate coverage. The plant matter sample tube and a control (blank) 50 mL tube were filled with enough CTAB extraction buffer (Teknova) to cover all plant matter and tightly capped. Both tubes were inverted five-ten times (as necessary) to ensure all litter was in contact with the buffer. Tubes were placed on ice and transported to the University of Illinois eDNA lab for extraction and analysis.
Tick sampling was conducted via drag method using a 1-m2 white denim fabric cloth attached to a wooden dowel pulled with white cotton cord (Bouseman et al., 1990). A distance method was selected in accordance with CDC tick surveillance guidelines (CDC, 2022), with each site representing approximately 750 m2, divided among the three linear transects (~250 m2 each) located in different habitat types (open prairie, mowed prairie/forest edge, and mixed deciduous understory). Tick sampling always occurred after eDNA samples were collected, or eDNA sampling and tick dragging were conducted by separate people to ensure there was no cross contamination from ticks collected on drags with plant matter samples. Drag cloths were pulled along transects at a walking pace and checked every 10–15 m for attached ticks. Any ticks that were found were removed with tweezers and placed into a 1.5 mL snap top vial filled with 70% ethanol. Vials were transported back to the University of Illinois College of Veterinary Medicine where they were identified to species and life stage using a compound microscope (Keirans & Durden, 1998; Keirans & Litwak, 1989).
2.4 Sample extraction and qPCR
All field samples were extracted 30 days post-collection via a modified chloroform: isoamyl alcohol method (Renshaw et al., 2015). Briefly, 450 μL of CTAB was drawn from each field sample and incubated at 65°C for 1 h. Then, 450 μL of chloroform:isoamyl alcohol (24:1) was added to each tube. Tubes were vortexed and centrifuged for 5 min at 15,000 × g. The supernatant was removed and transferred to a new tube where 350 μL of freezing cold isopropanol and 175 μL of room temperature 5 M NaCl was added. Samples were left at −20°C overnight for DNA to precipitate. The next day, DNA was centrifuged at 15,000 × g for 10 min. We decanted the liquid and then washed each pellet twice with 70% ethanol. Following the second wash, tubes were left at room temperature for 10 min to dry followed by drying in a vacufuge for 15 min. DNA was eluted in 100 μL of TE buffer and stored at −20°C until qPCR could take place.
All samples and field blanks were tested in triplicate for eDNA from all 3 target species using the primer/probe assays developed above. All reactions contained 12.5 μL of TaqMan Environmental Master Mix 2.0, 1 μL of forward and reverse primers (10 μM), 1 μL of probe (10 μM), 6.5 μL of molecular grade water, and 3 μL of DNA. Three replicates of seven gBlock standards containing target DNA from each species were run alongside samples from each plate in order to estimate copy number. Concentration of the seven standards ranged from 1 to 106 copies/μL by a factor of 10. Three no template controls (NTCs) containing molecular grade water instead of DNA were included on each plate. Thermocycler conditions included an initial denaturation step at 95°C for 5 min followed by 45 cycles of 95°C for 30 s, the optimal annealing temperature from in vitro validation for 30 s, and extension at 72°C for 1 min.
3 RESULTS
3.1 Results from in silico testing
In silico testing resulted in an optimal primer/probe set for each species (Table 1). Each of these sets successfully amplified all COI sequences on Genbank for the target species and to the exclusion either nontarget species. When testing the primers against other Parasitiformes species, all three sets showed evidence of specificity, as there were no nontarget species to which both the forward and reverse primers were predicted to bind to.
3.2 Results from in vitro testing
In vitro testing demonstrated that the optimal annealing temperatures for the A. americanum assay, D. variabilis, and I. scapularis assays were, respectively, 56.5, 57, and 57.5°C. None of the wells containing only nontarget DNA showed signs of amplification indicating that our assays were highly specific to the target species. Additionally, at each of the optimal annealing temperatures, we observed only a single peak in the melt curve, indicating that the other species did not co-amplify. These annealing temperatures also maximized sensitivity by providing the lowest Cq values. The optimal primer and probe volumes were recorded to be 1 μL across all three species. This volume provided the lowest Cq values and showed consistent amplification and the least variation in Cq values among replicates.
We developed values for efficiency, LOD, and LOQ for each assay (Table 2). We achieved an efficiency of 97% or greater for both the D. variabilis and I. scapularis assays; however, efficiency of the A. americanum assay was lower at 85.9%. The A. americanum assay generally performed worse, as the LOD occurred at 19 copies/μL in a single reaction, compared to the D. variabilis and I. scapularis assays which had an LOD around 1.7 and 6.6 copies/μL, respectively (Table 2). Our effective LOD (i.e., the LOD when performing three replicates) was even lower, under 1 copy/μL for both the D. variabilis and I. scapularis assays. The I. scapularis assay had the lowest LOQ at 105 copies/μL followed by the A. americanum assay at 181 copies/μL (Table 2). Despite the high efficiency and low LOD of the D. variabilis assay, we did encounter constraints related to consistency of copy estimates as the assay had the highest LOQ at 211 copies/μL.
| Assay | R 2 | Efficiency | Limit of detection (1 replicate) (copies/μL) | Effective limit of detection (3 replicates) (copies/μL) | Limit of quantification (copies/μL) |
|---|---|---|---|---|---|
| DVCO1 | 0.955 | 97.3% | 1.7 (0.86–2.6) | 0.80 (0.59–1.0) | 211 |
| AACO1 | 0.991 | 85.9% | 19 (11–27) | 7.6 (5.5–9.7) | 181 |
| ISCO1 | 0.981 | 97.0% | 6.6 (0–18) | 0.73 (0.76–1.2) | 105 |
- Note: Values in parentheses after limit of detections indicate outer ranges of 95% confidence intervals.
3.3 Results from field sampling
Across the 12 sites in eight counties, we collected 389 A. americanum individuals, 137 D. variabilis individuals, and 2 I. scapularis individuals in sites where eDNA samples (n = 51) were collected in central and southern Illinois (Table 3). Nymphs (n = 303) and adult females (n = 120) were the most commonly found life stage and sex. Most ticks were collected in Williamson and Pope counties (n = 240 and n = 114, respectively) and found during the months of June and July.
| County (number of sites) | Tick species | eDNA detections | Total ticks collected | Life stage | Sex | |||
|---|---|---|---|---|---|---|---|---|
| Adult | Nymph | Larvae | Male | Female | ||||
| Champaign (n = 2) | Amblyomma americanum | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Dermacentor variabilis | 0 | 22 | 22 | 0 | 0 | 14 | 8 | |
| Ixodes scapularis | 0 | 1 | 1 | 0 | 0 | 1 | 0 | |
| Total | 0 | 23 | 23 | 0 | 0 | 15 | 8 | |
| Ford (n = 1) | A. americanum | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| D. variabilis | 0 | 11 | 11 | 0 | 0 | 5 | 6 | |
| I. scapularis | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Total | 0 | 11 | 11 | 0 | 0 | 5 | 6 | |
| Hamilton (n = 1) | A. americanum | 0 | 44 | 13 | 31 | 0 | 0 | 13 |
| D. variabilis | 0 | 53 | 53 | 0 | 0 | 29 | 24 | |
| I. scapularis | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Total | 0 | 97 | 66 | 31 | 0 | 29 | 37 | |
| Jackson (n = 1) | A. americanum | 0 | 6 | 0 | 6 | 0 | 0 | 0 |
| D. variabilis | 0 | 1 | 1 | 0 | 0 | 0 | 1 | |
| I. scapularis | 0 | 0 | 0 | 0 | 0 | 0 |
0 |
|
| Total | 0 | 7 | 1 | 6 | 0 | 0 | 1 | |
| Jefferson (n = 2) | A. americanum | 0 | 37 | 7 | 31 | 0 | 3 | 4 |
| D. variabilis | 0 | 2 | 2 | 0 | 0 | 2 | 0 | |
| I. scapularis | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Total | 0 | 39 | 9 | 31 | 0 | 5 | 4 | |
| Perry (n = 1) | A. americanum | 0 | 9 | 8 | 1 | 0 | 1 | 7 |
| D. variabilis | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| I. scapularis | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Total | 0 | 9 | 8 | 1 | 0 | 1 | 7 | |
| Pope (n = 2) | A. americanum | 0 | 65 | 17 | 48 | 0 | 8 | 9 |
| D. variabilis | 0 | 37 | 37 | 0 | 0 | 17 | 20 | |
| I. scapularis | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Total | 0 | 102 | 54 | 48 | 0 | 25 | 29 | |
| Williamson (n = 2) | A. americanum | 0 | 228 | 42 | 186 | 0 | 21 | 21 |
| D. variabilis | 0 | 11 | 11 | 0 | 0 | 4 | 7 | |
| I. scapularis | 0 | 1 | 0 | 1 | 0 | 0 | 0 | |
| Total | 0 | 240 | 53 | 187 | 0 | 25 | 28 | |
- Note: All ticks were collected along transects by dragging a cloth sheet along a transect. Drags were pooled and summed by count in Illinois. The same transects were also used for sampling plant matter for eDNA analyses.
We tested all 51 field samples and field blanks resulting in zero confirmed eDNA detections across all three assays. One replicate out of three was positive for high amounts of A. americanum DNA, but all follow-up tests (six additional replicates were run for this sample) were negative, and this result was removed for suspected laboratory contamination of the synthetic gBlock. No field blanks or NTCs showed signs of amplification indicating that we did an adequate job of minimizing contamination. All gBlock standards ran with the field samples amplified correctly, indicating that the assays were working but that there were no positive eDNA detections from the field samples.
4 DISCUSSION
Environmental DNA remains an important tool in monitoring the distribution of species. Previous studies have successfully used eDNA methods to delimit range expansions (Valsecchi et al., 2022) and provide early monitoring of invasive species (Allen et al., 2021; Jerde et al., 2011). Moreover, eDNA methods have been shown to require less effort and are often more sensitive than conventional gears at identifying fish (Jerde et al., 2011), insects (Allen et al., 2021; Valentin et al., 2020), amphibians (Moss et al., 2022), pollinators (Harper et al., 2022; Johnson et al., 2023), and small mammals (Lyman et al., 2022). We developed three species-specific qPCR assays to identify three disease-causing ticks.
Tick ranges have been rapidly expanding in recent years due to climate change, bringing serious diseases (e.g. Lyme disease) to areas that had never experienced them (Greer et al., 2008; Süss et al., 2008). All three of our target species have shown evidence of range expansions and increased abundance in recent decades (Sonenshine, 2018). Population and speed of spread models have predicted rapid rates of range expansion for the blacklegged tick (Clow et al., 2017; Ogden, Maarouf, et al., 2006; Ogden, Trudel, et al., 2006). Species distribution models based on climatic suitability also project an increase in potential range area and significant northward expansion of D. variabilis and A. americanum (Minigan et al., 2018; Sagurova et al., 2019). Active surveillance of ticks in communities along the expansion front is important to alert physicians and public health officials, but active surveillance remains an arduous process that is prone to false negatives and often lags behind the true expansion front (Koffi et al., 2012; Ripoche et al., 2018).
We anticipated that eDNA methods might provide a more reliable method to detect ticks earlier in the expansion sequence. Our qPCR assays showed high sensitivity and specificity during in silico and in vitro testing. We were able to achieve consistent and species-specific amplification at just a few copies per microliter. However, when applying our qPCR assays to samples from the field, we were unable to record any positive detections. Our assays were functioning appropriately, as we detected DNA in the positive controls running alongside the field samples, even when the concentration in the positive controls was as low as 10 copies/μL.
It is possible that our lack of field detections could be a result of laboratory methods, including inhibition in the field samples, degradation of DNA, and assays not optimized for local genetic diversity. However, we find each of these explanations unlikely. Storing plant tissues in directly CTAB to later be extracted via the chloroform-isoamyl alcohol method has shown to be an effective method for detecting eDNA from pollinators on the surface of flowers (Harper et al., 2022; Johnson et al., 2023). Plant tissue is known to be high in various PCR inhibitors including polysaccharides, humic acids, and tannins (Schrader et al., 2012), which could have leached into the CTAB. We do not believe that inhibition impacted our results, however, as the chloroform-isoamyl alcohol extraction has shown to be rather robust to the presence of inhibitors in environmental samples (Deiner et al., 2015; Renshaw et al., 2015). Additionally, we reran 24 samples with each assay, but included a spike-in of extracted tick DNA. Every replicate amplified indicating that if tick DNA had been in the samples, we would have detected it. Degradation of tick DNA while the samples sat in CTAB is a more plausible explanation, but still one that we find unlikely. Although CTAB is capable of preserving DNA for more than 30 days (Rogstad, 1992), the DNA does start to degrade over time, so it was possible that small amounts of tick DNA had already degraded by the time we performed extractions. Another possible reason could be that our assays were designed around sequences in online repositories that do not reflect the genetic diversity within our study sites. Again, we find this explanation unlikely as all of our assays were tested in vitro against DNA from four separate specimens, all obtained from the state of Illinois. All assays performed well against these specimens indicating that they should work against the local genetic diversity in Illinois.
While we cannot completely rule out methodological concerns leading to the lack of tick DNA in the field samples, the more likely explanation was that our collection method was unable to obtain sufficient amounts of eDNA. We attempted to collect low-lying grasses and leaf litter along transects and then preserved eDNA on the surface in CTAB. Sampling individual plants has proven to be successful for detection of eDNA from small rodents (Lyman et al., 2022) and pollinators (Johnson et al., 2023). Ticks, however, are extremely small and have a metabolism 12% lower than the average arthropod (Lighton & Fielden, 1995). We posit that it is plausible they shed minute amounts of DNA. Additionally, the plant material sampled by Lyman et al. (2022) was used as nesting material by the small rodents which likely increased the amount of eDNA on the surface of these plants. Species like I. scapularis and D. variabilis, however, employ a sit-and-wait host-seeking strategy, waiting for mammals to move by them, so they are often not moving through a field and shedding DNA on plants they pass by (Sonenshine & Roe, 2014). While A. americanum utilizes both ambush and hunting strategies for host-seeking, the combination of lower metabolism and time in which they are immobile may still prevent enough DNA from being shed in the environment. As a result, a single grassland field or forest plot, which can have thousands of individual grass blades per square meter, is possibly absent of tick DNA except for a few individual blades of grass. Only sampling a few handfuls may not yield sufficient sampling density to capture tick eDNA. A different sampling approach would likely be necessary to capture the small amounts of residual tick DNA spread across a large grass field or forest plot. This consideration particularly applies to I. scapularis, a specie currently more abundant in the northern third of the state (Kopsco et al., 2023; Rydzewski et al., 2011, 2012), and is relatively scant in central and southern Illinois, where our sampling occurred, compared to the other three species.
One more promising method to capture of tick eDNA would likely be grass-rolling. This method is similar to the tree-rolling employed by Valentin et al. (2020). Cotton sheets could be sprayed lightly with water and then rolled over or even dragged across the surface of grasses. Any eDNA on the surface of the grasses would likely be collected on the surface of the cotton sheet which could then be sprayed over a collection container to capture eDNA (Valentin et al., 2020). Another alternative method could be spray aggregation where plots in a forest or grassland are sprayed with water and that water is collected into a container (Valentin et al., 2020). This method has been shown to be successful in detecting eDNA from spotted lanternflies in forests (Valentin et al., 2020) and agricultural fields (Allen et al., 2021). This method could be useful for sampling understory vegetation in forests, but would have to slightly modified for deployment in grasslands, likely by instead of allowing water to drip into a collection vessel, relying on the force of the water from a highly pressurized sprayer to dislodge eDNA from the surface of grasses and into a trough or container placed behind the grasses being sprayed. The water from both methods can then be filtered, and DNA can be extracted from the filter as is common in aquatic eDNA studies (Allen et al., 2021; Valentin et al., 2020). Both of these methods have the benefit of obtaining a much larger sampling area, ostensibly maximizing the chance of collecting tick eDNA (Valentin et al., 2021). The grass-rolling method has the additional benefit of being very similar to conventional tick sampling where a white sheet is dragged through a field collecting individual ticks on the surface of the sheet (Falco & Fish, 1992; Lyons et al., 2021). We recommend testing both of these methods to see if they are more successful at collecting tick eDNA.
Other factors may have also hampered our ability to use eDNA to monitor tick populations. It is possible that our lack of eDNA detections in the field were a result of rapid degradation of tick eDNA in grassland and forest ecosystems. In terrestrial systems, the persistence of eDNA is largely determined by its exposure to ultraviolet light and frequency of rain events (Curtis et al., 2021; Kessler et al., 2020; Valentin et al., 2021). The degradation rates of eDNA in grasslands have not been studied, but Valentin et al. (2021) found that extracellular eDNA left in exposure to full sun (as a large grassland would be) reduces the probability of detection and is undetectable after 4 days. Some of our sampling days took place soon after precipitation events which can also wash eDNA away from vegetation and into the soil or waterways (Valentin et al., 2021). Further work must focus on production and removal of eDNA from terrestrial systems in order to identify the optimal conditions for future sampling.
While collection of environmental samples may never yield enough eDNA to reliably to detect ticks, our assays could still be used for identifying bulk collections of ticks. Identifying each individual collected from a conventional tick drag can be a time-consuming process especially when large number of nymphs or larvae are collected. At these life stages, ticks of different species are difficult to distinguish and require manual identification under a scanning electron microscope (Keirans & Clifford, 1978), if they are able to be identified at all (Clifford et al., 1961). Our assays could alleviate that problem. Instead of identifying each individual tick, all ticks may be extracted together and tested for the presence of our three target species using each of our assays, similar to what is often done with bulk collections of terrestrial insects from traps (Piper et al., 2019).
Our assays were designed in accordance with current guidelines on in silico, in vitro, and in situ validation of qPCR assays (Thalinger et al., 2021). All three of our assays meet the requirements for the first two levels of validation. We conducted in silico and in vitro validation with the target species and closely related species. Our in vitro validation did only consist of specimens contained from Illinois, so further validation would be needed to verify assay sensitivity across the entire range of these species and against other closely related taxa beyond the three species in this study. Additionally, we developed optimal PCR conditions to maximize sensitivity of target DNA. Due to our absence of detections from field samples, our assays were unable to meet higher levels of the validation scale. However, we were able to partially meet criteria from levels four and five of the validation scale. We determined LOD and LOQ for all three assays through serial dilutions of target DNA. Additionally we performed extensive in silico testing on both co-occurring and non-co-occurring Parasitiformes species, increasing confidence that our assays are highly specific to our target species. We also performed limited in vitro testing on two co-occurring nontarget species with Ixodidae which provided further proof of specificity.
In order to meet the criteria for higher levels of validation for these assays, positive detections from environmental samples will be necessary (Thalinger et al., 2021). This could be accomplished, first, in an experimental setting first where individual ticks are placed in a container with grass for several hours and then the grass can be sampled for the presence of eDNA. Once positive detections are determined in an experimental setting, these assays can then be deployed in the field again. Once positive detections are obtained, the next goal should be to pursue optimal sampling conditions, and perhaps proceed to using an occupancy modeling framework (MacKenzie et al., 2002) to calculate detection probabilities in different conditions in local habitats. We invite others to continue validation of these assays using different sampling methods than we applied in this study.
5 CONCLUSIONS
Tick-borne diseases are among the most concerning emergent threats to human health (Herrmann et al., 2014; Paddock et al., 2016). As the climate continues to warm, ticks will continue to extend their ranges to higher latitudes be active throughout more of the year, increasing the prevalence of diseases that were previously considered afterthoughts in the medical community (Greer et al., 2008; Süss et al., 2008). Monitoring of future range expansions is vital to provide warnings to both medical providers and the public. Environmental DNA analysis has been shown to be successful in monitoring terrestrial species, often with increased sensitivity and resolution compared to conventional methods (Allen et al., 2021; Valentin et al., 2020). Here we provide qPCR assays for detection of eDNA from three pathogen-carrying ticks in North America. Our assays showed sufficient sensitivity in the laboratory, but were unable to successfully detect ticks in the field, despite their presence in conventional sampling. We believe that this stems from methodological constraints coupled with, perhaps, low eDNA deposition rates by target taxa. If appropriate capture methods can be found and paired with our qPCR assays, eDNA methods could improve early detection monitoring of ticks and provide earlier warnings to areas expected to see increased disease prevalence.
AUTHOR CONTRIBUTIONS
HK, PG, RS, and MD have made major contributions to the conception and design of the study. NI, HK, and SM have made major contributions to the acquisition, analysis, and interpretation of the data. NI, HK, and SM have made major contributions to the writing of the manuscript. PG, RS, and MD have made comments and edited the manuscript.
ACKNOWLEDGMENTS
The authors would like to acknowledge Alex Doran, Caryll Chiu Lee, Johnathan Tinoco, and Elly Uruchima for their assistance in the collection of ticks and eDNA samples.
FUNDING INFORMATION
Department of Defense, Grant number: TB180052.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest.
Open Research
DATA AVAILABILITY STATEMENT
The qPCR results used to generate limit of detection and limit of quantification values, and all qPCR results from field samples are openly available in Dryad at doi: 10.5061/dryad.q2bvq83s3.




