High-volume plankton tow net sampling improves eDNA detection of invasive zebra mussels (Dreissena polymorpha) in recently infested lakes
This draft manuscript is distributed solely for purposes of scientific peer review. Its content is deliberative and pre-decisional, so it must not be disclosed or released by reviewers.
Abstract
Aquatic invasive species are a serious and growing threat to biodiversity. Zebra and quagga mussels (Dreissena polymorpha and D. rostriformis bugensis) are freshwater invaders causing substantial ecological and economic damage across Europe and North America. Early detection of invasive mussels and other non-indigenous species is increasingly needed to prevent their establishment and spread. Environmental DNA (eDNA) techniques potentially offer higher sensitivity monitoring tools to complement more conventional methods for surveying adult and juvenile mussels and veliger larvae. eDNA assays are typically performed on small-volume (0.5–5 L) water samples that are concentrated by filtration prior to extraction and downstream processing. Sampling using a towed plankton net of a larger (64 μm) pore size can process orders of magnitude larger water volumes with the potential for increasing eDNA detection sensitivity. We compared the sensitivities of high-volume plankton tow net water sampling to filter sampling in three recently infested lakes in Canada and Minnesota, USA. Paired filtration and tow net samples were analyzed for Dreissena DNA using an established quantitative polymerase chain reaction assay for the genus. Higher yields of Dreissena eDNA (more DNA copies) were recovered from plankton tow than from filtered samples in all 33 paired comparisons. In some cases, plankton tow samples were positive for Dreissena eDNA while lower-volume filtering produced a false negative detection. Our results demonstrate the effectiveness of plankton tow net sampling for eDNA early detection of invasive mussels, a method that can be used exclusively or as a supplement to filter sampling. Our results further suggest that eDNA testing could be incorporated into monitoring programs that routinely use plankton tows for visual detection of invasive mollusk larvae, as well as other aquatic invasive and non-invasive species ranging from plankton to metazoans, including many fish.
1 INTRODUCTION
Invasive species are among the top threats to global biodiversity (Linders et al., 2019; Sepulveda, Amberg, & Hanson, 2019; Tilman et al., 2017; Van Rees et al., 2022). Non-indigenous species invasions are escalating with increased human activity and climate warming, and they cost billions of dollars in environmental damage worldwide, every year (Bax et al., 2003; Gervais et al., 2020; Mack & D'Antonio, 1998; Pimentel et al., 2001). Early detection rapid response (EDRR) is commonly recommended as an effective strategy for invasive species control; the theory being that concentrating efforts in the early stages of an invasion will lead to more successful population suppression than after an area has been fully colonized (Davidson et al., 2015; Zanden et al., 2010). EDRR is often an economically sound approach considering the high costs associated with eradication or management of established invasive species populations (Keller et al., 2008). With the growing threat of aquatic invasive species (AIS) invasions, an EDRR approach offers the benefits of increasing our probability of early detection and improves opportunities to effectively limit the spread of damaging species (Sepulveda, Schmidt, et al., 2019).
Among the highest-impact invasive species in North America are zebra mussels (Dreissena polymorpha) and quagga mussels (D. rostriformis bugensis), members of the bivalve family Dreissenidae. Firmly established in the Great Lakes and Mississippi Basins and spreading, Dreissenids are sweeping across North America toward other regions including the Columbia River Basin in the Pacific Northwest with recent detections in the nearby upper Missouri River drainage divide (e.g., Lake Mitchell and Lake Kampeska: South Dakota Game, Fish and Parks, 2022). The Columbia remains the largest dreissenid-free watershed in the contiguous United States, and if invaded, economic costs could reach billions annually (Strong, 2000; WRP-ANS, 2010). Economic impacts on hydroelectric facilities and infrastructure in Montana alone are projected to be $47 million USD per year (Nelson, 2019; Sepulveda et al., 2020). As ecosystem engineers, the effects of dreissenids are often far-reaching and include changes in ecosystem sediment composition, “benthification” of aquatic systems and changes in nutrient cycling, biomass reduction and altered composition of planktonic communities, and increases in the abundance of benthophagous fishes (Elgin et al., 2022; Higgins & Zanden, 2010; Hosler, 2011; Karatayev et al., 2007; Wong & Gerstenberger, 2011). While the consequences of a dreissenid invasion in the Columbia River headwaters and other pristine areas could be enormous, an invasion is not inevitable if EDRR is properly implemented (Sepulveda, Amberg, & Hanson, 2019). To make suppression and eradication feasible and to protect remaining untouched watersheds, we must target invasive mussels with a cost-effective and sensitive approach and consider new methods as they become available for EDRR (Van Rees et al., 2022; Wimbush et al., 2009).
Environmental DNA (eDNA) monitoring collects and detects the DNA shed by an organism into the environment by testing samples of water, soil, or air (Deiner et al., 2021; Taberlet et al., 2012). As the technology evolves, studies have evaluated and improved all aspects of the eDNA monitoring pipeline between sampling, extraction, analysis, and bioinformatic methodologies (e.g., Bedwell & Goldberg, 2020; Djurhuus et al., 2017 and Pansu et al., 2021). eDNA is a highly sensitive tool for the early detection of AIS (Darling & Mahon, 2011; Hunter, Meigs-Friend, et al., 2019; Mahon et al., 2013) whose power could be magnified by expanding sample volume (Schabacker et al., 2020; Sepulveda, Amberg, & Hanson, 2019; Sepulveda, Schabacker, et al., 2019; Sepulveda, Schmidt, et al., 2019). Research indicates that for traditional filtration protocols, increased sample water volume leads to greater likelihood of eDNA capture and detection (Bedwell & Goldberg, 2020; Capo et al., 2019; Hunter, Ferrante, et al., 2019; Wilcox et al., 2018; Wittwer et al., 2018). Studies have surpassed the throughput of the traditional ≤1 L sample volume, analyzing 5–100 L samples by employing larger pore size filters up to ~1.5 μm (Goldberg et al., 2011; Pont et al., 2018; Valentini et al., 2016; Wittwer et al., 2018). However, the amount of water that can be filtered, even using larger filtration devices, ultimately plateaus when filters clog. These volumes may be sufficient for smaller aquatic habitats, but for systematic surveys of large waterbodies (where DNA may be patchily distributed or less concentrated in its environment), more water likely must be processed. Increasing the volume of water processed per sample (e.g., >1000 L processed per sample) could be cost-effective by increasing the surface area of a water body examined without dramatically increasing the number of samples collected, as required with current techniques.
Two recent studies (Schabacker et al., 2020; Sepulveda, Schabacker, et al., 2019) increased eDNA sample volumes dramatically (e.g., 1000–7000 L of water processed per sample) by using a larger (64 μm) pore-size tow net to sample larger volumes and increase detection sensitivity (sensitivity meaning the likelihood of true positive detection of target DNA as defined in Furlan et al., 2016). DNA captured from tow nets likely includes organismal DNA (e.g., larvae) and extra-organismal DNA (see Rodriguez-Ezpeleta et al., 2021) which we recognize departs from the traditional definition of eDNA, being DNA sampled in the environment without capturing the source organism (Taberlet et al., 2012). Nonetheless, enacting EDRR carried out through high-volume eDNA approaches such as these to achieve earlier detection and systematic sampling has the potential to transform the current detection strategy for one of the world's most prolific invaders.
We investigated if sensitivity of detection of eDNA can be increased by larger volume samples. We compared conventional field sampling methods (low-volume water filtration) to high-volume plankton tow sampling. A paired sampling strategy was implemented at three recently infested lakes to compare the sensitivity of both methods for detecting zebra mussel and quagga mussel DNA using a quantitative polymerase chain reaction (qPCR) assay for both zebra and quagga mussels.
2 METHODS
2.1 Field sampling
We collected 108 samples (81 eDNA samples and 27 field blanks passing only dH2O through the net) from three separate lakes: Lake Winnipeg, North Star Lake, and Lake Bemidji (Figure 1; Table S1).
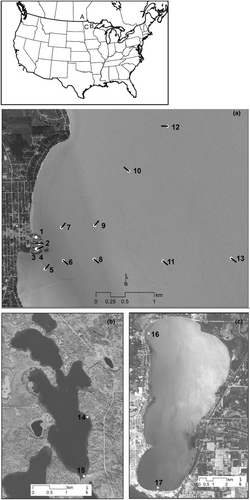
2.1.1 Lake Winnipeg
On August 13 and 14, 2018, 13 geographic locations were sampled around Gimli Harbor on Lake Winnipeg in Manitoba, Canada (Figure 1a). Sampling locations were chosen at five distances (0 m, 500 m, 1 km, 2 km, and 3 km) from Gimli Harbor, a known point of infestation of zebra mussels. One plankton tow sample and two to three replicates of filter samples were taken at each site along with field blanks of each sample type.
We used a 64 μm pore size plankton tow net that was 30 cm by 90 cm, 30 cm in diameter and had a dolphin bucket attachment (~7000 L per tow, Schabacker et al., 2020). All items (net, cod end, funnel, etc.) that would have met raw site water were sterilized in a 50% bleach solution for a minimum of 20 min with agitation followed by soaking and a thorough rinse with deionized water sprayed from a sterile garden weed sprayer. The plankton tow net assembly was finally triple-rinsed onsite in lake water prior to the 100-m drift sampling to remove potential residual bleach. Deionized water poured through the plankton tow net served as the negative control and was taken before each tow. The negative control was omitted for the plankton tow at Site 4 due to human error. Samples were stored in a minimum of 80% ethanol depending on the amount of bulk material present in the tow. Cells, gametes, and small veligers are smaller than the tow net pores and thus are likely attached to larger particles when captured (unless net pores become partially clogged and smaller than 64 um). Samples were stored on ice during collection and transport to the lab.
Water for filter samples was taken with sterilized 1.5 L Nalgene bottles at the initial point of the plankton tow drift. Bottles were decontaminated for 20 min with a 50% bleach solution followed by a thorough rinse with deionized water. At the sampling location sample bottles were triple-rinsed with site water to remove any residual bleach. A field blank was collected at each site using deionized laboratory water in one of the sterilized Nalgene bottles.
Water samples were stored on ice until processing. Filtration took place outdoors on land on the day of sampling by passing the samples through a 47-mm-diameter 1.2 μm glass microfiber filter disc (GF/C; Cytivia) using 250-mL single-use analytical filter funnels (Thermo Scientific) and peristaltic pump. Gloves were changed frequently and between every water sample. Each bottled sample was inverted before and during filtration to re-suspend any settled material. Between 500 and 1500 mL was filtered for each replicate depending on how much sample was able to be processed before each filter became clogged. Filters were stored in individual 15 mL conical tubes containing 95% ethanol. Filtered eDNA samples were placed on ice and kept in the dark until they were overnight shipped to University of Montana Conservation Genomics Group laboratory (Missoula, MT, USA) on ice packs where they were frozen at −20°C. Samples were collected and transported under Manitoba Aquatic Invasive Species Permit, AISP26-18, and Manitoba Scientific Collection Permit, SCP54-18.
The first zebra mussel in Lake Winnipeg was reported in October 2013 by a member of the public after it was found attached to the hull of a boat. The detection triggered an EDRR effort which resulted in the detection of additional mussels at four harbors on the lake. In May and June 2014, the four harbors were treated with potash to eradicate the invasive mussel. However, in late July, mussels were discovered outside of the treated harbors by a research vessel. Since the initial discovery of zebra mussel in Lake Winnipeg, the invasive mussel has been detected in several systems in the Lake Winnipeg basin including the third largest lake within Manitoba in 2021.
2.1.2 North star Lake
On July 31, 2018, we collected 20 total eDNA samples and two field blanks at the Big Timber Resort boat dock and the public boat dock on North Star Lake in Minnesota (Figure 1b). At each location, five replicates of plankton tow and filter samples were collected, plus one blank tow net sample per site. One-liter filter samples were taken by collecting 333 mL on each side and end of a dock just below the surface. Tow nets were pulled for 50 meters just below the surface 1 m away from the dock using the protocol described above.
Filter samples were collected following the Lake Winnipeg protocol except samples were collected in 3.8-L sterile (distilled water) milk-jug-style container. Each filter water sample was immediately processed on land by passing each sample through a 1.5 μm glass microfiber filter disc using a peristaltic pump. Filters were immediately stored in 50 mL conical tubes fully submerged in 20 mL of 95% ethanol. Samples were placed on ice blocks and kept in the dark until they reached the laboratory.
Zebra mussels were first detected in North Star Lake and confirmed by SCUBA divers in the summer of 2017 (Figure S1). Mussels were seen at multiple locations on the east-central shore near the Big Timber Resort area (our sample site 14), and at two locations in the far northwest corner of the lake. No individual mussels were detected by the survey in the southern part of the lake near the public boat dock (site 15) that we sampled in the summer of 2018.
2.1.3 Lake Bemidji
Twenty eDNA samples were collected from two public boat docks in Lake Bemidji on August 23, 2019. Five replicates each using the water filter and 50-meter tow sampling methods described above were collected at site 16 (North Woods public boat dock) and five replicates of both field methodologies were collected from site 17 (Double Tree Hotel boat dock). The time duration of each tow net pull through the water was similar between sites (5–7 min and 1–2 miles per hour), although the tow rate does not influence the total volume of water sampled. Water filtration samples were taken by filling a sterilized 250 mL single-use disposable water filtration cup four times with surface water to achieve 1 L per sample. 250 mL collections took place along the plankton tow transect around the dock. Filtration took place immediately on land using a 1.45 μm filter and protocol identical to North Star Lake (above).
The first zebra mussel on Lake Bemidji was detected in the gut of a yellow perch in July 2018 during a fishing class. On August 19, 2018, a plankton tow found one zebra mussel veliger larva near the north end of Lake Bemidji, but an August 28 follow-up dive also found no conclusive zebra mussels. The infestation was confirmed when mussels were found in October 2018 at the Northwoods public boat launch and also the easternmost Nymore Beach public access (boat launch).
2.2 Environmental DNA isolation
DNA isolation of both plankton tow and filter samples occurred in a facility dedicated to environmental DNA handling separate from any PCR product or tissue-based DNA extractions. Nucleic acid yield was obtained following the bead-based high-volume extraction protocol described in Sepulveda, Schabacker, et al. (2019) for both sample types. Half of the filter paper was extracted for each filter sample, and 20–25 mL of each plankton tow sample was used per extraction. The remaining sample was stored for re-analysis. The two 50 mL plankton tow samples taken at each site in Lake Winnipeg were pooled together to be extracted as one homogenized sample. Plankton tow samples were homogenized using a vortexer before being aliquoted. 200 μL of DNA suspension buffer (Teknova) was used for all sample types in the final elution. A negative extraction control which consisted of 80% EtOH and 20% high-performance liquid chromatography (HPLC) water was included with each extraction and treated in the same manner as the eDNA samples to measure for contamination during the DNA isolation process. Filter tips were used throughout the extraction process to prevent contamination between samples through aerosolized DNA. Laboratory benches were cleaned using a 20% bleach solution of concentrated 8.25% sodium hypochlorite. Total nucleic acid yield and purity, including 260/280 and 260/230 ratios, were quantified using a NanoDrop™ 2000 spectrophotometer (Thermo Fisher Scientific).
2.3 Quantitative PCR and PCR inhibition
Environmental DNA was amplified using a quantitative PCR (qPCR) protocol. We used the 16S rRNA qPCR assay and cycling conditions from Gingera et al. (2017) with optimized forward primer, reverse primer, and probe concentrations of 900, 500, and 250 nM, respectively. This 16S rRNA marker is specific to the Dreissena genus, amplifying the DNA from both zebra and quagga mussels (Gingera et al., 2017), although only zebra mussels are suspected to be present at our study sites. The assay was subjected to specificity testing with known and potential sympatric species including DNA of several Gastropoda (D. polymorpha, D. rostriformis bugensis, Ferrissia species, Fluminicola fuscus, Potamopyrgus antipodarum, Physella acuta, Physella gyrina, Stagnicola caperata, and Stagnicola elodes) at two concentrations (0.1 and 0.01 ng/uL) to ensure no off-target amplification would occur in environmental sample analysis. None of the off-target species produced a positive detection at any dilution with the Gingera 16S genus-specific assay used in the study.
The assay was duplexed with the DNA sequence from a unique mouse gene, HemT (Xue et al., 1999) which served as a custom exogenous internal positive control (IPC) to test for PCR inhibition. 1000 copies of the HemT gBlock® Gene Fragment (Integrated DNA Technologies) was added to every sample to detect inhibition. We measured the shift in Ct between unknown samples and control samples (IPC DNA spiked into Tris buffer) known to have no inhibition. To calculate ΔCt values and estimate relative inhibition, we subtracted the average Ct value observed for IPC controls from the average Ct value among replicates of our unknown samples. If the ΔCt was greater than 3.5 cycles, then the sample was considered inhibited. These inhibited samples were rerun at a dilution factor of 1:2, 1:3, or 1:10 dependent on Ct delay observed at full concentration.
Three qPCR technical replicates were run per sample. Each qPCR replicate had a total reaction volume of 20 μL containing 4 μL of DNA template, 6 μL of assay mix, and 10 μL of Inhibitor Resistant Genotyping PCR ReadyMix™ (Sigma Aldrich®). Cycling conditions were optimized for use with the QuantStudio™ 3 Real-Time PCR System (Applied Biosystems) and performed with a hot-start denaturation of 30s at 95°C followed by 50 cycles of denaturation for 5 s at 95°C, annealing for 15 s at 60°C, and extension for 10s at 72°C per the Master Mix manufacturers recommendations for 3-step cycling (Sigma Aldrich®). A 134-bp gBlock® Gene Fragment (Integrated DNA Technologies) published in Gingera et al. (2017) was used to quantify DNA copy number in each sample. The standard curve was created using a 10-fold serial dilution of 10 million down to 10 copies of the amplicon per μL. All plankton tow samples were run at a 1:3 dilution, due to the high overall concentration of nucleic acid in the samples and then back-calculated by the dilution factor to obtain copy number at full concentration. Six negative control replicates were included per qPCR plate where DNA suspension buffer was used in place of dreissenid template.
We determined the limit of detection (LOD) for the 16S assay using the standard curves (1 million to ten copies serial dilution) of synthetic target DNA included in the plates analyzed for this study. Per Klymus et al., 2020, we defined the LOD as the lowest copy number standard that amplified 95% of the time. Similarly, to assess the assay's ability to precisely quantify copy numbers, we calculated the limit of quantification (LOQ) for the g16S assay using the same series of analyte dilutions. For our purpose, we defined the LOQ as the lowest standard concentration that could be quantified with a CV below 35% (Klymus et al., 2020).
2.4 Sanger sequencing
To confirm positive qPCR results, PCR product from the Lake Winnipeg samples was Sanger sequenced using the assay's reverse primer by the Molecular Research Core Facility of Idaho State University. A small subset was also sequenced in the forward direction to determine if this improved sequence identification. Sequence Scanner Software 2 (Thermo Fisher Scientific) was used to read traces, and then each sequence was subject to an NCBI nucleotide-nucleotide Basic Local Alignment Search Tool (BLAST) search to confirm the target taxa.
3 RESULTS
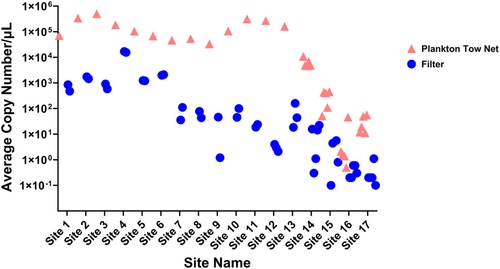
We estimated the LOD and LOQ for the g16s assay are both 10 copies and use LOD/LOQ as a threshold for dreissenid mussel detection. Tow nets detected more dreissenid DNA at all 17 sites with paired samples (13 sites from Lake Winnipeg, 2 from North Star, and 2 from Bemidji). Of the 30 total plankton tow samples across all sites, only one sample detected under 10 copies per μL in an average of 3 PCR replicates per sample. Of the 44 total filter samples across all sites, 26 samples (59.1%) detected less than 10 copies per μL of the target amplicon.
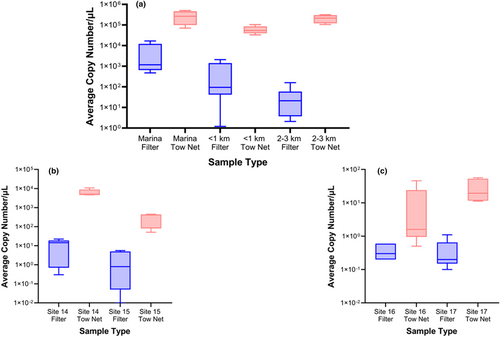
Lake Winnipeg tow net samples had significantly more dreissenid mussel qPCR product (DNA copies) than the filter samples (Figures 2 and 3). For example, at site 13 in Lake Winnipeg, the tow net yielded 160,459 DNA copies/μL from one field replicate, whereas three 1-L filter replicates detected only 18, 159, and 44 DNA molecules, respectively (Table S1). At site 12, all three filter samples were negative for dreissenid DNA based on our 10-copy threshold (4, 3, and 2 copies per μL detected, respectively), whereas the one single plankton tow replicate was positive for five orders of magnitude more copies (270,893; Table S1). A total of 13 plankton tow samples across all Lake Winnipeg sites accumulated an average of 173,652 copies of target DNA per μL (SD = 137,965). The 28 total filter samples detected an average of 1630 copies of target amplicon per μL (SD = 4091). Target amplicon copy number per μL detected in tow net samples ranged from 33,215 to 503,398 among Lake Winnipeg sites. Copy number per μL (PCR) of the target amplified with filtration samples ranged from 1 to 16,771 at Lake Winnipeg sites. None of the field blanks amplified any target DNA by the assay.
From Lake Winnipeg, the total genomic DNA extracted was higher for the large-volume tow samples than filter samples (3047.2 ng/μL (SE = 380.9) versus 27.9 ng/μL (SE = 1.7)) with a mean difference of 3019.3 ng/μl (Table S1). Plankton tow samples had higher 260/280 NanoDrop® absorbance readings than filter samples, on average (plankton tow: mean = 2.12, standard error = 0.06; filter: mean = 1.60, standard error = 0.01). Plankton tow samples also had higher 260/230 readings (mean = 2.21, standard error = 0.04) than filter samples (mean = 0.66, SE = 0.04).
In North Star Lake, Minnesota, the plankton tow nets detected more DNA than filters. Tow nets yielded (mean) 3350 DNA copies per μL (±standard deviation = 3482.2, range = 51–10,809), whereas filter samples yielded 6 copies/μL (±7.6, range = 0–22). The plankton tow method yielded positive eDNA detections (above 10 copies/ μL) in all five samples at both sites. At the Northern site (14) in North Star Lake, the five tow net samples yielded 4839, 6869, 4673, 4862, and 10,809 copies/μL. Filter samples at the same site yielded 16, 0, 1, 14, and 22 copies/μL. At the southern site (15), we detected dreissenid DNA from all five plankton tow samples (449–50 copies per μL), whereas no eDNA was detected in filter samples (≤7 copies for each sample, which is below our threshold of detection, the LOD).
Lake Bemidji also yielded more dreissenid DNA detections in plankton tow samples than in filter samples (Figure 2). Both sites 16 and 17 yielded dreissenid eDNA detections from plankton tows despite Lake Bemidji being lower in eDNA abundance (four replicates all belonging to Site 16 not amplifying ≥10 copies). No copies of the target amplicon were detected from any filter samples, except one qPCR replicate yielded a single copy per μL (17D) which was still below our threshold for positive detection. Twenty copies/μL (±20.9) were found across all 10 tows taken in Lake Bemidji. The average among 10 filter samples at Lake Bemidji was <1.
None of the extraction controls or PCR-negative controls amplified dreissenid DNA. One hundred percent of positive controls on each plate successfully amplified the spiked dreissenid amplicon of 1 million copies per μL in a tenfold dilution to ten copies per μL.
After qPCR analysis, we were able to purify and Sanger sequence the targeted fragment from 24 Winnipeg samples of both water filtration and plankton tow methods. The mean, median, and standard deviation of the contiguous read lengths were 81, 82, and 10, respectively, after the automatic trimming from Sequence Scanner Software 2 (Thermo Fisher Scientific). Trace quality scores were between 29 and 32 for all samples except for one not passing quality control (Figure S2). Restrictions to 100% query coverage and percent identity from the nucleotide BLAST recovered only Dreissena polymorpha from each sample (accession numbers: KY426898.1, KY426897.1, KY091877.1, KP052744.1, MZ400414.1, MT483676.1, EF414466.1, EF414465.1, EF414464.1, DQ280038.1, AF507049.1, AF038997.1, DQ333748.1, DQ333747.1). A 90-base pair consensus sequence of all 24 samples recovered only the same 14 accession numbers, all for the 16 s target.
4 DISCUSSION
Sanger sequence from the PCR product of both filter and plankton tow method field samples confirmed the detection of D. polymorpha using conventional databases of barcoded species. This demonstrates the pipeline's ability to detect species noninvasively and validate that detection with barcoded species from multiple reference sources across the globe. The length of the sequence traces was relatively short but yielded a high-quality 90-base pair consensus confirming D. polymorpha. Future research could benefit from an assay targeting a longer region to increase sequence coverage and potentially differentiate species, especially if more dreissenid species are present.
The large-volume plankton tow method yielded 1–5 orders of magnitude higher copy number of dreissenid mussel DNA than the traditional low-volume filter method in all three lakes studied (Figures 2 and 3). This suggests higher sensitivity and reliability for AIS detection of the tow net sampling method compared to traditional widely used filter sampling. Importantly, 16 filter samples detected no zebra mussel DNA above 1 copy per μL with an additional five filter samples detecting below our threshold of ten copies, whereas tow nets made positive detections in all samples (88% above 10 copies per μL) except for four field replicates (all occurring at a single site where a positive detection was also found), which is a critical difference for early detection of new invaders.
At site 15 (North Star Lake), all five field filter replicates failed to detect invasive mussel DNA above the assays LOD, and thus no management or further surveillance action would likely have followed if only filter samples were collected at this site (e.g., Sepulveda et al., 2020). At the same site, tow net samples yielded multiple dreissenid detections, which could lead to follow-up sampling or surveillance actions to confirm presence and manage spread. Similarly, at sites 16 and 17 (Lake Bemidji), target DNA was not detected using contemporary filtration sampling, but positive detections (above our threshold) were found at both sites using a tow net creating the need for further investigation. These two lakes have low-density dreissenid populations as they are recently established; the failure to detect these mussels (false negatives) using only the filtration method suggests managers and monitoring programs should include tow net sampling methods for detection, particularly in early-stage infestations.
NanoDrop® spectrophotometer readings revealed more total DNA from plankton tow samples than from filter samples, as expected. This is consistent with higher qPCR amplified dreissenid copy numbers from plankton tow samples and suggests that tow net samples recover more DNA (over more space) which might lead to higher effectiveness in detecting rare invasive species with little shed eDNA or eDNA unevenly distributed over space. Plankton tows were more prone to inhibition, with total nucleic acid concentrations up to 5985.9 ng/μL (Winnipeg mean Ct delay = 2.7). The highest delay was 4.8 cycles (site 6) and resulted in no amplification. When diluted 1:3, amplification increased to a mean of 68,123 copies (after dilution correction) and a delayed Ct mean value of 1.52 among the 3 replicate PCRs for site 6. Further dilution of 1:10 cleaned up to Ct value of 0.4 and an average of 74,539 copies. This suggests that researchers should monitor for inhibition (Ct delays) and correct for it when detected via dilution or commercially available sample purification kits. The increased susceptibility of tow net eDNA samples to inhibition compared to filter samples (at least in Lake Winnipeg) highlights the importance of monitoring and correcting for inhibition to maintain eDNA detection reliability and sensitivity.
This study demonstrates the potential cost-effectiveness of using large-volume sampling methods. We collected two or three filter samples to one tow sample in Lake Winnipeg and still retained more detection with the plankton tow. Laboratory processing fees would thus increase when providing multiple filter samples to create equal coverage as opposed to a single tow sample. Further, submitting multiple filter samples for extraction and analysis per plankton tow would still not achieve the sensitivity of one tow net sample (in the waters we sampled); the trade-off being that large-volume samples have increased processing times than low-volume ones.
Logistically, the implementation of plankton tow sampling into eDNA monitoring programs is feasible because hundreds of plankton tows are collected annually for microscopy (e.g., for larvae detection) (Sepulveda, Schmidt, et al., 2019; Trebitz et al., 2019). Replicate tows using much of the same gear and materials can be taken and the sample can be aliquoted for eDNA extraction. Only small modifications to existing sampling schemes will need to be made, such as net/site decontamination and sample preservation. Using eDNA as a non-invasive approach for management has been suggested by multiple authors (Adams et al., 2019; Barnes & Turner, 2016; Sepulveda et al., 2020), and studies are implementing eDNA sampling for assessing population structure, intraspecific genetic variation, and Ne estimation (Baker et al., 2018; Deiner et al., 2021; Parsons et al., 2018; Sigsgaard et al., 2016). However, Goldberg and Parsley (2020) established that the concentration of nuclear DNA in eDNA samples is a limiting factor in genotyping nuclear DNA. Adams et al. (2019) also find that one of the major challenges to applying eDNA to population genetics approaches is capturing enough initial DNA copy numbers to decrease PCR stochasticity and produce reliable datasets. Achieving high-quality data from plankton tows shows the promise of future use of eDNA for community and population genomics because we have successfully obtained the large quantities of DNA that are needed for population density estimates that is not achieved with filter samples.
A robust sampling effort is crucial in addition to appropriate laboratory replicates for determining limit of detection. The added managerial benefit to using large-volume sampling methods for aquatic eDNA surveillance includes the ability to sample waterbodies with increased particulate that may clog filter discs, the opportunity to capture metagenomic community data side-by-side, and the capacity to implement wide-scale systematic sampling schemes representing a whole lake that is less feasible with small-volume point filter sampling. Roussel et al. (2015) advocate for the implementation of proper detection rate estimation in eDNA field methodologies for AIS monitoring as one of the major critiques of eDNA as a surveillance tool. Due to the linear composition of plankton tow net sampling (as opposed to point), transect line sampling schemes can then be integrated into schemes like those demonstrated in Ferguson et al. (2019) for proper estimation of probability of detection.
Additionally, high-volume (40–60 L) autonomous filtration eDNA samplers have recently been incorporated as an eDNA sampling method (Govindarajan et al., 2022; Mirimin et al., 2021; Takahashi et al., 2023). In a side-by-side comparison of low-volume (~2 L) to 40–60 L autonomous filtration, Govindarajan et al., 2022 found increased total eDNA recovered from the higher volume samples as well as increased total reads mapping to target species in high-throughput sequencing of paired samples. Future research should evaluate sensitivity of detection in large-volume autonomous filtration to plankton tow sampling and consider the trade-off between both methods. Field-deployable digital droplet PCR (ddPCR) may also be assessed as this method offers turnaround time and cost–benefits; however, should be tested in-situ as available biomass in an EDRR setting may be a concern (Scriver et al., 2023).
Our tow net samples were taken during July and August. Seasonal tow net surveys (Jones & Montz, 2020) in nearby Lakes Cass, Leech and Mille Lacs (20, 40, and 160 km SE of Bemidji, respectively) show these months to coincide with midsummer peaks in veliger counts (MN DNR, 2023a, 2023b). Future research should repeat similar comparisons in this study outside of spawning season so that the signal from veliger DNA versus signal from DNA released into the environment by mussels (not from veligers) can be investigated. These results would inform the use of proper eDNA protocols to employ outside of spawning season, when it is difficult to use veliger counts for EDRR due to seasonal and site-to-site variation in positive detections when dreissenid populations are small (Evans et al., 2011; McCartney, unpublished).
We recommend the implementation of plankton tow net sampling as an alternative or additional field methodology for the eDNA surveillance of AIS for a wide range of taxa that shed DNA (including free-floating gametes, embryos, larvae, propagules including vegetative fragmentation, or even feces or pseudofeces). Although this study focused on mollusks (Dreissena), similar results have been produced using plants (Myriophyllum; Schabacker et al., 2020) and fish (Salvelinus; Sepulveda, Schabacker, et al., 2019) and echoed across eDNA metabarcoding studies including Fernandez et al. (2021). In summary, large-volume tow net sampling increased the sensitivity of detection up to 100-fold in this study and likely could improve the sensitivity of detection for species in other systems.
AUTHOR CONTRIBUTIONS
Conception or design of the study: DM, SA, RB, GL. Acquisition, analysis, or interpretation of the data: DM, SA, LH, RB, MM, GL. Writing of the manuscript: DM, SA, RB, MM, GL.
ACKNOWLEDGMENTS
We thank an anonymous donor who contributed to G.L. and the Flathead Lake Biological Station to help fund this study. Special thanks to Justin Shead (Fisheries and Oceans Canada) and Darcy Pisiak (Manitoba Natural Resources and Northern Development) for assisting in collecting field samples and to Zoe Dalquist for assisting with data. We are also grateful to Rich Rezanka (Minnesota Department of Natural Resources) and Bruce Anspach (Beltrami County, Minnesota) for kindly sharing information on their survey work on North Star Lake and Lake Bemidji, respectively. We disclose that the authors have no conflict of interest including any interests or relationships, financial or otherwise, that might be perceived as influencing our objectivity.
CONFLICT OF INTEREST STATEMENT
The authors have no conflict of interest to declare.
Open Research
DATA AVAILABILITY STATEMENT
We plan to archive the underlying data of this study in the following public GitHub repository: https://github.com/InvasiveH8ter/Miller_et_al_2023




