Flow cytometric CD34+ stem cell enumeration: Lessons from nine years' external quality assessment within the Benelux countries
Abstract
Background:
A biannual external quality assurance (EQA) scheme for flow cytometric CD34+ haematopoietic stem cell enumeration has been operational in the Benelux countries since 1996. In an evaluation of the results of 16 send-outs, we studied the effects of the methods used on assay outcome and whether or not this exercise was effective in reducing between-laboratory variation.
Methods:
Data were analyzed using robust multivariate regression. This approach is relatively insensitive to outliers and is used to assess the effect of methodological aspects of CD34+ cell counting on the bias and variability.
Results:
Five variables were associated with significant bias of absolute CD34+ cell counts: (i) unique laboratory number (ULN), (ii) gating strategy; (iii) CD34 mAb fluorochrome; (iv) type of flow cytometer, and (v) method of sample preparation. In addition, ULN and platform methodology (i.e., single vs. dual) contributed significantly to the variability of this assay. Overall, the variability in results of CD34+ cell enumeration has declined with time; in particular, after a practical workshop in which participants were trained to use the “single platform ISHAGE protocol.”
Conclusions:
Between-laboratory variation in CD34+ cell enumeration can be reduced by standardization of methodologies between centres. Our approach, i.e., EQA with targeted training and feedback in response to reported results, has been successful in reducing the variability of CD34+ cell enumeration between participants. © 2007 Clinical Cytometry Society
Haematopoietic stem cell (HSC) transplantation has been successfully used to reconstitute hematopoiesis after myeloablative therapies in man since the late 1950s (1, 2). HSCs have the capability of homing into the marrow microenvironment and regenerating multi-lineage haematopoiesis in relatively short time (3). Nowadays, many clinical studies have established that transplantation of CD34+ stem cells is a potentially curative therapy for many patients with malignant and nonmalignant haematological diseases (4-6). Initially, autologous and allogeneic HSC transplants have been performed utilizing bone marrow HSCs (7). In the last decade, increasing numbers of transplantations have been carried out using HSCs from mobilized peripheral blood (PB) or umbilical cord blood. Haematopoietic recovery is dependent on the number as well as the colony-forming capacity of the transplanted HSCs. This emphasizes the need for an adequate prediction of engraftment potency of HSCs in patients after receiving myeloablative therapy followed by HSC transplantation (8-11). Furthermore, enumeration of CD34+ cells has been shown to be useful in considerations of peripheral blood stem cell harvesting (12-16) and in comparing the efficacy of various PB stem cell mobilization strategies (reviewed by Fruehauf and Kessinger and Sharp) (17, 18). Furthermore, CD34+ cell enumeration is useful for monitoring the stem cell yield during graft manipulations, such as immunomagnetic selections, ex vivo expansion, or genetic modifications of HSCs. The advent and use of a CD34 monoclonal antibody enabled the detection of these cells by flow cytometry (19). Several flow cytometric studies have shown a direct correlation between the CD34+ cell content of PB in patients mobilized with chemotherapy (with or without growth factors such as granulocyte colony stimulating factor [G-CSF]) and the number of CD34+ cells collected by apheresis. Additionally, the number of transplanted HPCs as defined by flow cytometry was shown to correlate better with engraftment post SCT than that assessed using colony-forming unit culture assays (20). Initial enumeration methods for CD34+ cells (19-24) have been improved by distinguishing viable from nonviable cells (25, 26). To ensure that CD34+ cell counts are widely valid for clinical decision making, these must be accurate and precise. Therefore, various national and international guidelines for CD34+ cell enumeration have been formulated (24, 26-28). Nevertheless, in various multicentre studies (29-40) large variations between results of individual centers were observed. The outcome of CD34+ cell enumeration assays depends on several variables, such as sample source, sample processing, technical experience, and data analysis techniques. In the Benelux countries, an external quality assessment (EQA) scheme for CD34+ cell enumeration has been in place since 1996. Since then, participants have been trained and standard protocols have been provided (37). In this article we review our experience with this EQA scheme during its first 9 years. We addressed the impact of sample (e.g., origin, preparation, stabilization), flow cytometric CD34+ cell enumeration techniques (e.g., instrumentation, reagents, and analytical strategies), and laboratory experience on assay outcomes. In particular, we were interested in the efficacy of our EQA scheme to improve results, i.e., to reduce the variability and/or bias in CD34+ cell counts between laboratories.
MATERIALS AND METHODS
Study Design
This study consisted of 16 send-outs comprising 64 samples (PB, apheresis products, and cord blood), which were distributed to laboratories that had participated in the biannual EQA scheme for flow cytometric CD34+ cell enumeration organized within the Benelux. This scheme was run under the auspices of the Foundation for Immunophenotyping in Hemato-Oncology (SIHON), the Foundation for Quality Control in Medical Laboratories (SKML; all in The Netherlands), and the Belgian Association for Analytical Cytometry (BVAC/ABCA). The samples were obtained from various sources as shown in Table 1. Short-term stabilization was performed with TransFix™ (Cytomark Ltd, Buckingham, UK) or StabilCyte™ (BioErgonomics, St Paul, MN), and performed by WAK Chemie (Bad Soden, Germany) and Streck (Omaha, NE), respectively. Long-term stabilization was performed by UK NEQAS for Leucocyte Immunophenotyping (Sheffield, UK). The samples were divided in 1-mL aliquots and shipped by overnight courier to the participants. Each participant was requested to: (i) perform flow cytometric CD34+ cell enumeration according local protocols and (ii) provide relevant methodological details. On some occasions (i.e., 16 samples in 4 send-outs) the CD34+ cell enumeration was performed in triplicate to obtain intra-institutional reproducibility results. Reports of results and methodologies were to be carried out within 14 days upon receipt of the samples. Data analysis for debriefing of the EQA results was performed by the SKML data center. For each send-out, an overall debriefing report was issued and discussed at biannual participant meetings. In the Spring of 2000, the participants were invited to a workshop in which a new standard protocol (ISHAGE single platform) was introduced (37). Participants who were performing insufficiently were offered a hands-on training at the coordinating laboratory (Erasmus MC-Daniel den Hoed).
| Sample origin | Shelf life | Stabilization method | n |
|---|---|---|---|
| Peripheral blood | n.a. | No stabilization | 18 |
| Short-term | TransFix™ | 3 | |
| StabilCyte™ | 3 | ||
| WAK Chemie | 1 | ||
| Streck | 1 | ||
| Long-term | UK NEQAS | 17 | |
| Apheresis product | n.a. | No stabilization | 13 |
| Short-term | StabilCyte™ | 5 | |
| Cord blood | Short-term | WAK Chemie | 3 |
- n.a., not applicable.
Data Processing and Parameter Classification
For this study, the results of all CD34+ cell enumerations as well as the responses to the questionnaires were taken into account. Where necessary, incorrect data entries were corrected after consultation with the submitting participants. In addition, printed output of data analyses of the two 2004 send-outs were centrally reviewed to check for inconsistencies in the reported usage of gating strategies. Data processing and statistical analyses were performed using the STATA™ software (StataCorp., College Station, TX) as indicated in the text. Each laboratory was assigned a unique number for referral purposes. The absolute CD34+ cell number was assigned as response variable. The following 13 categorical variables were assumed to influence the outcome of CD34+ cell enumeration assays. These are summarized in Table 2 and discussed in detail later.
| Variable | Categories |
|---|---|
| EQA send-out | 1–16a |
| Laboratory expertise | WSN membership |
| EBMT membership | |
| No membership | |
| Workshop 2000 participation | Yes |
| No | |
| Sample source | Peripheral blood |
| Apheresis product | |
| Cord blood | |
| Sample stabilization | No stabilization |
| Short term stabilization | |
| Long term stabilization | |
| Gating strategy | Milan |
| Bender | |
| ISHAGE | |
| SIHON | |
| ProCOUNT™ (BD Biosciences) | |
| Stem-Kit™ (Beckman-Coulter) | |
| Platform methodology | Single |
| Dual | |
| Flow cytometer | FACScan™ |
| FACScalibur™ | |
| FACStar™ | |
| Epics XL™ | |
| Cytoron™ | |
| Haematology analyzer | Technicon-Bayer |
| Sysmex | |
| Beckman-Coulter | |
| Abbott | |
| Other | |
| Beads | TruCOUNT™ (BD Biosciences) |
| FlowCount™ (Beckman-Coulter) | |
| Perfect Count (Cytognos) | |
| Volumetry | |
| Unknown | |
| CD34 monoclonal antibody (mAb) | HPCA-1 [My10] (BD Biosciences) |
| HPCA-2 [8G12] (BD Biosciences) | |
| 581 (Coulter-Immunotech, IQ products, and Sanquin Diagnostics) | |
| Birma-K3 (DAKO) | |
| Labeling CD34 mAb | FITC |
| PE | |
| APC | |
| None | |
| Sample preparation | Lyse and wash |
| Lyse no wash | |
| No lyse no wash | |
| Mononuclear cells |
- a Send-outs were numbered sequentially as a function of time.
EQA send-out
Each send-out, from Spring 1996 to Autumn 2004 was chronologically assigned with a unique number (i.e., 1–16). In this way, we analyzed any effect of the EQA program on the results of the participants as a function of time.
Laboratory expertise
As actual figures of the number of performed CD34 enumerations per laboratory per year were not available, a surrogate parameter for laboratory expertise was used. We classified the laboratories into three mutually exclusive groups based on estimated experience: (i) laboratories that had only participated in the our EQA scheme but had had no daily practice in CD34 enumeration in the setting of a SCT program; (ii) laboratories participating in the Dutch Working Party of Stem Cell Laboratories (WSN; an organization of laboratories that perform the processing of stem cell transplants); and (iii) laboratories that were members of the European Group for Blood and Marrow Transplantation (EBMT), but were not members of the WSN. We reasoned that WSN participants were expected to have outstanding experience in CD34 enumeration because of the regular exchange of information on this issue between these laboratories, whilst EBMT members had only had an active stem cell transplantation program.
Participation at “Workshop 2000.”
This workshop, featuring a hands-on training in CD34+ cell enumeration, was organized for all participants in the Benelux CD34 EQA scheme. In this workshop, the single-platform ISHAGE method for CD34+ cell enumeration was introduced and recommended as the state-of-the-art technique (37). We analyzed the effects of this educational activity on systematic differences and variability in CD34+ cell enumeration.
Sample source
Three different sample sources were distinguished: (i) PB; (ii) apheresis product; and (iii) cord blood. We expected that the sample source would affect the quality and complications of CD34+ cell enumeration. For example, apheresis products may contain relatively high proportions of platelets and dead or dying (CD34+) cells; cord blood suspensions usually contain high proportions of nucleated red blood cells (RBC).
Sample stabilization
We expected that if the samples were stabilized before shipment, the variation between laboratories in preventing sample decay would be reduced. We distinguished three categories: (i) no stabilization, (ii) short-term stabilization, and (iii) long-term stabilization.
Gating strategies
We distinguished six strategies (methods); (i) Milan; (ii) Bender; (iii) ISHAGE; (iv) SIHON; (v) ProCOUNT™; and (vi) Stem-KIT™.
The Milan protocol is a “whole blood, stain-lyse-wash” method (20, 22). The gating procedure is based on selection of leukocytes as denominator set on forward scatter (FSC) and side scatter (SSC), excluding debris and aggregates. Within these leukocytes, a positive fluorescence analysis region is set on cells with SSClowtointermediate in a control sample stained with an isotype-matched control mAb. Within this fluorescence analysis region, the number of events of the CD34 mAb-stained samples are counted and used as numerator in the calculation of %CD34+ cells.
The Bender protocol (21) is the first multicolor analytical strategy in which CD45 fluorescein isothiocyanate (FITC) is included as a leukocyte marker in addition to CD34 phycoerythrin (PE) to eliminate nonleukocytes and debris from the analysis and to generate a stable denominator. CD45 staining is used to establish a more stable and precise denominator by including only nucleated white blood cells in the analysis. CD45+ events are then analyzed in a similar manner to the Milan protocol using an isotype control and CD34 staining versus SSC analysis to enumerate CD34+ cells.
The ISHAGE protocol (24) utilizes the maximum information available of four parameters; forward-light and side-light scatter and the intensity of CD34 and CD45 staining. These four parameters were combined in a sequential Boolean gating strategy that can be used to enumerate HSCs from a variety of sources.
An alternative sequential Boolean gating approach is the SIHON protocol of the Dutch Foundation for Immunophenotyping in Hemato-Oncology (SIHON) (36). Here, the laser dye solution LDS-751 that stains DNA and to a lesser degree RNA, is used to identify nucleated cells and to exclude debris, platelets, and unlysed erythrocytes. The denominator is nucleated cells (i.e., LDS-751bright). To exclude the effects of Fcγ receptor mediated mAb binding by monocytes and granulocytes from subsequent analyses, PE-conjugated antibodies to CD14 and CD66e are used to gate out these cell types. Thereafter, a region is set on the CD34+, SSClow cluster, identified using a class III antibody labeled with FITC, which also is used for the analysis of the isotype control. Any events stained by the isotype are subtracted from the CD34+ test result.
The ProCOUNT™ single-platform kit by BDBiosciences (San Jose, CA) is based on counting beads (TruCOUNT™ tubes). The denominator for nucleated cells is a nucleic acid dye (NAD). A threshold is set on nucleated cells and during software-driven data acquisition, sufficient events are recorded to ensure a 10% precision in absolute CD34+ cell counts. Data analysis is also software-driven. The gating strategy is aimed at identifying CD34+ cells; first by gating on NADpos, SSClowtointermediate events and, second, by gating on CD45negtonormalpositive events. Within these events the CD34pos events are analyzed. A similar analysis is run for the isotype-matched control. The TruCOUNT™ counting beads are gated on their bright fluorescent signals they emitted in the FL1, FL2, and FL3 channels.
Finally, Stem-KIT™ from Beckman Coulter (Miami, FL) consists of a two-color fluorescent CD45 FITC + CD34 PE, and CD45 FITC + isotonic control PE murine mAb reagent, the nucleic acid viability dye 7-amino-actinomycin-D (7-AAD), NH4Cl lysing reagent, and Stem-Count™ fluorospheres (Beckman-Coulter) to directly generate absolute CD34+ cell counts. Analysis was performed in a similar way to the single-platform ISHAGE protocol (25). Automated enumeration is also possible with the Stem-ONE™ software (Beckman Coulter).
Platform methodology
(i) Single and (ii) dual platform methods were distinguished. The use of single platform methodologies are expected to reduce the between-laboratory variation in CD34+ cell enumeration by eliminating the leukocyte differential count from additional haematology analyzer as a source of variation.
Flow cytometric instrumentation
As there were major differences between the various instruments in use, we investigated this parameter as a possible source of variation.
CD34 mAb clone
We distinguished four categories: (i) My10 (HPCA-1), (ii) 8G12 (HPCA-2), (iii) 581, and (iv) Birma-K3. All these mAb except My10 (Class I) were of Class III (41).
CD34 mAb fluorochrome
We assigned two categories: (i) FITC and (ii) PE. We expected that the fluorochrome brightness (i.e., PE > FITC) would affect between-laboratory variation of CD34+ cell enumeration.
Sample preparation
Four categories were distinguished: (i) Lyse and wash (LW); (ii) Lyse no wash (LNW); (iii) No lyse no wash (NLNW); and iv) gradient separation of mononuclear cells (MNC). Variable cell losses, due to different sample preparation techniques, would affect between-laboratory variation.
Statistical Analysis
First, a descriptive analysis of raw data of CD34+ cell enumerations was performed. This analysis revealed that the measurements had a low variability at low cell counts and a high variability at high cell counts, which is often observed with cell enumeration data. As standard statistical techniques require an approximately constant variability over the whole cell count range, the data were logarithmically transformed.
To assess the effect of multiple variables on the log-transformed CD34+ cell enumeration data, robust multivariate regression was used (42). This approach is less sensitive to outliers than standard multivariate regression analysis. We then addressed two aspects of the quality of the log-transformed CD34 measurements: bias (i.e. systematic differences) and variability (i.e. random differences) in separate analyses. Analysis of the mean of the log-transformed data revealed which variables caused systematic differences (bias) in the mean CD34+ cell counts. Subsequently, the bias was removed; the residuals of this analysis were used to investigate the variability of the CD34+ cell counts. To this end, the absolute values of these residuals (termed absolute error, which is related to the standard deviation) was used. A robust multivariate regression analysis of the absolute error of the log-transformed data was then performed to assess which variables affected the variability of CD34+ cell counts. For both multivariate analyses a step-down procedure was followed, i.e., at each iteration of the analysis the least significant variable was removed. This procedure was continued until all nonsignificant variables were removed. Similar analyses were performed on percentages CD34+ cells to check for inconsistencies in procedures and outcomes (data not shown).
RESULTS
Methods Used and Change of Usage Patterns with Time
From 1996 to 2004, 56 laboratories participated to 16 send-outs in our EQA scheme (between 37 and 44 participants per send-out). The central review of printed output regarding the reported gating strategies of the two 2004 send-outs showed no major inconsistencies. One participant used an alternative Boolean gating strategy for the ISHAGE approach, one participant used the ISHAGE approach instead of the reported Bender approach, one participant used the Bender approach instead of the reported Milan approach, and one participant used an additional CD45 mAb in the SIHON approach. Regarding the ISHAGE approach, 95% (19/20) of the participants applied the sequential Boolean gating strategy as described in Ref.24. In general, we could assume that the reported information regarding the gating strategies is consistent during the survey period. Of the 64 distributed test samples, 43 were blood specimens that had either not been stabilized (n = 18), short-term stabilized (n = 8), or long-term stabilized (n = 17). Long-term stabilized samples have mostly been distributed since the Spring of 2002. Eighteen test samples had been derived from apheresis products (13 nonstabilized, 5 short-term stabilized) and the remaining three test samples were short-term stabilized cord blood specimens (Table 1). For analysis of list-mode data, various gating strategies have been used (Fig. 1, panel A). The use of less sophisticated analytical strategies tends to decline with time: (i) the Milan approach (from 21% in 1996 to 5% of participants in 2004), (ii) the Bender approach (11% in 1996 and 11% in 2004), and (iii) the SIHON approach (from 55% in 1996 to 11% in 2004). In contrast, methods based on sequential gating strategies became more widely used with time: (i) the ISHAGE approach (from 13% in 1996 to 55% in 2004), (ii) the Stem-KIT™ assay (from 0% in 1996 to 11% in 2004), and (iii) the ProCOUNT™ assay (from 0% in 1996 to 7% in 2004). As shown in Figure 1, panel B, the use of FITC as fluorochrome for the CD34 mAb by laboratories decreased from 55% in 1996 to 5% in 2004. Consequently, the use of PE as fluorochrome by laboratories increased from 39% in 1996 to 95% in 2004. To establish absolute counts (Fig. 1, panel C), only 5% of the laboratories had adopted the single platform technique in 1996, which had increased to 53% of the laboratories in 2004. As a result of the workshop held in the Spring of 2000, in which 75% (42/56) of the participants were introduced to and trained in the ISHAGE single platform technique (37), the number of laboratories using single platform techniques clearly increased. For sample preparation (Fig. 1, panel D), the use of “LNW” methods increased with time at the expense of those of “LW” methods. With time, all single platform users and a small proportion of dual platform users adopted the “LNW” method preparation. As for laboratory expertise, 45% (25/56) participants had no daily practice in CD34 enumeration, 18% (10/56) participants were members of the WSN and participated in EBMT, and 55% (31/56) of participants participated in EBMT only. The participants operated mainly flow cytometers manufactured by BD Biosciences (FACScan™ [from 68% in 1996 to 11% in 2004], FACSCalibur™ [from 5% in 1996 to 53% in 2004], and FACStar™ [from 13% in 1996 to 5% in 2004]), followed by Beckman-Coulter (Epics XL™ [from 8% in 1996 to 34% in 2004]) and Ortho (CytoronAbsolute™ [from 11% in 1996 to 0% in 2004]). In dual platform techniques, haematology analyzers from Technicon-Bayer (from 21% in 1996 to 5% of participants in 2004), Sysmex (from 21% in 1996 to 16% in 2004), Beckman-Coulter (from 26% in 1996 to 16% in 2004), and Abbott (from 3% in 1996 to 8% in 2004) have been used. In single platform techniques, TruCOUNT™ beads (BD Biosciences [range 3–11% of participants]), FlowCount™ beads (Beckman-Coulter [range 3–18%]), Perfect Count™ (Cytognos [3%]), unknown beads (range 3–20%), and volumetry (range 2–3%) have been used. The majority of the laboratories used clone HPCA-2 (BD Biosciences) as CD34 mAb (range 76–93% of participants), followed by clone 581 (Coulter-Immunotech, (Beckman Coulter, Miami, FL), IQ-products (IQ-products, Groningen, The Netherlands), and Sanquin Diagnostics (Sanquin Diagnostics, Amsterdam, The Netherlands) (range 7–24%).
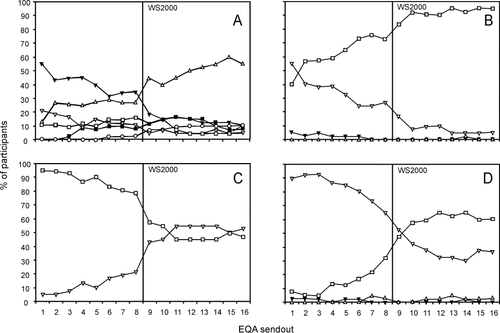
Change of methodology usage patterns over time. Panel A, labeling gating strategies: ∇ = Milan, □= Bender, ▵ = ISHAGE, ▾ = SIHON, ▪ = ProCOUNT™, ○ = Stem-KIT™. Panel B, labeling fluorochrome CD34 mAb: ∇ = FITC, □ = PE, ▵ = APC, ▾ = none. Panel C, labeling platform techniques: ∇ = Single platform, □ = Dual platform. Panel D, labeling sample preparation: ∇ = Lyse and wash, □ = Lyse no wash, ▵ = No lyse no wash, ▾ = MNC. WS2000: the vertical line indicates the timing of the educational workshop during which the single-platform ISHAGE protocol was introduced to the participants of this EQA scheme (see Materials and Methods).
Factors Affecting the Outcome of CD34+ Cell Enumerations
We studied which of the 13 categorical variables (Table 2) significantly influenced the outcome of CD34+ cell count measurements. The five variables having significant effects are shown in Table 3 and are discussed below.
| Variable (overall P) | Category | P-value |
|---|---|---|
| Gating strategy (<0.01) | Milan | <0.01 |
| Bender | n.s. | |
| ISHAGE | n.s. | |
| SIHON | n.s. | |
| ProCOUNT™ | n.s. | |
| Stem-KIT™ | <0.01 | |
| Laboratory (<0.01) | n.a. | |
| Labeling CD34 mAb (0.01) | FITC | <0.01 |
| PE | n.s. | |
| Flow cytometer (0.02) | FACScan™ | n.s. |
| FACScalibur™ | 0.03 | |
| FACStar™ | n.s. | |
| Epics XL™ | n.s. | |
| Cytoron™ | 0.04 | |
| Sample preparation (0.03) | LW | n.s. |
| LNW | 0.05 | |
| NLNW | n.s. | |
| MNC | n.s. |
- Only categorical variables with significant effects are shown.
- n.a., not applicable; n.s., not significant.
Gating strategy (Fig. 2, panel A)
Most observations have been made using the ISHAGE gating strategy. As a result, this strategy was used as benchmark and assigned a factor value of 1. In comparison, two strategies stood out by yielding significantly lower outcomes: the Milan method and the Stem-Kit™ assay. The outcomes of the remaining three strategies, i.e., Bender, SIHON, and ProCOUNT™, were not significantly different from that of ISHAGE.
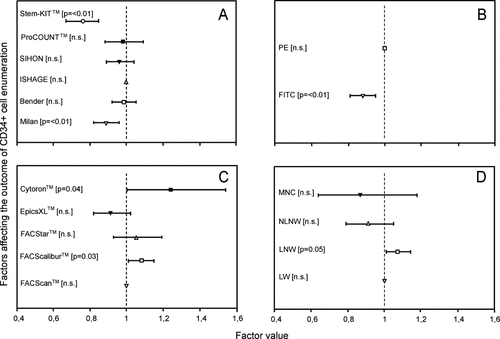
Factors significantly affecting the outcome of CD34+ cell enumeration. Panel A: gating strategies. Panel B: fluorochromes CD34 mAb. Panel C: platform techniques. Panel D: sample preparation. Factor value = 1 for the category with the most observations. The factor value mirrors the relative difference of the other categories related to the category with factor value = 1. The line reflects the 95% confidence interval of the estimation of the factor value. The P-value is shown between brackets.
Laboratory (as defined by ULN; Fig. 3)
Laboratory no. 22 was chosen as benchmark because it had no missing observations. Relative to this laboratory, six other laboratories stood out by systematically higher results (indicated with arrows). A search of possible explanations for these outlying results yielded that laboratory no. 38 had participated only on a few occasions in the EQA program (i.e. 5 send-outs) and hence may have accumulated only limited experience, and that laboratory no. 11 used a nonstandard gating strategy (i.e. SIHON method extended with CD45 gating).
Fluorochrome (Fig. 2, panel B)
PE was most commonly used as fluorochrome. FITC-labeled CD34 mAb yielded significantly lower results than PE-labeled CD34 mAb. A similar pattern was observed when CD34+ cell enumeration results were expressed as proportions of leukocytes, indicating that the effect of fluorochrome was independent of the technique of absolute cell count generation (data not shown).
Flow cytometric instrumentation (Fig. 2, panel C)
Most of the results were obtained using FACScan instruments. In comparison, significantly higher results were obtained using FACScalibur and Cytoron instruments; the latter stood out as a remarkably wide variation. A similar pattern was observed when CD34+ cell enumeration results were expressed as proportions of leukocytes, indicating that the effect of the type of instrument was independent of the technique of absolute cell count generation (data not shown).
Sample preparation (Fig. 2, panel D)
Most of the results were obtained using “LW” methods. In comparison, significantly higher results were obtained using “LNW” methods, whilst the results obtained with “NLNW” methods and those obtained using mononuclear cell isolation were not significantly different.
The effects of the remaining eight categorical variables (Table 2) on the outcomes of CD34+ cell enumerations were not significant.
Factors Affecting the Variability of CD34+ Cell Enumerations
Thereafter, we studied which of the 13 categorical variables significantly influenced the variability of CD34+ cell measurements. As shown in Table 4, two variables had significant effects.
| Variable (overall P) | Category | P-value |
|---|---|---|
| Platform methodology (<0.01) | Single platform | n.s. |
| Dual platform | 0.01 | |
| Laboratory (<0.01) | n.a. |
- Only categorical variables with significant effects are shown.
- n.a., not applicable; n.s., not significant.
Platform methodology
Most of the results were obtained using the single-platform method. Therefore, this category was taken as benchmark. The variability of CD34+ cell enumerations by dual-platform methods were significantly higher than that of single-platform methods (factor value 1.27 [95% confidence interval 1.05–1.55]).
Laboratory (as defined by ULN; Fig. 4)
Again, Laboratory no. 22 was chosen as benchmark because it had no missing observations. In relation to this laboratory, seven other laboratories stood out by a significantly larger variability in their results (indicated with arrows). One of these labs (no. 38) also stood out by the large systematic difference of its CD34+ cell enumeration results from the benchmark laboratory (Fig. 3). It must be noted that only one of these laboratories was an EBMT member, and none of them had participated in WSN. This information suggests that the seven laboratories with high variability of results were relatively inexperienced (see Materials and Methods).
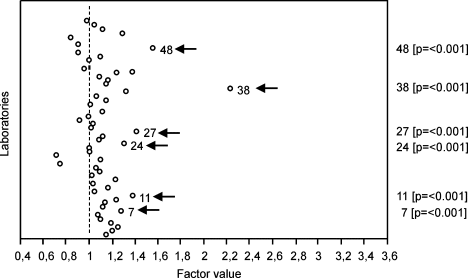
Factors significantly affecting the outcome of CD34+ cell enumeration (continued). Unique laboratory number. Factor value = 1 for laboratory no. 22 which had no missing data (“benchmark”). The factor value mirrors the relative difference of the other categories related to the category with factor value = 1. P-values are shown between brackets for laboratories with a significantly devious outcome than the benchmark laboratory.
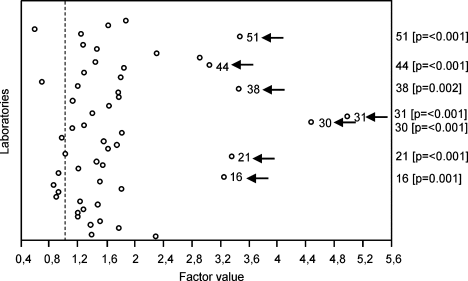
Analysis of the variability of CD34+ cell enumeration. Unique laboratory number. Factor value = 1 for laboratory no. 22 which had no missing data (“benchmark”). The factor value mirrors the relative difference of the other categories related to the category with factor value =1. P-values are shown between brackets for laboratories with significantly higher variability than the benchmark laboratory.
EQA send-out number (Fig. 5)
The first send-out was chosen as benchmark. The variability of results obtained in send-outs 2–7 did, by and large, not significantly differ from that of the first send-out. From send-out 8 onwards, significantly smaller variations were observed as compared to the first send-out except for send-out 12. The standardization workshop (37), in which most laboratories in this EQA scheme had participated, was held between the distribution of send-outs 8 and 9. Seven of the 8 “postworkshop” send-outs had a relatively low variability in CD34+ cell counts (indicated by factor value < 0.8; Fig. 5) versus only 1 of the 8 “preworkshop” send-outs (Fisher's 2-sided P = 0.01). This result suggests that the workshop had been effective in reducing the variability of CD34+ cell enumeration.
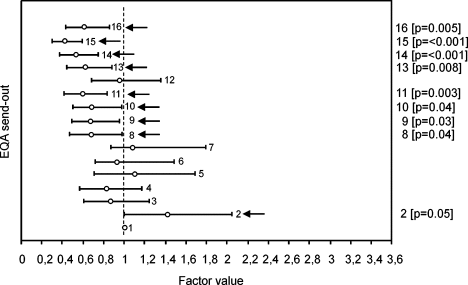
Analysis of the variability of CD34+ cell enumeration by EQA send-out. Factor value = 1 for the first EQA send-out in Spring 1996 (“benchmark”). The factor value mirrors the relative difference of the other send-outs related to the benchmark send-out. The horizontal lines reflect the 95% confidence intervals of the estimation of the factor value. P-values are shown between brackets for send-outs with significantly higher variability than the benchmark send-out.
DISCUSSION
An EQA program for flow cytometric CD34+ HSC enumeration has been operational in the Benelux countries since 1996. The results of nearly 3,000 assays reported by up to 56 laboratories, in the context of methodological information provided in questionnaires that were issued with each send-out, have been analyzed. We were specifically interested in identifying factors affecting the outcome of CD34+ cell enumeration (i.e., bias) and in identifying factors influencing the variability of this assay. Last but not least, we focused on the efficacy of the EQA program to reduce between-laboratory variation of CD34+ cell enumeration as a function of time.
The influence of 13 variables on the outcome of CD34+ cell enumeration was studied. Besides the individual laboratories, four other variables had significant effects: (i) gating strategy; (ii) labeling of CD34 mAb; (iii) flow cytometer type; and (iv) sample preparation. Among the factors influencing the variability of CD34+ cell enumeration, platform methodology (i.e., single vs. dual) had significant impact, whilst significant differences in variability between individual laboratories were also observed. Importantly, the variability of CD34+ cell enumeration also declined significantly with time. The effects of the remaining variables, i.e., laboratory expertise, workshop-2000 participation, sample source, sample stabilization, make of haematology analyzer, beads manufacturer, and clone of CD34 mAb, on bias or variability of the absolute CD34+ cell count were not significant.
Among the gating strategies, the Milan and Stem-KIT™ approaches generated significantly lower CD34+ cell counts in comparison to other approaches. With the Milan approach, the selection of leukocytes as denominator is based on FSC and SSC. Light scatter alone cannot exclude debris, RBC, and platelets, which increase the denominator. Furthermore, only bright CD34+ cells were counted, whilst dim CD34+ cells were not included. These findings may explain why lower absolute counts of CD34+ cells were obtained with the Milan gating strategy as compared to other strategies that also included dim CD34+ cells. Another explanation would be the use of an isotype control mAb in the Milan approach. When calculating absolute CD34+ cell counts, events reactive with the isotype control mAb are often subtracted from the experimental result. The effect of this subtraction is relatively large when the numbers of CD34+ cells are low and those of isotype control mAb-reactive cells relatively high (34). However, we had expected a similar effect for the Bender and SIHON approaches in which isotype control mAb-reactive cells were also subtracted, but this was not the case. We do not have an explanation for the relatively low CD34+ cell counts observed with the Stem-KIT™ gating strategy, which is similar to that of the ISHAGE protocol; therefore, similar CD34+ cell counts would have been expected with both approaches. The four participants using the Stem-KIT™ gating strategy also used Stem-KIT™ reagents but not the dedicated analysis software (Stem-ONE™). Inspection of the data analysis files from these laboratories did not reveal methodological inconsistencies. Furthermore, the four participants did not have another methodological feature (such as type of flow cytometer) in common. In this context it should be noted that the use of the ISHAGE method with Flow-Count beads (i.e., similar to the Stem-Kit protocol) yielded similar outcomes as the dual-platform ISHAGE method.
As for labeling of CD34 mAb, the FITC conjugates yielded lower absolute CD34+ cell counts than the PE conjugates. This difference may be caused by the fact that PE emits stronger fluorescent signals and therefore allows better separation between positive and negative populations than does FITC. This notion is confirmed by our observation that the use of PE-conjugated CD34 mAb not only yielded higher absolute counts of CD34+ cells but also larger proportions of CD34+ cells within the leukocytes in comparison to FITC (data not shown).
With the FACScalibur™ and Cytoron™ flow cytometers, relatively high CD34+ cell counts were obtained in comparison to other instruments (i.e., FACScan™, FACStar™, and Epixs XL™). As a volumetric instrument, the CytoronAbsolute™ is less suitable for enumeration of samples with low CD34+ cell counts as it can only acquire and analyze a limited volume of sample and cells in a single run (43). The wide 95% confidence interval for this instrument is due to the low number of observations. We do not have an explanation for the systematically higher CD34+ cell counts observed with FACScalibur™ instruments as compared to FACScan™ instruments, as their optical systems are highly similar. FACScalibur™ instruments are generally newer than FACScan™ instruments, which may have resulted in a somewhat reduced sensitivity for dim CD34+ populations.
The LNW technique generated slightly higher absolute CD34+ cell counts compared to the LW technique; washing may have resulted in (selective) cell losses. The use of NLNW was relatively infrequent, and therefore associated with a wide 95% confidence interval. A problem with this approach may be underestimation of CD34+ cell counts because of the large proportions of red cells in the unlysed samples. The use of MNC separation was discouraged because of the risk of selective cell loss (22), and therefore generally abandoned after the first send-outs. Therefore, only few observations with this method were made resulting in a wide 95% confidence interval.
It is well known that dual-platform techniques are associated with higher variability than single platform techniques (29, 37, 44). First, single platform technologies bypass the denominator issue, i.e., the percentage CD34+ cells should be reported as a proportion of leukocytes, total nucleated cells, or total events scattering above a FSC threshold. Second, single platform methodologies avoid the variability arising from hematology analyzers used to enumerate total nucleated cells or leukocytes (26). Third, single platform methodologies avoid inaccuracies by rounding up or down low percentages of CD34+ cells in dual platform techniques used for calculations of absolute CD34+ cell counts. Our current analysis of 9 years' experience with EQA for CD34+ cell counting confirms and extends the studies by Barnett et al, 2000; Chang et al, 2004; and Gratama et al, 2003. Furthermore, it shows that the implementation of single platform methodology is highly effective in reducing between-laboratory variability of this assay.
An analysis of bias and variability of CD34+ cell counts as a function of individual laboratories (as defined by ULN) revealed that some laboratories stood out by obtaining relatively high CD34+ cell counts, and others by obtaining a relatively large variation in CD34+ cell counting results. As the participants had not been requested to keep flow cytometric list mode data on file for this purpose, it was not possible to retrace the causes of bias and variability for this study.
Finally, we addressed whether or not the CD34 EQA was effective in reducing the variability of CD34+ cell enumerations over a period of time. Indeed, this analysis revealed that the variability in results of CD34+ cell enumeration in this EQA program had declined with time. As most send-outs with a relatively small variability in CD34+ cell counts fell after the 2000 standardization workshop, it seems reasonable to state that this exercise has been effective in reducing between-laboratory variability.
In conclusion, our 9-year EQA exercise of CD34+ cell enumeration has been highly successful in reducing the variability of CD34+ cell enumeration between participants. A major factor has been that a large percentage of participants have adopted a common approach which was—and still is—considered as the state-of-the-art methodology, i.e., the single-platform ISHAGE protocol. Crucial in this respect was the organization of a practical workshop in which this protocol was introduced to the participants, followed by targeted training and feedback in response to reported results. Whilst the goal of the Benelux EQA program for CD34+ cell enumeration has primarily been educational, results of EQA programs will, in general, increasingly serve as a basis for laboratory accreditation. With these regulatory aspects coming into practice, it is imperative that the EQA programs themselves meet the rigorous quality demands. These demands include documentation of the quality of distributed samples and the use of validated procedures for evaluating results. For this purpose, international collaboration should facilitate the development of uniform and statistically appropriate methods which, at the same time, should allow for comparable results between the different laboratories and the monitoring of a laboratory's poor individual performance, where necessary.
Acknowledgements
Authors gratefully acknowledge Mr. Michael Keeney (London Health Sciences Center, London, ON) and D. Robert Sutherland, PhD, (Princess Margaret Hospital, University Health Network, Toronto, ON) for their advice, and David Barnett, PhD, and colleagues (UK NEQAS for Leukocyte Immunophenotyping, Sheffield, UK) for providing long-term stabilized test specimens. This study was performed under the auspices of the Dutch Foundation for Quality Control of Medical Laboratory Diagnosis (SKML) and the Belgian Association for Analytical Cytometry (BVCA/ABCA) with the participation of (in alphabetical order): H. Aaldenberg (Immuno Quality Products, Groningen); R. Berger (UMC Utrecht, Utrecht); F.A.T.J.M. van den Bergh (Hospital “Medisch Spectrum Twente,” Enschede); M.H. Bernier [Institute Bordet, Brussel (B)]; D. van Bockstaele [UZA, Antwerpen (B)]; X. Bossuyt [Universital Hospital KU Leuven Gasthuisberg, Leuven (B)]; E. Braakman (Erasmus MC, Rotterdam); T. Braeckevelt (Hospital “Zusters van Barmhartigheid,” Ronse); B. Cantinieux [Hospital “St. Pieters,” Brussel (B)]; A. Criel [Hospital “Sint-Jan, Brugge (B)]; S. Darwood [Beckman Coulter, Bedfordshire (UK)]; X. Dicato [Center Hospital, Luxemburg (L)]; H. van Dijk (Meander Medical Center-Lichtenberg, Amersfoort); R.B. Dinkelaar (Hospital “Albert Schweitzer,” Dordrecht); X. Dromelet [University Hospital de Mont Godinne, Yvoir (B)]; A.A.M. Ermens (Hospital “Amphia,” Breda); X. van Erum [Hospital “Henri Serruys,” Oostende (B)]; W. Fibbe (LUMC, Leiden); H.S.P. Garritsen [Institute for Transfusion Medicine, Munster (G)]; J.W. Gratama (Erasmus MC-Daniel den Hoed, Rotterdam); A. ten Haaft (AZM, Maastricht); J.L. d'Hautcourt [Hospital “Warquignies,” Boussu (B)]; H.Y. von Hegedus (Sanquin Blood Bank South East Region, Eindhoven); R.M.J. Hoedemakers (Hospital “Jeroen Bosch,” 's Hertogenbosch); A. Honohan (Sanquin Blood Bank South West Region, Rotterdam); H. Hooijkaas (Erasmus MC, Rotterdam); N. Hougardy [Hospital “Sud Luxembourg,” Arlon (L)]; A.J. van Houte (Hospital “St. Antonius,” Nieuwegein); H. Jongen [Hospital “Virga Jesse,” Hasselt (B)]; S. van Keer [Becton Dickinson Biosciences, Erembodegem (B)]; J.C. Kluin-Nelemans (LUMC, Leiden); P.A. Kuiper-Kramer (Hospital “Isala Klinieken,” Zwolle); R. Malfait [Hospital “Middelheim,” Antwerpen (B)]; A.M. Masson [UCL Ecole de Sante Publique, Brussel (B)]; P. Meeus [Hospital “Onze Lieve Vrouwe Kliniek,” Aalst (B)]; M. de Metz (Hospital “Canisius Wilhelmina,” Nijmegen); E. Moreau [Hospital “Heilig Hart,” Roeselare (B)]; E. Mul (Sanquin CLB Diagnostics, Amsterdam); A.B. Mulder (Hospital “Jeroen Bosch,” 's Hertogenbosch); W.J. Nooijen (Hospital “Antonie van Leeuwenhoek,” Amsterdam); J. Pattinama (Hospital “St. Franciscus Gasthuis,” Rotterdam); J. Phillipé [UZG, Gent (B)]; O. Pradier [Hospital “Erasme,” Brussel (B)]; F.W.M.B. Preijers (UMC-St. Radboud); M. Rijpert-van Son (Hospital “Tweesteden,” Tilburg); A. Roeling (Eijkman-Winkler Institute, UMC Utrecht); C.E. v.d. Schoot (Sanquin CLB Diagnostics, Amsterdam); P. de Schouwer [Hospital “Stuivenberg,” Antwerpen (B)]; K. Sintnicolaas (Sanquin Blood Bank South West Region, Rotterdam); J.W. Smit (AZG, Groningen); C.Th. Smit Sibinga (Sanquin Blood Bank North East Region, Groningen); H.L. Vader (Maxima Medical Center-Veldhoven, Veldhoven); W. Veenendaal (Hospital “Leyenburg,” Den Haag); R. Vet (AMC, Amsterdam); M. de Waele [Universital Hospital VUB, Brussel (B)]; G. Wallef [Hospital “de Jolimont,” Haine-Saint-Paul (B)]; S. Weeks [Hospital “Wilrijk,” Wilrijk (B)]; J.W.J. van Wersch (Hospital “Atrium Heerlen,” Heerlen); G. Westra (VUMC, Amsterdam); R. van Woersel (Hospital “Catharina,” Eindhoven); G. Wouters [Cryo-cell Labs, Mechelen (B)]; B.J.M. Zeegers (UMC “Wilhelmina Kinderziekenhuis” (Utrecht); Hospital “Saint Luc,” Bouge (B).