Real-world evidence for preventive effects of statins on cancer incidence: A trans-Atlantic analysis
Bjoern-O Gohlke and Fabian Zincke as well as Ulrike Stein and Robert Preissner contributed equally to this article.
Dear Editor,
Cancer metastases severely limit therapeutic options: their aggressiveness and treatment resistance1 demand novel therapeutic targets, new/repositioned drugs, or alternative interventions. We previously discovered that lovastatin restricts colorectal cancer (CRC) growth and metastasis via transcriptional inhibition of a tumour-promoting “metastasis-associated in colon cancer 1″ (MACC1) gene.2 MACC1, identified by our group,3 is a known prognostic and predictive biomarker for more than 20 solid tumour entities.4 In our present high-throughput drug screen with HCT116/pMACC1-Luc cells (Figure 1A) we identified novel transcriptional MACC1 suppressors, for example, fluvastatin, as promising inhibitors. Atorvastatin and simvastatin also reduced MACC1 mRNA and protein expression in a concentration-dependent manner (Figure 1B). We confirmed the inhibitory effects of statins in pancreatic and gastric cancer cells (Figure S1).
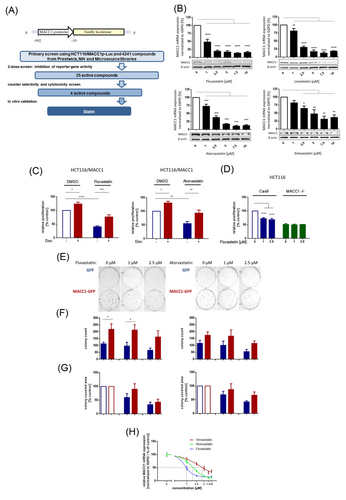
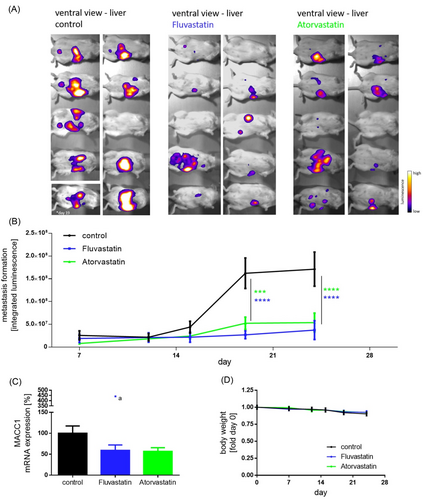
MACC1-dependent proliferation and cell clone forming abilities were inhibited by statins and partly rescued by induced overexpression (Figure 1C–G). Knocking-out MACC1 reduced proliferation (not further reducible by statin treatment). In vivo, we treated CRC xenografted SCID-beige mice with a human equivalent statin dose, which reduced MACC1 expression, tumour burden and metastasis formation (day 24; control vs. statin treatment p < .0001, Figure S2 and Figure 2). Thus, at a molecular/mechanistic level, we provide experimental evidence that statins act, at least partly, by inhibiting transcription of the tumour-promoting and metastasis-inducing MACC1 gene.
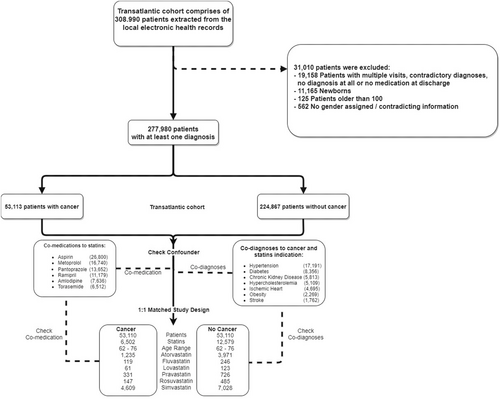
Further, we used real-world evidence (RWE) approach to examine associations between statin use and the prevalence of various cancers among patients with positive diagnoses. We conducted a retrospective, two-centre (University Virginia, VA, USA and Charité Universitätsmedizin Berlin, Germany) observational, cohort-based, nested case-control study, and evaluated all statin-taking patients relative to control groups.
We considered 308 990 patients enrolled between 2008 and 2018. After data pre-processing (Figure 3, Figure S3, and Table S1), 277 980 patients were included. Simvastatin and atorvastatin (71% and 19% of the study populations), pravastatin (5%), fluvastatin (2%), rosuvastatin (2%) and lovastatin (1%) were considered.
Notably, statin intake correlated with a significant reduction in cancer incidence (odds ratio [OR], .72; 95% confidence interval [CI], .70–.74). For statin-taking patients, we calculated a higher cancer survival probability versus subjects not taking any statins (Cox proportional-hazards model; hazards ratio [HR], .64; 95% CI, .48–.86). We found no difference for low-dose (20 mg: HR, 1.1; 95% CI, 0.89–1.37) versus high-dose (80 mg: HR, 1.11; 95% CI, .83–1.48) treatments; similarly, low-dose (10–20 mg: HR, .80; 95% CI, .59–1.09) and high-dose atorvastatin regimes (80 mg: HR, .72; 95% CI, .51–1.02) were comparable. When considering each statin separately, we found a strong cancer-preventive effect for atorvastatin (OR, .41; 95% CI, .38–.43) and significant effects for fluvastatin (OR, .7; 95% CI, .57–.85), pravastatin (OR, .63; 95% CI, .56–.71) and rosuvastatin (OR, .43; 95% CI, .36–.51); simvastatin showed only a weak cancer-preventive effect (OR, .9; 95% CI, .87–.94), and lovastatin effects were not readily assessed (relatively few patients; broad confidence intervals; Table S2).
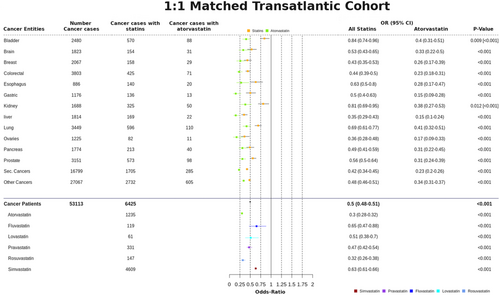
We also considered the clinical data as a 1:1 matched-study design, using propensity score-matched sub-cohorts to better control for confounding associations that might stem from different distributions of age and gender between the whole dataset and the subset of cancer patients. We discovered further evidence supporting the cancer-preventive effect of statins (Figure 4 and Figure S4): (i) all statins considered together had an OR of .5 (95% CI, .48–.51), with (ii) atorvastatin .30 (95% CI, .28–.32), fluvastatin .65 (95% CI, .47–.88), lovastatin .51 (95% CI, .38–.7), pravastatin .47 (95% CI, .42–.54), rosuvastatin .32 (95% CI, .26–.38), and simvastatin .63 (95% CI, .61–.66).
To account for confounders, we examined drugs prescribed alongside statins (Figure S5). Only aspirin and furosemide exhibit ORs < 1; all other co-medications did not show any cancer-preventive effect. After excluding aspirin and furosemide in our 1:1 matched study design, we still found a cancer-preventive effect for all statins (OR of .756, 95% CI .72–.80) and atorvastatin alone (OR of .63; 95% CI, .57–.70) (Table S3). Statin-specific co-diagnoses with cancer were sorted in descending order of appearance/occurrence (Table S4). The overall outcome shows a slight OR increase, but the cancer-preventive effect of statins remains unaffected. We conclude a real (significant) signal indicative of chemopreventive effect for statins, despite any influence of confounders.
RWE revealed a statistically significant link between statin usage and cancer development in patients. Statins exert cancer-preventive effects (up to 50% risk reductions), relating different cancer types and different statins. Superior chemoprevention via statins exists for liver cancer (OR .35, 95% CI .29–.43; confirming the meta-analysis5) and CRC (OR .44, 95% CI .39–.506) or secondary neoplasms (OR .42, 95% CI .30–.45).
Finally, we extended the RWE results by utilizing a clinical research platform (TriNetX) to access a large, international cohort of anonymized electronic health record (EHR) data (aggregate statistics only). Our initial RWE was based on 53 000 cancer patients in our two independent trans-Atlantic cohorts; the results from the TriNetX analysis, reflecting another 132 072 patients, are consistent with these single-site findings of the cancer-preventive effect of statins.
This study extends our previous work2 by corroborating, broadening (via EHR data) and deepening (via MACC1-based experiments) prior knowledge about the cancer-preventive effect of statins.5-10 Many clinically employed statins diminish MACC1 expression in CRC, pancreatic and gastric cancer cells, confirming the cross-entity–wide effects detected by our RWE study. The chemopreventive influence of simvastatin (OR .63, 95% CI .61–.66) and atorvastatin (OR .3, 95% CI .28–.32) correlates with their capacity to reduce MACC1 expression, with IC50 values of 3.1 and 1.6 μM, respectively (Figure 1H).
In conclusion, our study revealed strong evidence for cancer-preventive effects of statins in a large trans-Atlantic cohort, comprised of long-term statin users. We link this beneficial effect to MACC1 transcriptional inhibition, yielding inhibited tumour growth and metastasis formation. These two lines of evidence suggest statin use in treating cancers, for which MACC1 serves as a predictive biomarker and interventional target necessitating prospective, randomized clinical trials.
ACKNOWLEDGEMENT
We thank members of our laboratories for helpful discussions about this work.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
FUNDING INFORMATION
This work was supported by TRR295, KFO339 and DFG PR1562/1-1 (RP). Portions of this work were also supported by the University of Virginia School of Data Science and by NSF CAREER award MCB-1350957.
This work was also supported in part by the German Cancer Consortium (DKTK) and SPARK-BIH Berlin (US).




