Tackling aggression: Translating preclinical insights into clinical relevance
1 AGGRESSION AS A CLINICAL CHALLENGE ACROSS BRAIN DISORDERS
Aggression is a clinically significant and often disruptive behavioural symptom that spans a wide range of brain disorders. Despite distinct pathologies, both psychiatric and neurodegenerative diseases frequently manifest aggression, complicating disease management, threatening safety and worsening long-term outcomes.1
In psychiatric disorders, particularly schizophrenia, aggression is frequently observed during acute psychotic episodes marked by paranoia, hostility, and agitation. In inpatient settings, the prevalence of aggression among individuals with schizophrenia ranges from 10% to over 50%.2 Broader epidemiological studies, such as the Epidemiologic Catchment Area project, have demonstrated that individuals with schizophrenia, bipolar disorder, mania or major depression are approximately five times more likely to engage in violent acts—a figure that increases 16-fold when substance abuse is also present.3
Aggression is not limited to psychiatric disorders. It is also a prevalent and challenging symptom in neurodegenerative conditions such as Alzheimer's disease (AD). Nearly 28% of patients with AD and around 7% of those with mild cognitive impairment exhibit aggressive behaviours, including verbal hostility, physical aggression and resistance to care.4 Aggression is a leading cause of institutionalization, with one study reporting that 34.2% of dementia-related hospitalizations were triggered by aggressive episodes.5 Notably, male patients tend to display higher levels of physical and verbal aggression, alongside other disruptive behaviours such as disinhibition and wandering.6
Taken together, these findings underscore aggression as a transdiagnostic symptom that cuts across disease boundaries. Its prevalence across disparate conditions suggests the involvement of shared neural substrates, warranting further investigation into the underlying circuit and molecular mechanisms. Such insights could pave the way for novel interventions that target aggression regardless of disease category.
2 A CRUCIAL ROLE FOR THE CORTICAL AMYGDALA IN SHAPING AGGRESSION
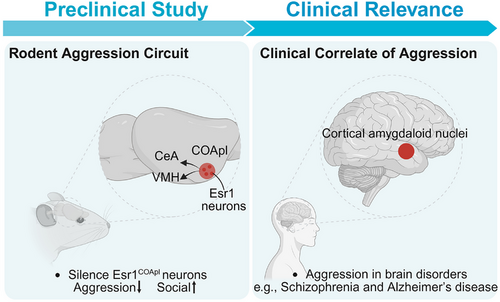
Our recent study, published in Nature,7 uncovers a previously unrecognized neural circuit centered on estrogen receptor 1-expressing (Esr1⁺) neurons within the posterolateral cortical amygdala (COApl) that selectively regulates male aggression. Using an integrative approach—encompassing whole-brain cFos mapping, in vivo calcium imaging, viral tracing and genetic perturbation—we found that Esr1COApl neurons act as a key node encoding the motivational drive for aggression.
Activity mapping revealed that the COApl is consistently activated in aggressive male mice, acting as a functional hub that orchestrates coordinated neural activity across multiple aggression-related regions. In-vivo fiber photometry further demonstrated that Esr1COApl neurons are not only active during the act of aggression but also during the anticipatory phase of a social investigation that precedes the attack. This temporal pattern suggests these neurons encode the motivational salience or perceived threat level of social stimuli, rather than aggression per se.
Importantly, chemogenetics and optogenetics silencing of Esr1COApl neurons led to a robust and selective suppression of aggression, while simultaneously enhancing prosocial behaviours such as close investigation and affiliative sniffing. Crucially, these manipulations did not impair social reward and reinforcement behaviour, indicating that Esr1COApl neurons play a specific role in aggressive motivation without broadly disrupting social function. Anatomically, we found that Esr1COApl neurons project to two key downstream targets ventromedial hypothalamus and central amygdala. Both projections were functionally necessary for the aggression-promoting effects of the COApl, supporting the idea that this circuit exerts top-down control over deeper subcortical aggression centers (Figure 1).
3 CLINICAL IMPLICATIONS AND FUTURE DIRECTIONS
Clinically, one of the most pressing challenges is how to mitigate pathological aggression without impairing normal social functioning. The discovery of an Esr1COApl-centered circuit that specifically governs aggressive motivation—but spares prosocial behaviour—offers a promising solution. Unlike conventional pharmacological interventions such as antipsychotics or selective serotonin reuptake inhibitors, which often blunt social engagement, targeted modulation of this pathway reduced aggression while preserving, and even enhancing, social interaction. This circuit-level dissociation of aggression from sociability also sheds light on sex-specific mechanisms of aggression. The Esr1COApl pathway was found to be selectively active in male mice, implicating sex hormones and dimorphic neural architecture in shaping how the brain encodes social threats. These findings may help explain the marked male predominance observed in aggression-related disorders such as antisocial personality disorder and further suggest that sex-tailored interventions could be both necessary and feasible in clinical practice.
From a translational perspective, the COApl is part of the conserved “ cortical amygdaloid nuclei ” in humans, which is implicated in the generation of learned and innate behaviours, as well as positive and negative valence processing.8 This cross-species conservation enhances the translational relevance of our findings and supports the feasibility of non-invasive imaging of homologous human circuits. Advances in high-resolution functional magnetic resonance imaging and positron emission tomography imaging may enable the development of circuit-based biomarkers to detect aggression-related dysfunction, stratify patients, predict treatment responses or inform personalized care plans. In parallel, the involvement of ESR1 introduces a neuroendocrine dimension to aggression regulation. Meta-analytic data suggests a positive association between estradiol levels and aggression in humans,9 and our data provide a cellular substrate for this relationship. These findings open the door to exploring hormonal therapies or selective ESR1 modulators as precision treatments for individuals with endocrine-related aggression phenotypes or hormone-sensitive conditions.
Looking forward, it will be important to determine whether analogous or distinct circuits govern aggression in females. Although less frequently studied, female aggression is clinically relevant—particularly in contexts such as trauma, mood disorders or postpartum psychiatric syndromes. Bridging our preclinical findings to human studies—through neuroimaging, behavioural phenotyping, and longitudinal approaches—will be essential for clinical translation.
ACKNOWLEDGEMENTS
This work was supported by the Beijing Natural Science Foundation (grant Z240012 to L.L.)




