Comparative impact of proton versus photon irradiation on triple-negative breast cancer: Role of VEGFC in tumour aggressiveness
Frédéric Luciano and Gilles Pagès are the co last authors.
Dear Editor,
In this study, we demonstrated that proton (P) and photon (X) radiotherapies (RT) lead to different molecular changes in triple-negative breast cancer (TNBC) cells. P-irradiated tumours tended to make larger tumours, while X-irradiated ones exhibited increased aggressiveness. Both types of radiation increased gene expression related to angiogenesis (blood vessel formation) and lymphangiogenesis (lymph vessel formation), which are associated with more aggressive cancer behaviour. We also found that targeting the lymphangiogenesis-related gene, vascular endothelial growth factor C (VEGFC), alongside either type of RT, could improve the prognosis for TNBC patients.
Breast cancer (BC) is the most common type of cancer among women.1 Its aggressive forms, like TNBC, tend to be highly vascularized and often have an increased network of lymphatic vessels, which allows the cancer to metastasize more rapidly.2 Standard treatment involves with a combination of surgery, chemotherapy and RT to target both local and systemic diseases. Despite these treatments, recurrence remains a significant challenge in aggressive forms of BC.3
Proton therapy, a newer form of RT, offers more precise targeting than conventional X-RT, potentially reducing side effects by narrowing the radiation field.4 Ongoing clinical trials are investigating whether P-RT might offer advantages over conventional X-RT, as recent research suggests promising advantages.5
Here, we investigated how irradiation impacts TNBC cell behaviour and their microenvironment, building on our prior study of P- and X-RT effects on head and neck cancer.6 Specifically, we investigated whether irradiation might inadvertently promote tumour growth by altering cells to release growth factors or cytokines that support tumour survival and progression.
To examine these effects, we developed TNBC cell populations (MDAMB231 and BT549) that are resilient to repeated X- or P-RT. The traits of aggressiveness, such as proliferation and migration were evaluated in these multi-irradiated cells. While proliferation rates in irradiated cells were like controls (Figure 1A,B), migration abilities were enhanced (Figure 1C,D), suggesting that these cells could have a greater potential for metastasis. This increase in migration mirrors findings in X-resistant medulloblastoma cells.7
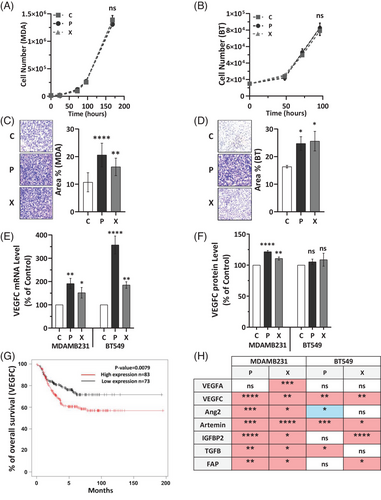
Since metastasis in TNBC frequently occurs via lymphatic vessels,8 we investigated the impact of X- and P-RT on the expression of VEGFC, a key regulator of lymphangiogenesis, in our TNBC cell lines which exhibit higher basal levels of VEGFC compared to cell lines of other BC subtypes (Figure S1). Both irradiation types significantly upregulated VEGFC mRNA expression (Figure 1E) and increased secretion of VEGFC protein (Figure 1F), (similar trend in BT549 cells). A comparable increase in VEGFC mRNA levels was also noted when comparing single versus multiple rounds of RT (Figure S2). Higher VEGFC expression has been associated with worse clinical outcomes in TNBC, as evidenced by patient survival data from existing databases (KM plotter software) (Figure 1G), suggesting that both baseline VEGFC levels and RT-induced VEGFC upregulation could contribute to worse prognosis in TNBC. This elevated VEGFC expression indicates a potential risk of enhanced lymphangiogenesis, and thus higher recurrence and metastasis.9
Other genes linked to poor survival, such as artemin, angiopoietin 2, IGFBP2, FAP and TGFβ were also differentially expressed after irradiation in both cell lines, further suggesting that radiation may subtly alter gene expression in ways that could increase relapse risk (Figure 1H). Individual data for each factor are presented in Figure S3. These findings emphasize the need to consider the distinct impacts of different radiation types on the tumour cells and the surrounding microenvironment when devising TNBC treatment strategies.
Furthermore, the aggressiveness and the capacity of the multi-irradiated cells to promote metastasis via lymphatic vessels was evaluated by permeability assay. Vessel-on-chip experiments using reconstituted lymphatic vessels surrounded by multi-irradiated X-irradiated MDAMB231 cells demonstrated increased vessel leakiness compared to vessels exposed to either control or multi-irradiated P-irradiated MDAMB231 cells (Figure 2A,B), suggesting enhanced metastatic potential. Considering the aggressive behaviour observed in vitro in TNBC cells adapted to repeated irradiations, we conducted experiments to assess the tumourigenic capacity of TNBC P- and X-adapted cells in nude mice. Tumours from P-adapted cells had higher incidence (100%) compared to 60% in X-adapted, and 40% in controls (Figure 2C) and were larger than those from X-adapted or control cells (Figure 2D,E). However, X-irradiated tumours, though smaller, showed higher expression of lymphatic (Lyve1) and vascular markers (CD31), indicating a more aggressive molecular profile than P-irradiated tumours (Figure 2F–I). Moreover, VEGFC protein expression was elevated in X-irradiated tumour lysates compared to both P-irradiated and control (C) tumours (Figure 2J). Transcriptomic analysis performed on human genes (tumours cells) and mouse genes (microenvironment) revealed that X tumours had more active pathways for angiogenesis, lymphangiogenesis and other genes associated with aggressiveness such as those involved in epithelial-mesenchymal transition (Figure S4A,B). This suggests that X-irradiated tumours may carry a higher risk of aggressive relapse.
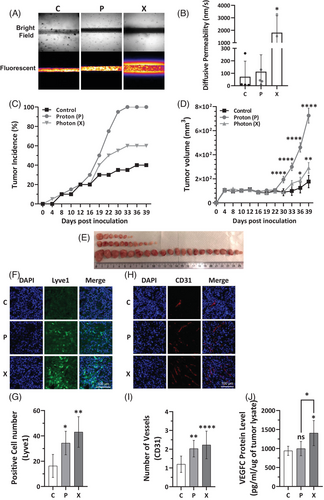
Proteomic analysis of the tumour tissues revealed distinct molecular profiles between the two irradiation types (Figure S5A). The unique protein signatures in P- and X-irradiated tumours were confirmed using Principal Component Analysis (PCA), which showed clear separation between the two types based on their protein expression (Figure S5B). This result underscores that P- and X-RT impact TNBC tumour biology in different ways.
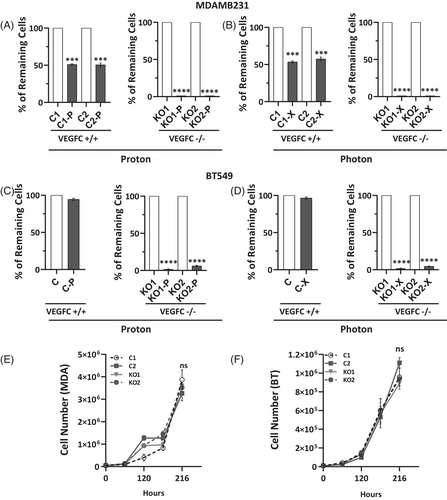
To investigate the role of VEGFC in radiation response, we used CRIPRS/Cas9 to knockout the VEGFC gene in MDAMB231 and BT549 cell lines (Table S1, Figure S6). VEGFC-deficient cells (VEGFC-/-) had significantly lower survival rates after X- or P-RT compared to controls, with almost no viable VEGFC-/- cells remaining (Figure 3A–D). However, VEGFC knockout did not affect the cells' response to chemotherapy, indicating VEGFC's specific influence on RT sensitivity (Figure S7A–C). Furthermore, VEGFC knockout did not affect cell proliferation in vitro (Figure 3E,F). To assess the role of VEGFC in modulating sensitivity to RT, MDAMB231 cells were exposed to high-dose RT (8 Gy) with or without preincubation with recombinant VEGFC protein. While both X- and P-RT significantly reduced cell numbers, preincubation with VEGFC conferred a protective effect (Figure S8). This finding is consistent with our previous observation that VEGFC-/- cells exhibit heightened sensitivity to RT.
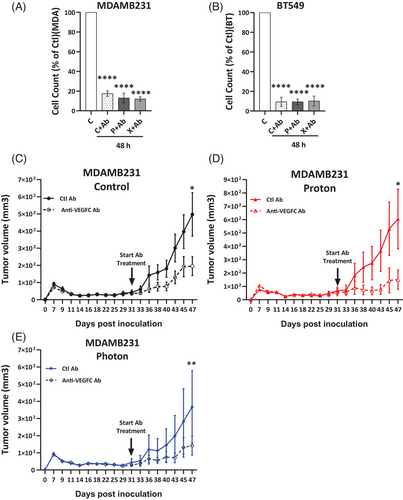
In a related in vitro experiment, treatment with an anti-VEGFC antibody reduced cell counts by up to 90% in both control and multi-irradiated X- and P-irradiated cells, suggesting that endogenous and RT-induced VEGFC function as an autocrine factor promoting proliferation and/or survival (Figure 4A,B). Anti-VEGFC antibody treatment inhibited the growth of experimental tumours in nude mice derived from control as well as multi-irradiated X- and P-irradiated MDAMB231 cells, with some tumours exhibiting near-complete regression (Figure 4C–E). This suggests that anti-VEGFC therapy, especially alongside RT, could be an effective strategy to limit TNBC progression.
In conclusion, our study reveals that P and X radiotherapies produce different molecular effects in TNBC cells, with P-irradiated tumours being larger and X-irradiated tumours displaying more aggressive molecular characteristics. These differences suggest that X-RT may lead to more aggressive relapses if resistant cells survive. Future research should focus on understanding these radiation-specific effects and developing strategies to manage risks associated with X-RT. Furthermore, our study emphasizes the potential of anti-VEGFC therapies, especially if administered before irradiation, to counteract radiation-induced molecular changes. Comparative clinical trials and further investigations into anti-VEGFC treatment schedules are anticipated to advance TNBC treatment strategies.
AUTHOR CONTRIBUTIONS
Conception and design: Gilles Pagès and Frédéric Luciano. Development of methodology: Saharnaz Sarlak. Acquisition of data: Saharnaz Sarlak, Delphine Marotte, Alessandra Pierantoni, Jessy Sirera, Meng-Chen Tsai, Arthur Karaulic and Roxane Sylvestre. Analysis and interpretation of data: Saharnaz Sarlak, Florent Morfoisse, Barbara Garmy-Susini, Frédéric Luciano, Gilles Pagès and Paraskevi Kousteridou. Writing, review: Saharnaz Sarlak, Florent Morfoisse, Meng-Chen Tsai, Frédéric Luciano and Gilles Pagès. Administrative, technical, or material support: Marie Vidal, Joël Hérault and Gilles Pagès. Study supervision: Frédéric Luciano and Gilles Pagès.
ACKNOWLEDGEMENTS
This work was performed using the microscopy (PICMI) and mouse facilities of IRCAN. The materials of the different facilities at IRCAN were supported by the Conseil Général 06, the FEDER, the Ministère de l'Enseignement Supérieur, the Région Provence Alpes-Côte d'Azur and the INSERM. The authors are grateful for financial support from CNRS, Université Côte d'Azur, the Research fund from the Canceropôle PACA, ANR, INCA, the H2020 TheraLymph Grant project ID: 874708, the Ligue Nationale contre le Cancer (Equipe Labellisée 2019), Fondation ARC de la Recherche contre le Cancer Programme Labellisé 2022 and program ARCAGEING2023020006332 and postdoc grant ARCPOST-DOC2021070004080, the Fédération Claude Lalanne, the grant from the Fondation Estée Lauder and the Pink Ribbon award and who did the bioinformatic analysis. The authors acknowledge MET’CONNECT a Structuring Action financed by the French National Cancer Institute (INCa), the Région Sud, and the Canceropôle Provence-Alpes-Côte d’Azur
CONFLICT OF INTEREST STATEMENT
The authors declare no conflicts of interest.
Open Research
DATA AVAILABILITY STATEMENT
All data generated or analysed during this study are available from the corresponding author upon reasonable request.
This study was carried out in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals. Our experiments were approved by the “Comite National Institutionnel d'Ethique pour l'Animal de Laboratoire (CIEPAL)” (reference: NCE/2023823).




