Mitochondria-derived vesicles: A promising and potential target for tumour therapy
Xueqiang Peng, Yu Gao, and Jiaxing Liu contributed equally to this work.
Abstract
Mitochondria-derived vesicles (MDVs) participate in early cellular defence mechanisms initiated in response to mitochondrial damage. They maintain mitochondrial quality control (MQC) by clearing damaged mitochondrial components, thereby ensuring the normal functioning of cellular processes. This process is crucial for cell survival and health, as mitochondria are the energy factories of cells, and their damage can cause cellular dysfunction and even death. Recent studies have shown that MDVs not only maintain mitochondrial health but also have a significant impact on tumour progression. MDVs selectively encapsulate and transport damaged mitochondrial proteins under oxidative stress and reduce the adverse effects of mitochondrial damage on cells, which may promote the survival and proliferation of tumour cells. Furthermore, it has been indicated that after cells experience mild stress, the number of MDVs significantly increases within 2–6 h, whereas mitophagy, a process of clearing damaged mitochondria, occurs 12–24 h later. This suggests that MDVs play a critical role in the early stress response of cells. Moreover, MDVs also have a significant role in intercellular communication, specifically in the tumour microenvironment. They can carry and transmit various bioactive molecules, such as proteins, nucleic acids, and lipids, which regulate tumour cell's growth, invasion, and metastasis. This intercellular communication may facilitate tumour spread and metastasis, making MDVs a potential therapeutic target. Advances in MDV research have identified novel biomarkers, clarified regulatory mechanisms, and provided evidence for clinical use. These breakthroughs pave the way for novel MDV-targeted therapies, offering improved treatment alternatives for cancer patients. Further research can identify MDVs' role in tumour development and elucidate future cancer treatment horizons.
1 BACKGROUND
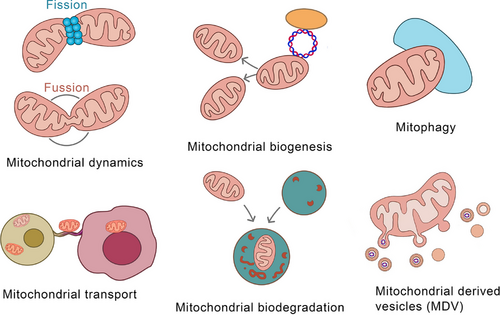
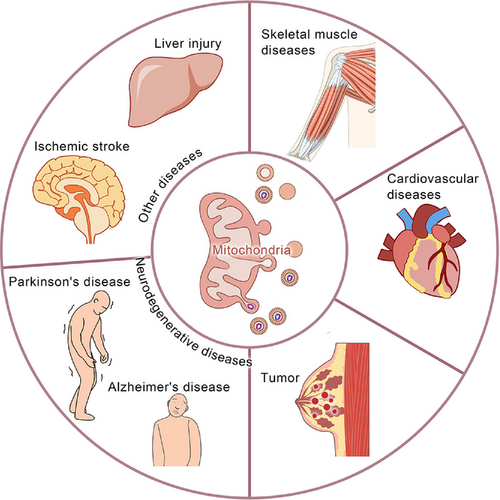
Mitochondria, eukaryotic ubiquities, are central to aerobic respiration,1 harbours respiratory enzymes that catalyse ATP synthesis – meeting ∼95% of cellular energy needs.2 Beyond bioenergetics, mitochondria regulate proliferation, apoptosis, signalling cascades, and calcium dynamics.3 This functional pleiotropy positions mitochondria as master regulators of cellular homeostasis.4 Dysfunctional mitochondria disrupt these networks, driving pathophysiological cascades,5 thus highlighting mitochondrial fidelity as a cornerstone of organismal viability.6 Mitochondrial quality control (MQC) is vital for safeguarding essential cellular processes and ensuring normal mitochondrial function and cellular homeostasis.7 This includes mitochondrial dynamics,8 biogenesis,9 mitophagy,10 transport,11 intracellular protein degradation,12 and mitochondria-derived vesicles (MDVs)13 (Figure 1). Mitochondrial dynamics mainly encompass fission and fusion. Phosphorylated Drp1 translocates to mitochondria, forming a ring-shaped oligomer for fission-induced membrane division.14 MFN1/2 restructure the outer mitochondrial membrane, enhancing fusion.15 OPA1 interacts with inner-membrane phospholipids to form polymers, promoting inner – membrane and matrix changes for fusion.16 PGC-1α regulates mitochondrial biogenesis.17 Mitophagy, a specialised autophagy, recruits damaged mitochondria and ATG proteins to form autophagosomes,18 occurring after specific damage.19 Mitochondrial transport depends on microtubules, actin filaments, and motor proteins like myosin and dyneins; nanotubes also aid transport.20, 21 The regulation of mitochondrial transfer and its impact on homeostasis are unclear, despite links between Ca2+ levels, volume, and fusion.22 The ubiquitin–proteasome system, with mitochondrial proteases and chaperones, degrades dysfunctional proteins for quality control.23 Cancer is the most formidable challenge to human health, with persistently high incidence and mortality rates.24 There have been certain advancements in traditional cancer treatment modalities such as surgery, radiation, and chemotherapy; however, they are still beset by numerous limitations, highlighting the urgent need for novel therapeutic targets and approaches.25 Cancer is predominantly characterised by abnormal cellular growth and can be divided into benign (non-invasive and non-metastatic) and malignant (cancerous, invasive, and metastatic) types.26, 27 Cancer development has multiple stages, which are influenced by both genetic and environmental factors. Treatment options include surgery, radiotherapy, chemotherapy, and others.28 Furthermore, it has become the 2nd leading cause of death worldwide.29 In recent years, MDVs have been extensively studied, which has increased our knowledge about their critical roles in many diseases30 (Figure 2). In 2014, Ayumu Sugiura et al.31 published a comprehensive review of MDVs, outlining their discovery and transport mechanisms. However, the review did not elucidate the role of MDVs in tumours. Several studies have indicated the critical involvement of MDVs in tumourigenesis and tumour progression.32-34 Emerging evidence implicates mitochondrial-derived vesicles (MDVs) in regulating oncogenic processes, including tumour invasion and metastasis. In breast cancer, MDVs encapsulate mitochondrial DNA (mtDNA) within extracellular vesicles (EVs), activating TLR9-mediated signalling to drive migratory and invasive phenotypes.35 Towers et al. further demonstrated that autophagy-deficient breast cancer and osteosarcoma cells bypass canonical mitophagy by upregulating mitochondrial fusion – mediated by MFN1/MFN2 dynamins – and amplifying MDV biogenesis. These SNX9-dependent vesicles shuttle damaged mitochondrial cargo directly to lysosomes or peroxisomes, with vesicle production markedly enhanced under pharmacologic stress. This adaptive rewiring of mitochondrial quality control underscores the metabolic plasticity of cancer cells under autophagy suppression, revealing SNX9 or MDV pathways as tractable targets for therapy-resistant malignancies. Intriguingly, MDV-mediated organelle crosstalk may extend beyond oncology, potentially contributing to neurodegenerative disorders marked by mitochondrial dysfunction.32 Therefore, MDVs can serve as a promising new target for cancer therapy. This review summarises the current data on the roles of MDVs in tumour development and progression, critically examining their potential as therapeutic targets. Moreover, this study also projects future research trajectories, emphasising the necessity for innovative experimental strategies and technologies to further elucidate the multifaceted functions of MDVs in cancer. By providing evidence for targeting MDVs, this review aims to pave the way for the development of more effective and personalised cancer treatments, thereby advancing the frontiers of oncological research.
2 MDVS OVERVIEW: DISCOVERY, FORMATION MECHANISMS, AND DISTINCTIONS WITH EVS
Mitochondria are crucial for cellular function and maintain cellular stability through fusion and fission via DRP1. In 2008, Neuspiel et al.36 indicated that MAPL overexpression generates DRP1-independent mitochondrial fragments, MDVs of 70–150 nm diameter lack cristae and resemble transport vesicles. MDVs selectively include mitochondrial cargo and function autonomously, not requiring ATG5 for formation.31 This mechanism parallels primitive bacterial processes, suggesting evolutionary conservation. In 2016, the MDV pathway was observed in Arabidopsis, suggesting its association with higher eukaryotes.37 Recent research has revealed that PD-related genes like Parkin and PINK1 are critical for MDV formation,38,39 mediate mitophagy-derived vesicles, and intensify under oxidative stress.40 Reactive oxygen species (ROS) activates PINK1 and Parkin, leading to oxidised protein accumulation and vesicle formation.41 The synthesis of MDV involves ROS-induced protein oxidation, phosphatidylcholine oxidation, and PINK1-Parkin activation. It has been observed that MDVs maintain mitochondrial quality and modulate substance exchange.13, 42 SNX9 interacts with Rab9, dynamin, AP-2, and clathrin to regulate MDV formation and release.43 Further research is needed to fully understand MDV formation mechanisms. MDVs are vesicles with diameters ranging from 70–150 nm,36 originating from mitochondria.31 They carry various cargoes including mitochondrial inner and outer membranes, proteins, DNA, RNA, and factors such as PINK1, Parkin, and MAPL.31, 44 MDVs play crucial roles in maintaining mitochondrial quality control, inter-organellar communication, signalling, metabolic regulation, and energy metabolism, and can also serve as delivery vehicles.45 Their ultimate fate may involve lysosomal degradation, transport to peroxisomes, intracellular reuse, or release and secretion.45-47 EVs, including exosomes, microvesicles, and apoptotic bodies, have diameters of approximately 30–150 nm, 100–1000 nm, and greater than 1000 nm, respectively.48-50 EVs carry proteins, nucleic acids, lipids, and metabolic products51-54 and are involved in intercellular communication, immune regulation, metabolic regulation, and disease progression, as well as serving as biomarkers and delivery vectors. Their fate includes uptake by recipient cells, transport and fusion within endosomes, release and function of contents, and intranuclear transport and function.55-58 MDVs interact with late endosomes, forming MDVs-EVs, which carry mitochondrial proteins and DNA59,60 and are involved in cell communication, immune regulation, clearance of damaged mitochondria, and regulation of tumour stem cells.61-64 They may enter the circulatory system as biomarkers or be degraded extracellularly65 (Table 1). Tim König et al. systematically summarised the primary markers of mitochondrial-derived vesicles (MDVs), which include TOMM20, MID49/51, MF, DRP1, and phosphatidic acid, among others.45 Concerning the detection of MDVs, there are currently two primary methods61: (1) morphological observation via transmission electron microscopy to assess MDV morphology and (2) detection based on MDV biomarkers, such as employing immunofluorescent co-staining or Western blot techniques to detect markers like TOMM20 and DRP1. Effective isolation of MDVs is a prerequisite for their detection. Soubannier et al. successfully extracted MDVs in vivo by digesting mitochondria isolated from the body with proteinase K.66
| EVs | ||||
|---|---|---|---|---|
| MDVs | MDVs-EVs | Other EVs | References | |
| Source | Budding of the mitochondrial inner or outer membrane | MDVs fuse with multivesicular bodies and are released extracellularly through contact with the plasma membrane |
Exosomes: multivesicular bodies Microvesicles: cell membrane |
31, 65-68 |
| Diameter | 60–150 nm | 70–150 nm |
Exosomes: 30–150 nm Microvesicles: 100–1000 nm |
43, 47-49 |
| Cargo | Mitochondrial related components (inner membrane, outer membrane, proteins, mtDNA, etc.) | Mitochondrial-related components (proteins, mtDNA, etc.) | Proteins, nucleic acids, lipids, etc. | 31, 43, 50-53, 60, 62, 69, 70 |
| Function |
Involved in mitochondrial quality control Involved in interorganellar communication Involved in intracellular signal transduction and metabolic regulation |
Intercellular communication (immune regulation, myocardial regulation, neural regulation, regulation of tumour cell malignancy, etc.) | Participate in intercellular cargo and information transmission | 44, 60-63, 70-73 |
| Fate |
Lysosomal degradation Transport to peroxisomes Transport to autophagosome Intracellular reuse Release and secretion |
Release into the extracellular space of the cell Can be taken up by receptor cells |
Release into the extracellular space of the cell Can be taken up by receptor cells |
44-46, 54-57, 64, 74 |
3 TRANSPORT PATHWAYS AND CARGO SELECTION OF MDVS
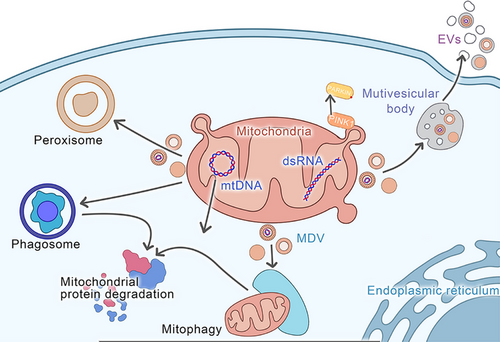
Studies have indicated that MDVs utilise two distinct pathways, where one targets the degradation by lysosomes, and the other targets the specific peroxisomal subpopulation.13 Furthermore, MDVs also target bacterial phagosomes and extracellular vesicles (EVs)75 (Figure 3). Soubannier et al.66 revealed that MDVs can selectively enrich oxidised proteins, such as subunits of complex II, III, and IV, but not of complex I and V, and lack any nuclear proteins. This indicates the distinctive densities and uniform diameters of single- and double-membrane vesicles, suggesting their ability to selectively carry cargo. Different types of cargo are selected by each transport pathway13 (Figure 3) (Table 2).
| Selection of cargo | Transport pathway | Mechanism | References |
|---|---|---|---|
| Pyruvate Dehydrogenase Complex(PDH) | Targeting lysosomes | The oxidative stress generated within mitochondria stimulates the entry of different cargo into MDVs, thereby targeting them to the lysosomes. | 76 |
| VDAC | Targeting lysosomes | The enzyme xanthine oxidase (XO) stimulates cells to produce H2O2 and induces the formation of single-membrane MDVs targeted to the lysosomes within mitochondria. | 77 |
| HOPS complex | Targeting lysosomes | Stx17, in conjunction with SNAP29 and VAMP7, assembles into a ternary complex, mediating MDV-lysosome fusion. | 78 |
| TOMM20 protein | Targeting lysosomes | Stx17, together with SNAP29 and VAMP7, forms a ternary complex, facilitating MDV-lysosome fusion. | 79 |
| Aberrant endoplasmic reticulum (ER) cargo | Targeting lysosomes | Tollip can interact with the endosomal sorting required for transport-0 (ESCRT-0) protein Tom1 within the intracellular system, promoting vesicle maturation. | 80 |
| TOMM20 protein | Targeting lysosomes | Tollip captures MDVs, facilitates their entry into the endocytic transport system, and recruits cytoplasmic Parkin. After processing and maturation within the endocytic system, cytoplasmic protein Rab7 binds to the vesicle surface, ultimately directing TOM20+ MDVs to the lysosome for degradation. | 41 |
| TOMM20 protein | Targeting lysosomes | VPS35 recognises MDVs containing the TOM20 protein and associates with the surface oligomers of DRP1, forming TOM20+/ DRP1+ MDVs. Subsequently, the DRP1 oligomers are engulfed by the MDVs, completing the translocation process from the mitochondrial surface to the lysosome for degradation. | 45 |
| MAPL | Targeting peroxisomes | DRP1 is abundant in MAPL. | 76 |
| MAPL | Targeting peroxisomes | The ROS generated during xanthine oxidase/xanthine reaction induce the formation of TOM20-positive single-membrane MDVs, which selectively transport MAPL to the peroxisomes in HeLa and osteosarcoma cells. | 81 |
| MAPL | Targeting peroxisomes | The recruitment of vesicle protein sorting-associated proteins VPS35 and VPS26 forms a complex, which in turn mediates the transport of MAPL to peroxisomes via MDVs. | 62 |
| DLP1 complex | Targeting peroxisomes | The Vps35 subunit binds to the cargo tail, thereby mediating the transport of MAPL to peroxisomes via MDVs. | 82 |
| SOD2 | Targeting phagosome | Infection of macrophages with methicillin-resistant Staphylococcus aureus stimulates the production of MDVs containing SOD2. The loaded MDVs are transported to phagolysosomes containing the bacteria. | 83 |
| DAMPS | Targeting EV | Optic atrophy 1 (OPA1) and SNX9 mediate the targeting of MDVs containing DAMPs to extracellular vesicles (EVs). | 63 |
| Alpha-synuclein | Targeting EV | When Parkin and PINK1 are mutated, MDVs can load α-synuclein and enter EVs, facilitating intercellular transfer through EVs. | 84 |
| mtDNA | Targeting EV | Cancer-associated fibroblasts can transfer mtDNA through EVs, restoring oxidative phosphorylation in hormonally treated breast cancer cells. | 83 |
| TOM20 protein | Targeting MVB | MDVs can directly target to multivesicular bodies (MVBs). | 13 |
| mtDNA | Targeting lysosomes | SNX9 mediates the targeting of mtDNA-enriched MDVs to the lysosomes. | 34 |
| mtDNA | Targeting EV | The trafficking of mtDNA in extracellular vesicles facilitates the transmission of oncogenic signals, promoting the therapy-induced reawakening of cancer stem cell-like cells. | 64 |
3.1 Transport pathway targeting lysosomes
The lysosomal pathway is primarily mediated by MDVs containing oxidised proteins and is closely associated with the PINK1/Parkin pathway.85 Evidence confirms MDVs selectively transport oxidised products to lysosomes for degradation, aiding mitochondrial stability; however, MDV entrance into lysosomes is complex.86 This underscores the intricate MDV-lysosome interaction, highlighting MDV's critical role in cellular homeostasis regulation. Therefore, it is crucial to understand that MDV targeting the lysosomal pathway is significant. Recent research has revealed that soluble N-ethylmaleimide-sensitive factor attachment protein receptor (SNARE) facilitates the fusion of mitochondrial transport vesicles with lysosomes,85 which is essential for intracellular transport and degradation. SNARE proteins are conserved and mediate typical membrane fusion. They have been observed to form a four-helix bundle via their coiled SNARE domain, promoting membrane fusion. Mitochondrial fusion is homotypic, SNARE-independent, and regulated by outer mitochondrial membrane fusion proteins.87 Moreover, MDV-lysosome fusion is heterotypic and SNARE involvement is critical for MDV targeting the lysosomal pathway.88 Syntaxin-17 (STX17), a SNARE complex subset, detects mitochondrial outer membrane curvature via a unique clamping structure; its absence reduces MDV transport to lysosome fusion, impeding the process.85 Studies on yeast have revealed that the phosphorylated ubiquitin-binding domain (P-UB) interacts with SNARE, and STX17 directly binds P-UB.89 Recent studies79 have shown that STX17, SNAP29, and VAMP7 form a ternary SNARE complex crucial for PINK1/Parkin-dependent MDVs targeting lysosomes, affecting MDV formation and maturation. Familial early-onset Parkinson's disease has been associated with biallelic PINK1 (PARK6) mutations and PARK2 gene functional loss, causing Parkin deficiency.90 Xanthine oxidase (XO) stimulates H2O2 production, thus inducing single-membrane MDVs targeted towards lysosomes, rich in outer mitochondrial membrane proteins like TOM20 or VDAC.45, 77 Tollip plays a critical role in MDV transport and acts as an adaptor molecule in the endosomal system, interacting with ESCRT-0 protein Tom1, facilitating vesicle maturation and cargo transport.80, 83 In addition, Tollip captures MDVs, aids their entry into the endosomal transport system, and recruits cytoplasmic Parkin, regulating TOM20+ MDV transport.91 Tollip and Parkin's interaction creates a sophisticated complex, with Rab7 adhering to mature MDVs, thereby directing them towards lysosomal degradation. Tollip and Rab7 are recycled within lysosomes for further functional utilisation, marking TOM20+ MDVs for degradation.82 VPS35, a retromer component, dissociates from the retromer, recognises TOM20-carrying MDVs, and binds them with DRP1 oligomers92 to form TOM20+/DRP1+MDVs, which engulf DRP1 oligomers and transport them from the mitochondrial surface to the lysosome for breakdown.
3.2 MDV pathways targeting peroxisomes
The transportation pathway of MDVs to peroxisomes is a classic transport process;89,93 however, its mechanism remains elusive as the pathway is specific to lysosomes.94 A crucial player in this process is MAPL, a mitochondrial small ubiquitin-related modifier (SUMO) E3 ligase that maintains the stability of DRP1, degrading mitofusin 2 (Mfn2), and facilitating mitochondrial fission.76 Several studies have demonstrated the widespread presence of MAPL in MDVs89 and its significance in the transport process. Moreover, the production of ROS via the XO/xanthine reaction can induce the formation of translocase of the outer membrane 20 (TOM20)-positive MDVs, which selectively target MAPL to peroxisomes in specific cell types.81 The complex of VPS35 and VPS26 is essential for transporting MAPL+ MDVs to the peroxisome within the cell. This complex not only facilitates the organised transport of MDVs but also plays a key role in supporting cellular metabolism and various biological functions.62, 95 In addition, silencing VPS35 or VPS26 has been found to reduce the co-localisation of MAPL+ MDVs with peroxisomes, which highlights the significance of these proteins in the targeting process.94 Although these findings highlight the possible targeting mechanism, further investigation is warranted to fully comprehend the complex processes involved in MDV's localisation to peroxisomes. The intricate interplay between the various proteins and cellular components indicates the complexity of MDV transport pathways and the need for comprehensive research to identify their precise mechanisms. Overall, the MDV pathways targeting peroxisomes comprise a series of intricate interactions involving MAPL-, ROS-, TOM20-positive vesicles, VPS35, and VPS26. Although the insights gained from these findings are valuable in understanding the process, further research is still necessary to fully comprehend the specific mechanisms involved in the targeting of MDVs to peroxisomes to further advance the understanding of cellular homeostasis and identify novel approaches for therapeutic interventions aimed at targeting these crucial cellular pathways.
3.3 Other MDV transport pathways
MDVs, which accumulate on bacterial-laden macrophage phagosomes,96 play a critical role in transporting SOD2 to phagolysosome to facilitate bacterial elimination.83 VPS proteins, such as VPS26 and VPS29, are integral to cargo sorting and transport to the TGN, thereby maintaining cellular homeostasis and efficient intracellular transport.97 During stress conditions, MVBs can serve as damage-associated molecular patterns (DAMPs) to promote inflammation, or cells can package MDVs into EVs for release, potentially protecting against cellular damage. Todkar et al.63 investigated the pathway involving OPA1 and SNX9 in EVs and revealed that the mutations in Parkin and PINK1 are related to the loading of α-synuclein into MDVs in EVs, which might be a cause of Parkinson's disease.84 MVBs with 200 to 1000 nm diameters contain numerous vesicles and can fuse with MDVs that carry TOM20.13
4 THE PHYSIOLOGICAL CONTRIBUTIONS OF MDVS
As mentioned above, MDVs are crucial for ensuring the balance and stability of the internal cellular environment. Its involvement in various physiological processes indicates its crucial role in the cell. Firstly, MDVs are involved upstream of mitochondrial autophagy and maintain mitochondrial homeostasis.98 Secondly, they can break down various dysfunctional mitochondrial proteins, which are crucial for cellular health and function.99 Furthermore, MDVs have an essential and multifunctional involvement in organelles communication, highlighting their multifaceted and crucial role within the cellular environment.100 Overall, these diverse functions highlight the significant impact of MDVs in the regulation of cellular function and maintenance.
4.1 MDVs act upstream of mitochondrial autophagy to maintain mitochondrial homeostasis
Mitophagy, a crucial mechanism for maintaining MQC,101 depends on the participation of PINK1 and Parkin, similar to MDVs.102 However, PINK1 or Parkin inhibition did not cause significant phenotypic alterations or neurodegeneration, indicating an alternate mechanism for mitochondrial engulfment and MDV formation in higher eukaryotes.103, 104 MDVs are released within a short frame of 2–6 h after exposure to mild stress, such as treatment with antimycin A, whereas mitophagy typically occurs between 12 and 24 h after the stress onset.99 This temporal distinction suggests that MDVs protect mitochondria from mitophagy, which is triggered by mitochondrial damage caused by the absence of PINK1 and Parkin.31 Furthermore, decreased MDVs have been associated with increased mitophagy, indicating the upstream regulatory role of MDVs in the maintenance of mitochondrial homeostasis. In addition, exposure of cells to lysosomal inhibitors such as bafilomycin A1 or pepstatin A/E-64D can result in a substantial accumulation of MDVs within the cytoplasm as well as multivesicular bodies.85 Moreover, an in vitro budding study performed a quantitative assessment of the cargo released into multivesicular bodies and revealed an expulsion rate of about 4% of specific proteins every hour, thus highlighting the importance of MDVs in the steady-state transport of cargo.66,85 McLelland et al. in 2016 indicated the pivotal role of MDVs in the intracellular trafficking of proteins.85 These findings provide valuable insights into the intricate mechanisms involved in the regulation of protein transport within the cell. During mild cellular stress, MDVs protect the structural integrity of mitochondria, potentially by impeding the progression of mitochondrial autophagy, thus serving as a pivotal upstream regulator of mitophagy.32 Moreover, during cellular homeostasis, the quantitative appraisal of MDV-mediated cargo transportation significantly participates in maintaining cellular stability.105 MDVs act as upstream regulators of mitochondrial autophagy and are indispensable for the preservation of mitochondrial robustness and operational efficiency.106 Therefore, a comprehensive examination of the interplay between MDVs and mitochondrial autophagy is indispensable to understanding the processes that regulate MQC within the cellular milieu.107 Furthermore, it is crucial to understand theoretically and practically the causes of mitochondrial dysfunction in order to develop effective treatments for mitochondrial diseases.
4.2 Involvement of MDVs in the degradation of damaged mitochondrial proteins
A study108 found that when mitochondria are stimulated by oxidative stress. Oxidation-induced damaged mitochondrial proteins can be encapsulated by MDV and then transported to lysosomes for degradation. Moreover, intracellular mitochondria can effectively germinate and form MDVs containing the mitochondrial oxidative proteins in the basal state or when cultured with galactose to increase aerobic metabolism. As previously stated, compared to mitochondrial autophagy, MDV, as the first line of defence, will be activated within a few minutes of mitochondrial stress, leading to a significant increase in production. Furthermore, before reaching the stress threshold and initiating mitochondrial autophagy for the clearance of damaged mitochondria, it is plausible for oxidised or impaired proteins in mitochondria to be transported through MDVs, alleviating mitochondrial damage.19 Thus, mitochondria can internally break down and remove damaged proteins, as well as promote mitophagy, which can facilitate the degradation of these proteins.109,110
4.3 The involvement of MDVs in inter-organelle communication
MDVs have been found to mediate inter-organelle communication.111 For example, to enhance its antimicrobial function, SOD2 shuttles as MDV cargo between mitochondria and phagosomes.83 The translocation of MAPL between the mitochondria and peroxisomes can promote the biogenesis of peroxisomes.112 The fusion of vesicles containing various peroxiproteins, proteins for peroxisome biogenesis, can stimulate the generation of immature peroxisomes. Furthermore, MDVs have also been implicated in the biogenesis of peroxisomes, which are essential for the different metabolic processes within cells. In addition, peroxisomal proteins PEX3 and PEX14 have been found to target the mitochondria and subsequently release MDVs, which then fuse with the vesicles derived from the ER containing PEX16, resulting in peroxisomal precursor structures.113 MDVs carrying the peroxisomal marker E3 ubiquitin ligase MAPL can also generate peroxisomes de novo or via the growth and division of the existing organelles. The literature has also reported the advantageous effects of the transfer of mitochondrial components via EVs.114 This translocation not only occurs between different organelles (such as the endoplasmic reticulum) but also extends across distant cells or tissues such as the oxidative mitochondrial components carried by EVs released from palmitate-stressed adipocytes.62 This horizontal mitochondrial transfer could be employed as a preventive signal against heart muscle damage, indicating potential implications for regenerative medicine. Moreover, the injection of EVs obtained from energy-stressed adipocytes in mice before coronary artery ligation has been observed to relieve damage caused by cardiac ischemia/reperfusion.62 These EVs have significant protective effects, as they are associated with improved cardiac function and decreased tissue damage.61 However, a study analysed Parkin knockout mice with reduced mitochondrial-enriched EV production in adipocytes and revealed that this cardiac protective effect was absent, highlighting the importance of mitochondrial components in EV-mediated cardioprotection.115 Researchers have also discovered that brown adipocytes when exposed to heat stress, release EVs containing oxidised mitochondrial components that can be internalised again by parental brown adipocytes. This process reduces peroxisome proliferator-activated receptor gamma (PPAR-γ) and uncoupling protein 1 (UCP1) protein levels in the recipient cells. These findings revealed the vital role of EVs in intercellular communication and the potential modulation of cellular metabolism and function. Some other studies have indicated similar mechanisms in the hearts of mice, where resident macrophages have been observed to engulf EVs that contain mitochondria released by cardiomyocytes.116 The tyrosine-protein kinase Mer is a macrophage receptor, which is crucially involved in the phagocytic uptake of EVs by recognising phosphatidylserine residues on their surface.117 Thus, understanding these intercellular communication pathways is crucial for identifying new avenues for the development of targeted therapies for cardiovascular and metabolic diseases. In addition, mice subjected to catecholamine or coronary artery ligation stress have indicated increased engulfment of EVs containing mitochondria. This indicates the possible role of these EVs in stress response and tissue regeneration mechanisms, further validating the significance of intercellular mitochondrial transfer in maintaining cellular homeostasis and responding to physiological as well as pathological stressors. Moreover, EVs released by neural stem cells (NSCs) have been found to contain mitochondrial proteins and intact mitochondria with complete membrane potential and respiration ability.107 The addition of EVs to L929 Rho0 cells lacking mitochondrial DNA (mtDNA), successfully revived mitochondrial function and increased cell viability, highlighting the therapeutic promise of these EVs for addressing mitochondrial dysfunction and cellular damage.118 Overall, this intricate interaction between MDVs and EVs, which transport mitochondrial components, reveals the fascinating mechanisms by which cells maintain cellular homeostasis and respond to stress. These data indicate the possibilities for treatments and additional exploration in regenerative medicine, cardiovascular disease, and metabolic disorders (Figure 3).
5 INDUCTION OF IMMUNE RESPONSES BY MDVS
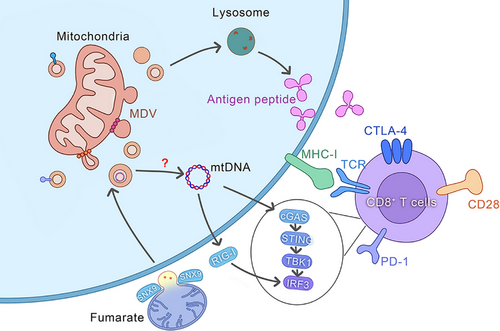
In immune cells, MDVs serve as pivotal signals that trigger the body's immune response. This process comprises a series of finely regulated steps, beginning with the targeted recognition of MDVs, followed by their internalisation, subsequent release of components, and signal transduction, culminating in the activation of innate immune responses (Figure 4).
5.1 Phagocytosis of MDVs
For immune cells to process exogenous substances, it is crucial that MDVs undergo phagocytosis and internalisation. Upon MDV recognition by specific receptors on the immune cell surface, a complex series of phagocytic mechanisms are initiated to ensure their efficient internalisation into the cell interior.62 Phagocytosis is typically mediated by phagocytic receptors on the cell surface,119 which recognise and bind to specific molecules on the MDVs. Activation of these receptors stimulates dynamic alterations in the cell membrane, such as cytoskeleton remodelling, particularly of actin and tubulin.120 Rapid remodelling of the actin network enables the formation of pseudopodia.121 The formation of phagosomes is a highly dynamic process requiring the concerted action of various intracellular proteins, such as the Rho family of small GTPases.122 These proteins regulate the dynamics of the cytoskeleton to ensure the effective formation and movement of phagosomes into the cell. In phagosome formation, activation of the membrane fusion mechanism is a key step, which involves the interactions between SNARE proteins and members of the Rab GTPase superfamily.123 These proteins facilitate the fusion between the cell and phagosomal membranes, ensuring the effective release of MDV contents into the cell. The phagocytosis and internalisation of MDVs are complex processes involving the recognition by cell surface receptors, remodelling of the cytoskeleton, and activation of the membrane fusion mechanism. The synergistic action of these steps ensures the effective MDV internalisation by immune cells, providing a basis for further immune recognition and response
5.2 Recognition and signal transduction of MDV components
After MDV's internalisation, PRRs can effectively recognise the release of mitochondrial DNA, proteins, and other components within the cell.123 This process is crucial for immune cells to identify and respond to exogenous substances.Upon contact between antigen-presenting dendritic cells (DCs) and T cells, T cells can deliver mitochondrial components (e.g., mtDNA) back to DCs via extracellular vesicles, triggering type I interferon responses and STING signalling in DCs.124 The cytosolic PRR cGAS can recognise and bind to exogenous or endogenous double-stranded DNA125 and catalyses the production of the second messenger cGAMP, which then binds and activates the STING receptor. STING is a transmembrane protein predominantly located in the cytoplasm and can recognise cytosolic cyclic dinucleotides, such as cyclic GMP-AMP (cGAMP).126 Once engulfed by DCs, the mtDNA in MDVs may be released into the cytoplasm, where it binds to cyclic GMP-AMP synthase (cGAS) and catalyses cGAMP synthesis.124, 127 The interaction of cGAMP with the STING receptor triggers the activation of downstream signalling cascades, including the phosphorylation of TANK-binding kinase 1 (TBK1) and interferon regulatory factor 3 (IRF3), activating type I interferon synthesis.128 Type I interferons are antiviral cytokines, which activate an antiviral state in immune cells, promoting their activation and migration.129 Other than the STING receptor, damaged MDVs may interact with immune cells via other receptors.44 For instance, Toll-like receptors (TLRs), which are pattern recognition receptors (PRRs) expressed on the surface of immune cells, can recognise pathogen-associated molecular patterns (PAMPs), such as bacterial lipopolysaccharides (LPS) or viral double-stranded RNA (dsRNA).130 Furthermore, Absent in Melanoma 2 (AIM2), a cytosolic inflammasome receptor can recognise double-stranded DNA and assemble it into an inflammasome.131 This process involves apoptosis-associated speck-like protein recruitment and caspase-1 activation.132,133 Activated caspase-1 cleaves the pro-inflammatory cytokines pro-IL-1β and pro-IL-18, maturing and releasing them extracellularly to promote the development of inflammatory responses.134 Activation of these PRRs is not limited to the aforementioned mechanisms and they have been observed to be activated by other signalling pathways, such as the activation of interferon regulatory factors (IRFs) and nuclear factor-κB (NF-κB).135 IRFs are transcription factors that, when recognised for viral infection or intracellular DNA damage, induce the expression of type I interferons, activating an antiviral state in immune cells.136 NF-κB, a key transcription factor for inflammatory responses, upon recognising inflammatory signals from both intracellular and extracellular sources can induce the expression of various inflammatory cytokines and chemokines, promoting the activation and migration of immune cells.137 The MDV components recognition and signal transduction is a complex process that involves the activation of multiple PRRs and the coordination of various signalling pathways.138 These mechanisms ensure that immune cells can effectively recognise and respond to signals carried by MDVs, thus maintaining immune homeostasis and defending against pathogen invasion.
5.3 MDV activates innate immune response
MDVs are involved in the transportation of mitochondrial antigens to lysosomes for antigen presentation, a process tightly regulated by numerous proteins. After undergoing degradation by lysosomal hydrolases, the antigenic peptides are transported to the cell surface, where they are identified by immune cells and elicit an immune response,139-141 triggering the innate immune response. Another pathway through which MDVs participate in the innate immune response involves early alterations in mitochondrial morphology and the release of mtDNA into the cytoplasm as a result of fumarate deficiency. This activates the cGAS–STING–TBK1 pathway, which stimulates the innate immune response in a manner partly reliant on RIG-I. This phenotype is mediated by succinate and selectively occurs through MDVs that rely on SNX9.34 MDVs play a vital role in phagocytic cells by effectively eliminating bacterial pathogens by transporting superoxide dismutase 2 (SOD2). In the innate immune system, when phagocytic cells like macrophages or neutrophils experience stress, they generate ROS by triggering NADPH oxidase or stimulating mitochondrial metabolism. Mitochondrial ROS (mROS) can kill bacterial pathogens, thereby exerting its bactericidal effect.91 These findings indicate the significant role of MDVs in the immune response, as they are essential for antigen presentation, early activation of the innate immune response, and clearance of bacterial pathogens. Therefore, the elucidation of the mechanisms and pathways underlying MDV involvement in immune regulation is crucial for identifying novel strategies for developing targeted immunotherapies and offering profound insights into intercellular communication and immune system modulation. Investigation of MDVs and their role is crucial for increasing our understanding of immune responses and the development of innovative therapeutic interventions (Figure 4).
6 MDVS PROMOTE TUMOUR PROGRESSION
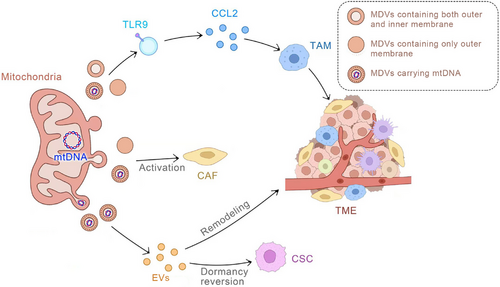
In cancer and other cell types with impaired mitochondrial engulfment, the generation of MDVs serves as a compensatory and adaptive mechanism to maintain mitochondrial health.32,105,142 When mitochondria cannot be effectively cleared through the engulfment process, MDVs are generated and then targeted for lysosomal clearance to enable the elimination of damaged mitochondrial particles. This process aids in preventing severe cellular consequences caused by imbalanced mitochondrial function by ensuring the elimination of dysfunctional mitochondrial fragments. MDVs can also transport mtDNA.71, 143 Dysfunctions in mtDNA, along with genetic mutations, are intricately associated with tumourigenesis and can contribute to varying degrees of tumour progression. For example, a prior study has reported that in hepatocellular carcinoma (HCC), aberrant mitochondrial fission mediated by DRP1 can effectively trigger a mtDNA stress response, leading to its release from mitochondria into the cytoplasm. This DRP1-mediated mtDNA stress can then activate the TLR9 receptor signalling pathway and upregulate the secretion of chemokine CCL2. This in turn promotes the infiltration of tumour-associated macrophages (TAMs) into the tumour microenvironment (TME), which causes immune tolerance of HCC tumour cells, thereby exacerbating HCC progression.144 Hence, MDVs carrying mutated mtDNA can promote tumour development through a complex mechanism that involves the MDV's interaction with the EVs pathway. It is well-recognised that tumours are complex systems engaged in continuous communication with the microenvironment.Therefore, once targeted by MDV, the resulting MDV-EVs play a crucial role in tumour development.145 Breast cancer cells release EVs rich in mtDNA that can enhance the expression of matrix metalloproteinase (MMP) and α5β1 integrin under glutamine deficiency conditions, promoting the invasiveness of recipient breast cancer cells.35 Acute myeloid leukaemia (AML) cells release increased levels of mitochondrial EVs during differentiation, and inhibiting the formation of these EVs can prevent myeloid cell differentiation.146 Melanoma cells release EVs rich in mtDNA that can induce cytokine production in macrophages, thereby inhibiting the cytotoxic T-cell immune response in the tumour microenvironment.125 Moreover, EV-mediated mitochondrial transfer may contribute to cancer drug resistance. In metastatic breast cancer patients with resistance to insulin therapy, circulating EVs rich in mtDNA were detected, which might act as a pro-oncogenic signal to induce endocrine therapy resistance in breast cancer cells dependent on oxidative phosphorylation.83 EVs released by chemoresistant triple-negative breast cancer cells can transfer functional mitochondria to sensitive triple-negative breast cancer cells, leading to increased chemoresistance and tumourigenesis.147 Similarly, EVs from tumour-activated stromal cells deliver mitochondria to malignant glioma cells, resulting in resistance to cancer radiotherapy and chemotherapy.148 In addition, in the plasma of patients with metastatic breast cancer, MDVs carrying mutated mtDNA have been detected. This mtDNA can be transferred by cancer-associated fibroblasts through MDVs to restore oxidative phosphorylation in hormone therapy-treated breast cancer cells71 (Figure 5).
7 POTENTIAL CLINICAL APPLICATIONS OF MDVS
The potential of MDV as a tumour therapy target primarily depends on two factors. Firstly, in clinical treatment, MDV can be employed as a vector to carry specific therapeutic agents, which can provide novel designs for immunotherapeutic strategies against tumours, and enhance treatment efficacy. Secondly, for diagnostics, MDVs are potential biomarkers for tumour diagnosis and prognosis assessment. By enabling earlier detection of tumours and evaluation of their progression, MDVs can assist physicians in providing more precise treatment regimens for patients.
7.1 The potential of MDV targeted therapy
As aforementioned, mtDNA serves as one of the cargoes transported by MDVs, effectively promoting tumour progression to some extent.44 This discovery not only highlights the significant role of mtDNA in tumour biology but also provides new avenues for developing novel cancer therapies. MDV-targeted therapies against tumours are based on this phenomenon, which aims to modulate the function of mtDNA within MDVs using mitochondria-targeted molecules. Key methods include the application of nanocarrier systems to deliver drugs specifically targeting mtDNA. Advancements in nanotechnology have offered new possibilities for drug delivery. For instance, Hall et al. utilised delocalised lipophilic cations to prepare mitochondria-targeting agents, designing molecular-based nanocarriers that selectively accumulate in mitochondria under a high transmembrane potential (ΔΨm) and deliver bioactive substances.149 This approach enhances drug targeting and efficacy while reducing normal cell toxicity by employing metal complexes for targeted delivery against mtDNA. It has been indicated that metal elements are significantly associated with biochemical reactions. Certain metals can participate in mitochondrial reactions, while others selectively bind to DNA and stabilise complexes. Jiang et al.150 developed a nanocatalytic drug, MSN-Ru2+/Fe2+, composed of mesoporous silica nanoparticles carrying Fe2+-Ru2+ complexes. Ru2+ complexes bind to mtDNA, and Fe2+ Fenton reagents promote ROS production in mitochondria through Fenton reactions, inducing apoptosis. Phototherapy is employed to activate specific drugs or substances targeting mtDNA to promote cancer cell destruction. It uses specific light wavelengths to activate drugs or substances for therapeutic purposes. Yang et al.151 precisely assembled biotinylated Pt (IV) prodrug derivatives with IR780 at a 1:1 molecular ratio, achieving mitochondria-targeted chemophotothermal therapy in A549R tumour cells under near-infrared (NIR) irradiation, nearly eliminating tumours. This method has combined advantages of chemotherapy and photothermal therapy and therefore, can precisely target tumour cells with minimal damage to surrounding normal tissues. In summary, the rapid development of mtDNA-targeted therapies suggests that MDVs can serve as potential carriers of mtDNA and have a significant potential as a target for cancer treatment. These innovative targeted strategies not only enhance therapeutic efficacy but also reduce side effects, offering new hope for cancer patients. Future advancements based on the comprehensive knowledge of the biological role of mtDNA and MDVs, as well as further development of nanotechnology and phototherapy, will yield more efficient and safer cancer treatments.
7.2 Potential of MDVs as biomarkers in tumour diagnosis and prognosis
MDVs hold significant clinical potential in tumour-targeted therapies, extending beyond therapeutic applications to encompass crucial diagnostic implications. As potential biomarkers, the detection of MDVs in blood offers a novel approach to assessing the vitality of mitochondria within the cardiovascular system, underscoring their pivotal role in diagnosis.152 It has been observed that circulating MDVs serve as indicators of mitochondrial dysfunction.153 Therefore, MDVs have the potential for diagnostic purposes and can serve as innovative biomarkers in the medical field to evaluate mitochondrial function and related diseases. By assessing the distribution and characteristics of MDVs across various tumours, it is possible to uncover their potential as biomarkers for diagnosing and assessing a range of diseases. This could pave the way for personalised treatments and novel clinical diagnostic strategies. The anticipated outcomes of this prospective research are poised to significantly advance the medical field, offering more comprehensive and precise support for existing therapeutic and diagnostic approache.154 Altogether, the potential applications of MDVs in diagnosis merit further and in-depth investigation, which can help provide valuable insights into the medical field and open new diagnostic and therapeutic avenues and methodologies in the future.155 It is imperative to acknowledge and enhance these potential applications through extensive research and collaborative efforts, as they are crucial for revolutionising and enhancing clinical diagnostic and therapeutic paradigms.
8 CONCLUSION AND FUTURE PERSPECTIVES
In summary, MDVs have become a focal point in cancer research due to their role in tumour progression and interaction with the TME. These vesicles carry bioactive molecules that modulate tumour cell behaviour and immune responses, making them potential therapeutic targets. Furthermore, they are being investigated as biomarkers are being explored for early diagnosis and prognosis assessment. Understanding how MDVs influence the tumour immune microenvironment could lead to novel targeted therapies, improving the efficacy of cancer treatments and offering new avenues for early diagnosis and personalised care. Future studies will aim to elucidate its biological functions and regulatory networks to develop more effective and safe cancer treatments.
AUTHOR CONTRIBUTIONS
XP, YG and JL conducted the research study and drafted the manuscript. XS and WL provided assistance during the revision and drafting process. YM, XL and HL contributed to the conceptual framework, supervised the study, and revised the manuscript. All authors carefully reviewed the final manuscript and approved it for publication.
ACKNOWLEDGEMENTS
The authors have nothing to report. This study was supported by the National Natural Science Foundation of China (No. 82303373), Liaoning Provincial Central Government Guiding Local Science and Technology Development Funds (2024020198-JH6/1008), Science and Technology Projects of Shenyang (21-104-0-04; 22-321-31-02), Support plan for young and middle-aged scientific and technological talents in Shenyang (RC230583).
CONFLICT OF INTEREST STATEMENT
The authors declare that they have no competing interests.




