Induction of HMOX1 by mesenchymal stem cell cytotherapy inhibits osteoclastogenesis and myeloma-induced bone disease
Dear Editor,
Multiple myeloma (MM) cells typically grow in focal lesions (FLs), which often turn into osteolytic lesions.1 Through the study of cytotherapy with mesenchymal stem cells (MSCs) for treating MM, we discovered that MSCs mediate HMOX1 expression in monocytes to balance differentiation of osteoclast precursors into osteoclasts. Lower expression of HMOX1 in the MM bone is associated with poor outcome and induction of HMOX1 pharmacologically resulted in suppression of MM-induced bone disease.
Previously, we showed that MM-induced osteolytic bone disease can be treated via direct cytotherapy with MSCs using our well-established SCID-hu and SCID-rab MM models2, 3 (see Methods and Discussion in Supplementary Information). By applying global gene expression profiling (GEP) on the whole human bone in SCID-hu mice we found that MSC cytotherapy induced expression of several genes associated with the macrophages and monocytes (Figure 1A, Table S1). Of the top upregulated genes, we focused on HMOX1, which encodes heme oxygenase 1, and known as an inducible factor that mediate oxidative stress, inflammation and bone remodelling.4 HMOX1 expression in bone was consistently upregulated following MSC cytotherapy in bones engrafted with 4 different MM cell lines (Figure 1B and C). Immunohistochemistry post-cytotherapy revealed induction of HMOX1 protein in monocytes and macrophages and some MM cells (Figure 1D). HMOX1 expression is highest among immune cells in MM bone marrow based on publicly available scRNA-seq data (Figure 1E–G).
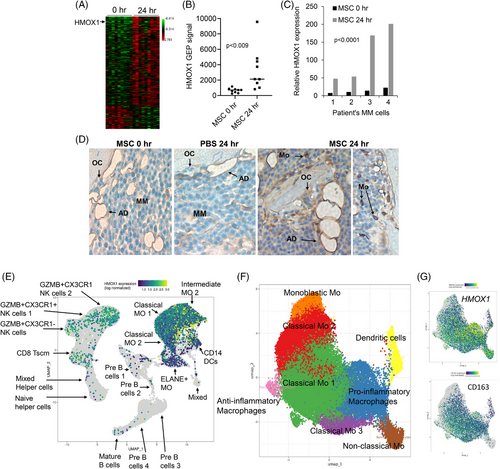
To study whether MSCs mediate osteoclastogenesis through HMOX1, we co-cultured MSCs with osteoclast precursors (pOC) and found that MSCs suppressed their differentiation into multinucleated osteoclasts, an effect that was associated with upregulation of HMOX1 at the RNA and protein levels, lower expression of the osteoclast markers: ACP5 (TRAP), CTSK, and VTNR, and lower secretion of HMGB1 (Figure 2A–G). RANKL is a master regulator of osteoclastogenesis that acts on pOC via TNFRSF11A/RANK. Using qRT-PCR, immunofluorescence and immunoblot we found that MSCs conditioned medium reduced TNFRSF11 expression and RANK levels in pOC (Figure 2H–J). MSCs secreted factors that restrain osteoclastogenesis are discussed in Supplementary Information.
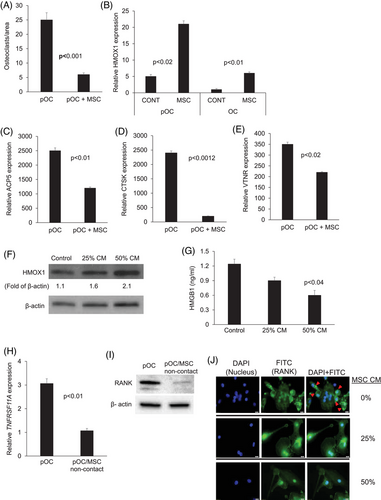
NFκB is induced by RANK/RANKL signalling and is a vital signalling pathway for osteoclastogenesis.5 MSC-conditioned medium inhibited cytoplasmic phosphorylated IκBα and NFκB p65 and nuclear NFκB p65 in pOC (Figure 3A–C).
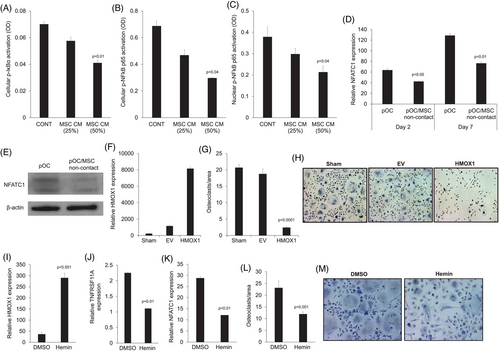
NFATC1 is a main downstream transcription factor activated by the RANKL/NFκB pathway in osteoclasts that induces expression of typical genes associated with osteoclasts, such as CTSK and APC5/TRAP.6 Compared to pOC cultured alone, pOC cultured with MSCs had lower expression of NFATC1 (Figure 3D). Immunoblots conducted on pOC confirmed reduced levels of NFATC1 in pOC cocultured with MSCs in a non-contact condition (Figure 3E). Taken together, these data indicate that MSCs downregulate RANK expression in pOC, resulting in reduced activation of the NFκB pathway, leading to lower activity of the main osteoclastic transcription factor, NFATC1.
We applied two different methods to shed light on the direct role of HMOX1 on osteoclast formation. To induce constitutive HMOX1 expression, we infected monocytes with lentiviral particles containing either HMOX1 cDNA or empty vector. Culturing these cells in osteoclast medium for 7 days induced formation of multinucleated osteoclasts in the control groups (i.e., noninfected cells [sham] and cells containing empty vector); in contrast, multinucleated osteoclasts failed to form in cells expressing HMOX1 cDNA (Figure 3F–H).
To further corroborate our finding, we used hemin, a pharmacological agent that induces HMOX1 expression.7 We confirmed by qRT-PCR that treatment of pOC with hemin induced HMOX1 gene expression in these cells (Figure 3I). We also tested expression of the RANKL receptor RANK, encoded by TNFRSF11A, and NFATC1. Both TNFRSF11A and NFATC1 were downregulated in hemin-treated pOC (Figure 3K–M). Treatment with hemin inhibited the formation of multinucleated osteoclasts in pOC continually cultured in osteoclast medium for 7 days (Figure 3M and N). Together, these findings indicate that induced expression of HMOX1 in pOC inhibits pOC differentiation into osteoclasts.
In vivo, we used our well-established SCID-rab model to test the effect of hemin on MM growth and MM-induced bone disease. Specifically, we engrafted a BM-dependent MM line into SCID-rab mice as previously described.8 Upon establishment of MM engraftment, mice were treated with hemin or control vehicle (DMSO) for 4 weeks. The bone mineral density (BMD) of the implanted myelomatous bone was reduced from pretreatment levels by 16% and 1% in the DMSO- and hemin-treated groups, respectively (p < .005) (Figure 4A). The X-rays showed more osteolysis and lytic lesions in DMSO-treated bones than in hemin-treated bones (Figure 4B). Although fewer osteoclasts were observed in hemin-treated bones, the number of osteoblasts were equivalent (Figure 4C and D). Further, total tumour burden analysed by circulating hIg ELISA were similar between the two groups (Figure 4E). Thus, these results indicate that hemin, the HMOX1 inducer, inhibited osteoclastogenesis and MM-induced osteolytic lesions in vivo.
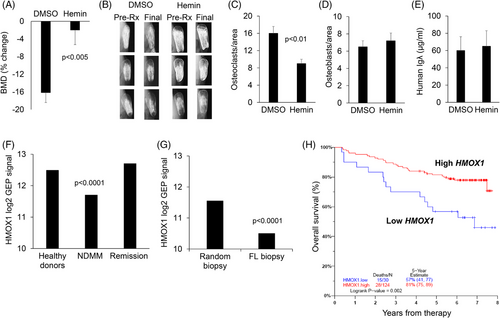
To explore the clinical relevance of our findings, we used publicly available GEP data from our institute to analyse expression of HMOX1 in whole bone biopsies from healthy donors (n = 68), patients with NDMM (n = 354), and MM patients in remission (n = 132).9 Consistent with our findings, HMOX1 expression was decreased in whole biopsies of patients with NDMM and returned to normal when patients were in remission (Figure 4F). We also used available data from paired random interstitial bone biopsy and FL biopsy from patients with NDMM (n = 49 patients). HMOX1 expression was lower in FLs than in interstitial bone samples (Figure 4G). Additionally, lower expression of HMOX1 was associated with poor overall survival in patients with NDMM enrolled in a TT3 clinical trial at University of Arkansas for Medical Sciences (Figure 4H).10 These observations indicate that lower HMOX1 expression in myelomatous bones is markedly suppressed in FLs and that lower expression in interstitial bone marrow is an adverse clinical parameter.
We conclude that MSCs are central in mediating differentiation of osteoclasts through maintaining high expression of HMOX1 in monocytes. Suppression of bone resorption by MSC cytotherapy is partially mediated by induction of HMOX1 in monocytes suggesting that approaches to induce HMOX1 expression may help control MM-induced osteolysis.
AUTHOR CONTRIBUTIONS
X.L. performed the in vitro and in vivo work, the GEP analysis, immunohistochemistry, immunoblots, qRT-PCR, and statistical analyses; X.L. was also one of the writers of the paper. W.L. performed in vitro and in vivo work and the immunohistochemistry. B.B. interpreted the data and provided clinical insight. S.Y. designed and directed the research, conceptualised the work, analysed and interpreted the data, and was one of the writers of the paper.
ACKNOWLEDGEMENTS
Editorial support was provided by the Science Communication Group at University of Arkansas for Medical Sciences.
CONFLICT OF INTEREST STATEMENT
The authors declare no competing financial interests.
FUNDING
This work was supported by a grant CA55819 (B.B.) from the National Cancer Institute and grant CA200068 (S.Y.) from the US Department of Defense.
ETHICS STATEMENT
All animal experimental procedures and protocols were approved by the University of Arkansas for Medical Sciences Institutional Animal Care and Use Committee.
Open Research
DATA AVAILABILITY STATEMENT
The GEP analyses of bone biopsies from MM patients and healthy donors are available as described by Danziger et al. (2020).9




