KMT2C/D mutations in newly diagnosed acute myeloid leukaemia: Clinical features, genetic co-occurrences and prognostic significance
Wenting Wang, Miao Yang and Xue Zhang contributed equally to this work.
To the Editor:
Acute myeloid leukaemia (AML) is a diverse and complex category of malignant disease with poor outcomes. Despite advancements in prognostication and treatment strategies, the molecular landscape of AML remains complex and is not fully understood. Epigenetic factors are acknowledged as crucial in tumour development and progression.1 The KMT2 (histone-lysine N-methyltransferase 2) family encompasses six key proteins: KMT2A/B, KMT2C/D, KMT2F and KMT2G, which is most notably associated with AML.2 KMT2A is associated with KMT2A-rearranged leukaemia.3 KMT2B is identified as a hotspot for rearrangements.4 KMT2C/D mutations occurred frequently in various malignancies.5 However, the clinical characteristics of KMT2C/D mutations in AML remain poorly defined. We found that KMT2C and KMT2D mutations are relatively rare and mutually exclusive in newly diagnosed AML, with KMT2C mutations enriched in CEBPA-mutated and KMT2D in NPM1-mutated AML subtypes, respectively. In general, KMT2CMUT and KMT2CWT, as well as KMT2DMUT and KMT2DWT AML, exhibit distinct mutational spectrums, similar clinical characteristics and survival outcomes.
We reviewed 1935 AML patients who underwent next-generation sequencing (NGS) analyses between 2015 and 2024. Of these, 1050 were eligible for KMT2C analysis and 1777 for KMT2D analysis. The KMT2C mutation rate was 1.90% (20/1050), consistent with previous studies.6, 7 The KMT2D mutation rate was 1.41% (25/1777), lower than that reported in a small-sample study.8 No patient had concurrent KMT2C and KMT2D mutations. Characteristics of AML patients with and without KMT2C/D mutation are presented in Table 1. Clinical characteristics did not differ significantly between wild-type and KMT2C/D-mutated AML patients, except that KMT2DWT patients had higher haemoglobin levels than KMT2DMUT patients (p < .001). Among KMT2C mutated patients, nine (45%) were male and their median age was 43.9 years. For AML classification, 10 of 20 patients were classified as AML with CEBPA mutation. Patients with KMT2D mutations included 11 (44%) males, with a median age of 42.0, and 11 (44%) were recognised as AML with NPM1 mutation. Patients with KMT2C and KMT2D mutations were enriched by AML with CEBPA and NPM1 mutations, respectively. Additionally, most patients had normal karyotypes, including 50% of KMT2CMUT patients and 72% of KMT2DMUT patients.
|
KMT2CMUT (n = 20) |
KMT2CWT (n = 1030) |
KMT2DMUT (n = 25) |
KMT2DWT | |||
|---|---|---|---|---|---|---|
| Characteristic | p1 | (n = 1752) | p2 | |||
| Age (years) | 43.9 (16–63) | 41.9 (14–79) | .598 | 42.0 (15–71) | 41.9 (13–82) | .976 |
| Gender | .518 | .428 | ||||
| Male (N) | 9 (45.0%) | 551 (53.5%) | 11 (44.0%) | 946 (54.0%) | ||
| Female (N) | 11 (55.0%) | 479 (46.5%) | 14 (56.0%) | 806 (46.0%) | ||
| Peripheral blood | ||||||
| WBC (109/L) | 23.4 (1.0–106.2) | 30.8 (.6–317.1) | .312 | 21.4 (1.1–132.9) | 33.0 (.5–404.1) | .093 |
| Haemoglobin (g/L) | 87.8 (57.0–135.0) | 84.4 (40.0–144.0) | .552 | 70.0 (47.0–97.0) | 86.1 (34.0–158.0) | <.001 |
| Platelets (109/L) | 58.0 (8.0–244.0) | 54.8 (2.0–376.0) | .811 | 60.0 (16.0–176.0) | 61.8 (2.0–1852.0) | .817 |
| AML classification | .127 | .022 | ||||
| AML with RUNX1::RUNX1T1 fusion | 1 | 154 | 4 | 284 | ||
| AML with CBFβ::MYH11 fusion | 0 | 93 | 2 | 132 | ||
| AML with DEK::NUP214 fusion | 0 | 2 | 0 | 8 | ||
| AML with BCR::ABL1 fusion | 0 | 3 | 0 | 8 | ||
| AML with KMT2A rearrangement | 1 | 63 | 0 | 104 | ||
| AML with NPM1 mutation | 2 | 192 | 11 | 317 | ||
| AML with CEBPA mutation | 10 | 211 | 0 | 340 | ||
| AML with myelodysplasia related | 4 | 148 | 5 | 248 | ||
| AML defined by differentiation | 2 | 164 | 3 | 311 | ||
| Chromosome karyotype | .011 | .410 | ||||
| Normal karyotype | 10 (50.0%) | 460 (44.7%) | 18 (72.0%) | 795 (45.4%) | ||
| T(8;21) | 1 (5.0%) | 154 (15.0%) | 4 (16.0%) | 279 (15.9%) | ||
| Inv(16) | 0 (0%) | 84 (8.2%) | 1 (4.0%) | 122 (7.0%) | ||
| T(v;11q23.3) | 1 (5.0%) | 36 (3.5%) | 0 (0%) | 56 (3.1%) | ||
| Del(7)/-7/abn(17p) | 2 (10.0%) | 14 (1.4%) | 0 (0%) | 30 (1.7%) | ||
| Del(5q) | 1 (5.0%) | 1 (.1%) | 0 (0%) | 5 (.3%) | ||
| Del(9q) | 1 (5.0%) | 19 (1.8%) | 0 (0%) | 30 (1.7%) | ||
| Trisomy | 1 (5.0%) | 57 (5.5%) | 1 (4.0%) | 112 (6.4%) | ||
| Other abnormal karyotype | 5 (25.0%) | 141 (13.7%) | 0 (0%) | 242 (13.8%) | ||
| ND | 0 (0%) | 64 (6.2%) | 1 (4.0%) | 81 (4.6%) |
- Note: p1: KMT2CMUT vs. KMT2CWT; p2: KMT2DMUT vs. KMT2DWT.
- Abbreviations: ND, not detected; WBC, white blood cell count.
We identified 22 mutation sites in the KMT2C gene (Figure 1A) and 30 in the KMT2D gene (Figure 1B). In all 95.5% of KMT2C mutations were nonsense and frameshift, consistent with the COSMIC database analysis by Rao et al.5 The PHD domain was the most frequently mutated region in KMT2C, with 27.3% (6/22) of mutations located there, of which 83.3% (5/6) were nonsense. In contrast, KMT2D mutations were dispersed without clustering in specific exons or domains. We also analysed co-mutations in KMT2C/D mutant patients. A total of 110 (median five mutations per patient) and 128 (median five mutations per patient) mutations had been found in KMT2C and KMT2D mutated AML, respectively. All KMT2C/D mutations were accompanied by additional gene mutations. CEBPA (n = 10, 50.0%) was the most frequent co-mutation with KMT2C, followed by NRAS (n = 7, 35.0%), GATA2 (n = 5, 25.0%), FLT3 (n = 5, 25.0%). Garg et al. reported that KMT2C co-mutates with FLT3.9 In addition, compared with KMT2CWT AML, KMT2CMUT AML had significantly higher frequencies of CEBPA (50.0% vs. 20.6%, p = .004), GATA2 (25.0% vs. 6.1%, p = .007) and BCOR (20.0% vs. 4.9%, p = .016) mutations (Figure 1C). Besides, all CEBPA mutations were CEBPAbZIP (Figure 1D). Correspondingly, FLT3 (n = 13, 52.0%) was the most frequent co-occurred mutation in KMT2DMUT AML, followed by NPM1 (n = 11, 44.0%) and IDH2 (n = 7, 28.0%). Compared with KMT2DWT AML, KMT2DMUT patients were more likely to co-occur with FLT3 (52.0% vs. 28.1%, p = .016) and NPM1 (44.0% vs. 19.2%, p = .004) mutations (Figure 1E,F).
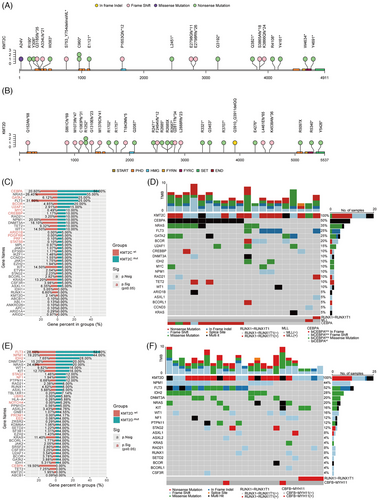
Further, we compared the characteristics of different mutation statuses of KMT2C/D in the CEBPAbZIP (Table S1) and NPM1 (Table S2) subgroups, respectively. In both subgroups, clinical characteristics were similar between wild-type and KMT2C/D-mutated AML patients, except for the higher haemoglobin in KMT2DWT patients (p = .035). In the CEBPAbZIP AML cohort, KMT2CMUT AML were more likely to have co-mutations of NRAS (50.0% vs. 21.1%, p = .05) than KMT2CWT. In the NPM1MUT subgroup, the prevalence of IDH2 co-mutations was significantly higher in KMT2DMUT AML compared with KMT2DWT AML (63.6% vs. 18.7%, p = .002).
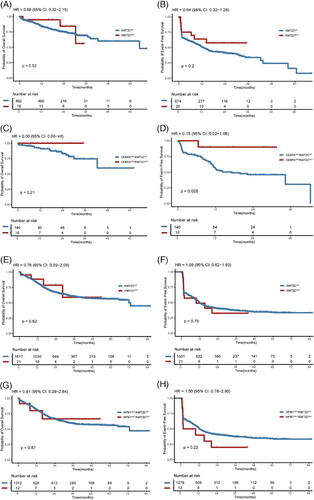
Finally, we examined whether KMT2C/D mutations influence the prognosis of AML. There was no difference in overall survival (OS) or event-free survival (EFS) between KMT2CWT and KMT2CMUT AML patients. The 1-year OS rates were 83.8% and 94.7% for KMT2CWT and KMT2CMUT groups (HR:. 69, 95% confidence interval [CI]:. 22–2.15, p = .52; Figure 2A), respectively. The 1-year EFS rate in KMT2CWT and KMT2CMUT groups were 50.5% and 63.8% (HR:. 64, 95% CI:. 32–1.28, p = .2; Figure 2B), respectively. Although no difference in OS was observed between CEBPAbZIP/KMT2CWTand CEBPAbZIP/KMT2CMUT patients (Figure 2C), the CEBPAbZIP/KMT2CMUT patients exhibited superior EFS, achieving 1-year EFS rate of 90.0% in contrast to 55.4% in the CEBPAbZIP/KMT2CWT patients (HR:. 15; 95% CI:. 02–1.06, p = .028; Figure 2D). Multivariate analysis also demonstrated that KMT2C mutation in CEBPAbZIP patients was associated with better EFS (HR:. 102, 95% CI:. 013–.766, p = .026) but not OS (Table S3). KMT2DMUT and KMT2DWT groups exhibited similar outcomes with 1-year OS rates at 81.5% and 88.4% (HR:. 78, 95% CI:. 29–2.09, p = .62; Figure 2E), 1-year EFS rate at 49.5% and 49.0% (HR: 1.09, 95% CI:. 62–1.93, p = .76; Figure 2F), respectively. Moreover, for NPM1MUT patients, KMT2D mutation did not affect EFS or OS, as shown by Kaplan–Meier survival analysis (Figure 2G,H) and multivariate analysis (Table S4). Additionally, we investigated the impact of KMT2C/D mutations on survival outcomes across different NCCN risk groups, yet found no significant effects in the favourable, intermediate or adverse risk groups (Figures S1 and S2). However, the effect of KMT2C/D on the prognosis of AML needs to be further explored and verified by more studies with larger samples.
AUTHOR CONTRIBUTIONS
HW and JW participated in concept design. MY, XZ, and WW were involved in data collection and analysis, drafting and revising the manuscript. JC, SQ, BL, and YM were responsible for interpreting the results. All authors have read and approved the final manuscript.
ACKNOWLEDGEMENTS
Not applicable.
FUNDING INFORMATION
National Key Research and Development Program of China, Grant Number: 2023YFC2508900; National Natural Science Foundation of China, Grant Number: 82370183; CAMS Innovation Fund for Medical Sciences, Grant Number: 2023-I2M-2-007; Tian Jin Natural Science Foundation, Grant Number: 23JCZXJC00310; Haihe Laboratory of Cell Ecosystem Innovation Fund, Grant Number: 22HHXBSS00040; Beijing Xisike Clinical Oncology Research Foundation, Grant Number: Y-SYBLD2022ZD-0031
ETHICS STATEMENT
This research was approved by the ethical committee in the Institute of Hematology and Blood Diseases Hospital, and all procedures were in accordance with the Helsinki Declaration. Written informed consent was obtained from each participant.




