Farletuzumab ecteribulin and MORAb-109, folate receptor alpha and mesothelin targeting antibody–drug conjugates, show activity in poor prognosis gynaecological cancer models
Dear Editor,
Few therapeutic options are available for aggressive, poor prognosis gynaecological cancers (GC), including uterine serous carcinoma (USC) and ovarian clear cell adenocarcinoma (OCCA). We observed deep and durable responses to the antibody-drug conjugates (ADC), farletuzumab ecteribulin (FZEC, previously known as MORAb-202) or MORAb-109, in GC patient-derived xenograft (PDX) models expressing corresponding target antigens, folate receptor alpha (FRA) or mesothelin (MSLN), providing evidence for clinical trial inclusion of these GC types. Resistance was observed in PDX models with high ABCB1 expression, highlighting this as a potential exclusion criterion in clinical trials of ABCB1 substrates.
The anti-microtubule agent (AMA), paclitaxel, is commonly used in first-line treatment for most GC, in combination with the platinum agent carboplatin, but resistance is common. The AMA, eribulin, has demonstrated efficacy in breast and non-small cell lung cancers1 and we have shown preclinical efficacy in treating aggressive, poor prognosis GC.2, 3 ADCs have enabled targeted delivery of potent cytotoxic agents, including in GC.4, 5 Both FRA and MSLN are not generally expressed in normal adult tissues, but given high frequency of expression in ovarian, uterine and other solid cancers, they have become attractive ADC targets.5 Here, we investigate the utility of the anti-FRA-eribulin ADC, FZEC, and the anti-MSLN-eribulin ADC, MORAb-109, in the treatment of aggressive GC.6-8
We screened 41 GC PDX models covering 11 poor prognosis GC subtypes to determine the frequency and distribution of FRA and MSLN expression (Table S1). For high-grade serous ovarian cancer (HGSOC), 14 PDX models were assessed for FRA expression, with 5/7 chemo-naive and 6/7 post-treatment models being designated as FRA positive (total FRA > 5%, with various staining patterns described; Figure 1A). Three post-treatment HGSOC models, reflecting the target patient group for clinical trials, were selected for FZEC treatment (Table S2). To investigate low versus high expression of FRA, the PDX models #111 (6% FRA+), #206 (32% FRA+) and #931 (43% FRA+) were chosen and treated with a 3-week regimen of eribulin, or with FZEC administered as a single dose (Figure 1A; the FZEC single dose equates to 0.25 mg/kg eribulin payload). We observed comparable eribulin and FZEC activity in all three models (Figure 1B, Table S3) and progressive disease (PD) was observed 85 days or more after treatment. We next investigated if repeated dosing with FZEC could extend response duration, testing this in PDX #111, which showed PD at 85 days with the single FZEC dose. Three weeks of weekly dosing produced a complete response (CR; tumour < 50 mm3 for ≥3 consecutive weeks) for all mice treated, with no PD by 120 days post-treatment initiation (Figure 1B, Figure S1 and Table S3).
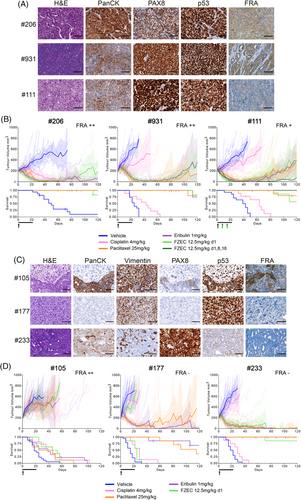
Eribulin has shown promising preclinical results in ovarian carcinosarcoma (OCS),2 therefore we assessed whether FZEC may offer an effective therapeutic avenue for this rare and aggressive cancer. OCS vary in their carcinoma/sarcoma composition, seen morphologically and by pan-cytokeratin and vimentin staining2 PDX #177, with FRA expression < 1%, was purely sarcomatous (Figure 1C, Table S4). PDX #105 and #233 both had mixed composition: for PDX #105, FRA expression was mainly observed in the carcinoma component (47% FRA+), for PDX #233 although predominantly carcinomatous in appearance, it had < 1% FRA positivity (Figure 1C, Table S4). Despite high FRA expression, PDX #105 was resistant to FZEC, as it also was to eribulin, and standard chemotherapies (Figure 1D). While PDX #177 was sensitive to eribulin (PD > 120 days), no tumour regression was observed with FZEC, consistent with the lack of FRA expression. Somewhat surprisingly, PDX #233 was equally sensitive to eribulin and FZEC with deep and durable responses (eribulin CR 7/8; FZEC CR 6/7; Figure 1D; Table S3), despite very low FRA positivity. Extreme eribulin sensitivity in PDX #233 may drive FZEC response via bystander effect, despite low target expression. FZEC binding by Fc/C-type lectin receptors on macrophages or neutrophils in the tumour microenvironment can also lead to internalisation, ADC cleavage and release of eribulin.4
Of the additional 27 non-HGSOC GC PDX models assessed, total FRA expression of ≥5% was seen in 13/27 (48%) and total MSLN expression of ≥5%, in 6/27 (22%) (Table S1 and Table S5). FRA expression was more common in epithelial cancers, OCCA (3/3) and USC (6/8). MSLN expression was not as frequent, however, certain PDX models expressed MSLN, without expressing FRA. Nine PDX models were selected for comparison of eribulin versus FZEC versus MORAb-109, with priority given to models with dual FRA and MSLN expression—three OCCA and six USC PDX models (Table S5, Figure S2). The dual FRA and MSLN expressing models were USC #33, USC #256, OCCA #108 and OCCA #279. PDX #256 had deep and durable responses to eribulin, FZEC and MORAb-109 (CR in 85%–100%; Figure 2 and Table S6). PDX #33 and #108 had relatively short eribulin responses (time to PD 50 and 67 days respectively), while FZEC and MORAb-109 significantly extended survival compared to eribulin (Table S6). OCCA PDX #279 was resistant to all treatments despite high FRA and MSLN expression (Figure 2).
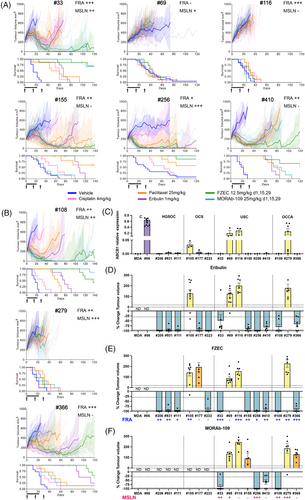
For USC PDX #155 and #410, and OCCA PDX #366, expression of FRA was 30–50% but MSLN expression was < 5%. (Table S5). Accordingly, FZEC response was more durable than for eribulin, and no MORAb-109 activity was observed for PDX #155 and #366 (Figure 2A,B and Table S6). USC PDX models #116, with high (> 50%) FRA expression, and #69 were resistant to all treatments.
Our data are consistent with the previously demonstrated on-target activity of FZEC and MORAb-1097,9 as PDX models #177 (FRA negative), #155 (MSLN negative) and #366 (MSLN negative) were sensitive to eribulin but resistant to eribulin-ADC when the target antigen was not expressed.
In total, four drug resistant PDX models were identified (Figures 1 and 2). Paclitaxel and eribulin are known substrates of P-glycoprotein 1 (P-gp; encoded by the ABCB1 gene) and P-gp expression correlates with paclitaxel resistance and poor patient outcomes.10 Four out of 15 PDX models had elevated ABCB1 mRNA expression, OCS #105, USC #69, USC #116 and OCCA #279; and all four were resistant to eribulin, as well as to FZEC and MORAb-109, all with PD as their best response (Figure 2C–F). Notably, these AMA-resistant PDX had all been derived from samples from patients who had received prior chemotherapeutics, including paclitaxel (Tables 1 and 2). Deriving a suitable diagnostic for transporter over-expression would be beneficial.11
| Tumour type | PDX | Stage at Dx | Age at Dx | Patient treated prior to PDX | Patient TTD from diagnosis (months) | No. of prior systemic therapies | Pre-treatment | Treatment in Pt subsequent to obtaining PDX | BROCA Molecular characterisation | BROCA panel version | paclitaxel response | eribulin response | % total FRA positive | FZEC response | ABCB1 RT-qPCR |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HGSOC | 206 | IV | 45-49 | YES | 44 | 1 | 1) Carboplatin and Paclitaxel |
1) Olaparib and Cyclophosphamide (PD) 2) Carboplatin and Caelyx (PD) 3) Paclitaxel (PR) 4) Etoposide (PD) |
germline BRCA1 NM_007294 c.3817C>T p.Q1273* TP53 NM_000546 c.451C>T p.P151S; rearrangement of BLM NM_000057 |
BROCA-HR v8 | yes | yes | 32 | yes | LOW |
| 111 | IV | 55-59 | YES | 31 | 4 |
1) Carboplatin and Paclitaxel (PR) 2) Anastrazole (maintenance) 3) Carboplatin, Paclitaxel, AKT inhibitor (SD) 4) AURK inhibitor (SD) 5) RTx |
1) Olaparib and Cyclophosphamide (PD) |
CCNE1 NM_001238 amplification (CN 24); EZH2 NM_004456 deletion; TP53 NM_000546 c.700T>A p.Y234N |
BROCA-GO v1 | yes | yes | 5.6 | yes | LOW | |
| 931 | IV | 50-54 | YES | 60 | 9 |
1) Carboplatin and Paclitaxel/ Gemcitabine (PR) 2) Carboplatin and Cediranib (SD) 3) Caelyx (PR) 4) Paclitaxel (1 month) (PD) 5) Carboplatin (PR) 6) Docetaxel (PD) 7) Carboplatin (1 month) (PD) 8) Cisplatin (SD) 9) Bevacizumab (PD) |
None |
BRCA2; c.1299_1331del, BRCA2; c.1300_1332del, BRCA2; c.1313dupA, TP53; c.743G>A p.R248Q |
BROCA-HR v8 | yes | yes | 42.9 | yes | LOW | |
| OCS | 105 | IIIC | 45-49 | YES | 37 | 1 |
1) Carboplatin and Paclitaxel (PD) 2) RTx |
1) Carboplatin and Caelyx (PR) 2) Olaparib (maintenance) 3) Carboplatin and Paclitaxel (PD) 4) Adavosertib (PD) |
RB1 NM_000321 c.1654C>T p.R552X TP53 NM_000546 c.725G>C p.C242S |
BROCA-HR v8 | minimal | minimal | 46.9 | minimal | MED |
| 177 | IIIC | 60-64 | YES | 16 | 1 | 1 cycle Carboplatin and Paclitaxel (PD) | 1) Carboplatin and Paclitaxel (CR) |
TP53 NM_000546 c.742C>T p.R248W TP53 NM_000546 c.818G>A p.R273H germline vus POLD1 NM_002691 c.2293G>A p.V765M |
BROCA-HR v8 | yes | yes | 0.2 | no | LOW | |
| 233 | IIIA | 50-54 | NO | 10 | 0 | None | 1) Carboplatin and Paclitaxel (PD) | no mutations detected | BROCA-HR v8 | yes | yes | 0.7 | yes | LOW |
- Note: Patient clinical history, PDX molecular characteristics, and summary of PDX analysis from this study. None of the patients had received prior ADC therapy.
- Abbreviations: Dx, diagnosis; PDX, patient-derived xenograft; PD, progressive disease; Pt, Patient; PR, partial response; RTx, Radiotherapy; SD, stable disease; TTD, time to death.
| Tumour type | PDX | Stage at Dx | Age at Dx | Patient treated prior to PDX | Patient TTD from diagnosis (months) | No. of prior systemic therapies | Pre-treatment | Treatment in Pt subsequent to obtaining PDX | BROCA Molecular characterisation | BROCA panel version | paclitaxel response | eribulin response | % total FRA+ | FZEC response | % total MSLN+ | MORAb-109 response | ABCB1 RT-qPCR |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| USC | 33 | IIIC |
45-49 (USC) 40-44(BC) |
YES | 44 | 2 |
1) RTx* 2) Docetaxel, Cyclophosphamide (CR)* 3) Tamoxifen (maintenance) * 1) Carboplatin and Paclitaxel (PD) 2) RTx 3) Caelyx (SD) |
1) Durvalumab (PD) |
TP53 c.844C>T, p.R282W TP53 c.451C>G, p.P151A PIK3CA c.3019G>C, p.G1007R XRCC2 c.731_732delAA; WRN c.1163delA; |
BROCA-HR v8 | SD then PD | SD then PD | 52.1 | yes | 38.1 | yes | LOW |
| 69 | IV | 40-44 | YES | 22 | 2 |
1) Carboplatin and Paclitaxel (MR) 2) Paclitaxel, Trastuzumab, Pertuzumab (PD) |
1) T-DM1 (PD) 2) Carboplatin and Gemcitabine (PD) |
CCNE1 NM_001238 amplification (CN 24); CDK12 NM_016507 amplification (CN 9); ERBB2 NM_004448 amplification (CN 127); TP53 NM_000546 c.713G>T p.C238F; Single-allele inactivation of BRCA1 and RPA2 due to genomic rearrangement |
BROCA-GO v1 | no | no | 4.1 | no | 7.7 | no | HIGH | |
| 116 | II | 60-64 | YES | 31 | 1 |
1) RTx 2) Carboplatin and Paclitaxel (SD) |
1) Lenvatinib (maintenance) 2) Letrozole (PD) |
ARID1A NM_006015 c.1820C>G p.S607X; CCNE1 NM_001238 amplification (CN9); FBXW7 NM_001349798 c.1394G>A p.R465H; TP53 NM_000546 c.730G>A p.G244S |
BROCA-GO v1 | no | no | 53.9 | no | 2 | no | HIGH | |
| 155 | IV | 70-74 | NO | 16 | 0 | None |
1) Carboplatin and Paclitaxel (PD) 2) Paclitaxel (SD) 3) Olaparib (PD) |
Germline PALB2 NM_024675 c.2257C>T p.R753X, wildtype allele retained; TP53 NM_000546 c.818G>A p.R273H |
BROCA-GO v1 | yes | yes | 29.9 | yes | 3.4 | no | LOW | |
| 256 | II | 55-59 | YES | 21 | 1 | 1) Carboplatin and Paclitaxel, PORTEC-style (SD) |
1) Trastuzumab. Pertuzumab, Paclitaxel (PR) 2) Carboplatin and Gemcitabine (PD) 3) T-DM1 (PD) |
ERBB2 NM_004448 amplification (CN 13); PIK3CA NM_006218 c.1636C>G p.Q546E; TP53 NM_000546 c.817C>T p.R273C; TP53 NM_000546 c.437G>A p.W146X; WNT7A—MYC rearrangement |
BROCA-GO v1 | yes | yes | 10.5 | yes | 58.9 | yes | LOW | |
| 410 | II |
70-74 (USC) 60-64 (CRC) 45-50 (BC) |
NO | 11 | 0 |
1) Doxorubicin and Cyclophosphamide (CR)* 1) 5FU/Capecitabine (PR)** 2) FOLFOX and Bevacizumab (CR)** |
1) Carboplatin and Paclitaxel (PD) |
KRAS NM_004985 c.38G>A p.G13D; PIK3CA NM_006218 c.1624G>A p.E542K; TP53 NM_000546 c.775G>T p.D259Y |
BROCA-GO v1 | yes | yes | 32.8 | yes | 1.3 | yes | LOW | |
| OCCA | 108 | IV | 40-44 | YES | 23 | 2 |
1) Carboplatin, Paclitaxel and Bevacizumab (SD?) 2) Ipilimumab and Nivolumab (PR) |
1) continued Ipi/Nivo (PR) 2) RTx (PD) |
PIK3CA NM_006218 c.334A>T p.I112F | BROCA-GO v1 | SD then PD | yes | 24.7 | yes | 22.6 | yes | LOW |
| 279 | IIIC | 55-59 | YES | 13 | 1 | 1) Carboplatin and Paclitaxel (CR) |
1) Carboplatin, Paclitaxel and Bevacizumab (CR) 2) Cisplatin, Gemcitabine and Bevacizumab (PD) |
SMARCA4 NM_001128844 c.3766C>T p.Q1256X; TP53 NM_000546 c.797G>A p.G266E; PIK3CA NM_006218 c.1624G>A p.E542K; WRN NM_000553 c.1676C>T p.S559L; ATM NM_000051 c.6452+138_6808-70delinsAAGTCCTCAATGAATGGTAGTTGCTGCTTTC |
BROCA-HR v8 | no | no | 32.8 | no | 60.7 | no | HIGH | |
| 366 | IV | 70-75 | YES | 10 | 1 | 1) Carboplatin, Paclitaxel and Bevacizumab (SD) | None |
ARID1A NM_006015 c.546_555del p.A182fs; ATR NM_001184 c.7219C>A p.R2407S; FANCM NM_020937 c.359T>C p.I120T |
BROCA-GO v1 | SD then PD | yes | 51.1 | yes | 1.3 | minimal | LOW |
- Note: Patient clinical history, PDX molecular characteristics, and summary of PDX analysis from this study.
- Two of the USC models were derived from chemo-naïve tumour samples (#155 and #410) with the remainder being derived from patients who had received treatment including carboplatin/paclitaxel, prior to the generation of the PDX. None of the patients had received prior ADC therapy. Two USC PDX models were HER2 positive, #69 and #256, in agreement with the patient tumour (Figure S2A), and for PDX #69 the patient had received chemotherapy combined with trastuzumab/pertuzumab treatment, prior to generation of the PDX model.
- Abbreviations: CR, complete response; Dx, diagnosis; PDX, patient-derived xenograft; PD, progressive disease; Pt, Patient; PR, partial response; RTx, Radiotherapy; SD, stable disease; TTD, time to death.
- * breast cancer (BC) treatment
- **colorectal cancer (CRC) treatment.
Whole genome sequencing of three of the drug resistant PDX identified a β-tubulin mutation in PDX #69 (TUBB2B ENST00000259818.8, c.743C>T, p.Ala248Val, 0.15 adjusted allele frequency) that may contribute to AMA resistance (data not shown).12
All 15 aggressive GC PDX models showed impressive responses to FZEC and MORAb-109 if positive for FRA and/or MSLN expression, respectively, and negative for high ABCB1 expression. Given the paucity of therapeutic options available to patients with advanced GC, our data highlight the urgent need for inclusion of GC in eribulin-ADC trials early in the GC journey; and exploration of high ABCB1 expression as a potential exclusion criterion in clinical trials of ABCB1/P-gp substrates.
AUTHOR CONTRIBUTIONS
Ksenija Nesic—extracted RNA, performed qPCRs, analysed data, drafted and edited the manuscript. Katherine Rybinski—performed FRA and MSLN IHC and analysis using HALO. Gayanie Ratnayake—histological review. Ratana Lim—curation of patient data, edited the manuscript. Marc Radke–BROCA assay data analysis. Chloe Neagle—extracted RNA, performed qPCRs, analysed data. Gwo-Yaw Ho and Matthew J. Wakefield—study design and initiation, edited the manuscript. Elizabeth M. Swisher—data analysis, edited the manuscript. Holly E. Barker—study design, IF analysis, edited the manuscript. Keiji Furuuchi—study design, IHC analysis using HALO, and edited manuscript. Clare L. Scott—study design and initiation, interpreted patient data, drafted and edited the manuscript. Cassandra J. Vandenberg—study design and initiation, developed and analysed preclinical models, led PDX treatment studies, analysed data, drafted and edited the manuscript. All authors read and approved the final manuscript.
ACKNOWLEDGEMENTS
We thank Bruce Littlefield and Toshimitsu Uenaka (Eisai Inc) for valuable discussions, and for the supply of eribulin, FZEC and MORAb-109. We thank Silvia Stoev, Kathy Barber, Rachel Hancock, Dan Fayle and Stephanie Bound and WEHI Bioservices for their excellent technical assistance. We thank the team at the WEHI Advanced Histotechnology Facility for providing high quality services. We acknowledge Briony Milesi, Amanda Lobley, Adriana Acciarino and the contribution of all scientific and clinical staff who have been involved in the WEHI-SFRCP and the patients who have participated in this program.
This work was made possible through the Australian Cancer Research Foundation, the Victorian State Government Operational Infrastructure Support and Australian Government NHMRC IRIISS.
CONFLICT OF INTEREST STATEMENT
K. Nesic, R. Lim, C. Neagle, M. J. Wakefield, H.E. Barker, C.L. Scott and C.J. Vandenberg report research support (paid to institution) for this work from Eisai Inc; and outside the submitted work from AstraZeneca Pty Ltd and Boehringer Ingelheim, and non-financial support from Clovis Oncology and Sierra Oncology. C.L. Scott reports unpaid advisory boards: AstraZeneca Pty Ltd, Clovis Oncology, Roche, Eisai, Sierra Oncology, Takeda, MSD. KR and KF were employees of Eisai Inc.
FUNDING INFORMATION
This work was supported by funding from Eisai Inc. and grants from the Stafford Fox Medical Research Foundation (KN, RL, CN, MJW, HEB, CLS, CJV) and the National Health and Medical Research Council (NHMRC Australia; Investigator grant 2009783 (CLS)).
ETHICS STATEMENT
Deidentified patient samples and corresponding clinical information were obtained from patients enrolled in the WEHI-Stafford Fox Rare Cancer Program (SFRCP); all patients provided informed written consent. Ethics approval for the WEHI-SFRCP was obtained from the Royal Melbourne Hospital Human Research Ethics Committee (Project number 2015.300) and governance review by the WEHI HREC (Project number G16/02). In addition, deidentified HGSOC samples were also obtained from patients consented to the Australian Ovarian Cancer Study (AOCS) at the Royal Women's Hospital (RWH HREC projects 01/56 and 10/57; WEHI HREC project 10/05).
Open Research
DATA AVAILABILITY STATEMENT
The datasets generated during the current study are available from the corresponding author on reasonable request.




