Aberrant DNA methylation of genes regulating CD4+ T cell HIV-1 reservoir in women with HIV
Abstract
Background
The HIV-1 reservoir in CD4+ T cells (HRCD4) pose a major challenge to curing HIV, with many of its mechanisms still unclear. HIV-1 DNA integration and immune responses may alter the host's epigenetic landscape, potentially silencing HIV-1 replication.
Methods
This study used bisulphite capture DNA methylation sequencing in CD4+ T cells from the blood of 427 virally suppressed women with HIV to identify differentially methylated sites and regions associated with HRCD4.
Results
The average total HRCD4 size was 1409 copies per million cells, with most proviruses defective and only a small proportion intact. The study identified 245 differentially methylated CpG sites and 85 regions linked to HRCD4 size, with 52% of significant sites in intronic regions. Genes associated with HRCD4 were involved in viral replication, HIV-1 latency and cell growth and apoptosis. HRCD4 size was inversely related to DNA methylation of interferon signalling genes and positively associated with methylation at known HIV-1 integration sites. HRCD4-associated genes were enriched on the pathways related to immune defence, transcription repression and host–virus interactions.
Conclusions
These findings suggest that HIV-1 reservoir is linked to aberrant DNA methylation in CD4+ T cells, offering new insights into epigenetic mechanisms of HIV-1 latency and potential molecular targets for eradication strategies.
Key points
- Study involved 427 women with HIV.
- Identified 245 aberrant DNA methylation sites and 85 methylation regions in CD4+ T cells linked to the HIV-1 reservoir.
- Highlighted genes are involved in viral replication, immune defence, and host genome integration.
- Findings suggest potential molecular targets for eradication strategies.
1 INTRODUCTION
The HIV-1 reservoir (HR) has posed a major barrier to an HIV cure. The provirus persists in resting or memory CD4+ T cells1, 2 because of cellular biological conditions during the early stage of infection. These conditions include near-silenced gene expression due to a lack of necessary host transcription factors, restrictive epigenetic features at the proviral promoter and sequestration of key transcription factors (like NFAT and NF-κB).3, 4 Such conditions enable the HR to evade the immune system, posing challenges in curing HIV. Other cell types, such as monocytes, are also of interest for HR, but little is known about their role in HIV reservoir. Due to the presence of HR, cessation of anti-retroviral therapy (ART) leads to HIV-1 rebound usually within approximately 2 weeks.5 The HR size decays slowly with an estimated half-life of 44 months for the total HR6 and 59 months for the intact provirus.7 At this decay rate, eradication would take over 60 years, necessitating lifelong ART for people with HIV (PWH).
In recent years, efforts to cure HIV have focused on latency reversal followed by immune-mediated clearance of infected cells. However, latency reversal agents have failed to effectively reduce HR size, presenting a major challenge.8, 9 Another obstacle is the limited understanding of how HR establishes, maintains, and rebounds in CD4+ T cells before, during, and after ART discontinuation. Addressing these challenges is particularly important among women with HIV (WWH), whose immune response to HIV-1 may differ from that of men. WWH exhibit lower HIV-1 viral load during the early stage of HIV infection but show higher immune activation and faster progression compared with men with HIV,10-12 which may influence HR latency and size for WWH. Furthermore, sex hormones can modulate immune function. For example, oestrogen has been shown to enhance the activity of certain immune cells, including HIV-1-targeted CD4+ T cells.13, 14 Hormonal fluctuations such as those occurring during menopause can affect the dynamics of HIV infection and the establishment of viral reservoirs. The underlying mechanisms of HR among WWH have not been understood.
Emerging evidence shows that epigenetic regulation of both the viral and host genomes is crucial to establishing and maintaining HIV-1 latency. Transcription of provirus depends upon the dynamic interaction between genomic alteration of the provirus and host immune activity.15 The HIV-1 provirus genome reduces chromatin accessibility in latently infected cells.16 Mechanistically, two CpG sites in the promoter region of long terminal repeats (LTR) of the provirus are hypermethylated, mediated by the host methyl-CpG binding domain protein 2 in infected CD4+ T cells.17 In contrast, some studies reported that methylation of LTR was not associated with HIV-1 latency.18, 19 Research on the host genome's DNA methylation and HR is still in its infancy. Some studies have reported that hyper- and hypo-methylated regions are associated with HR size and positively correlate with disease progression assessed by viral response to ART,20 but these findings are based on small sample sizes or ex vivo experiments, making it challenging to draw strong conclusions about in vivo host DNA methylation dynamics associated with HR.
Genome-wide DNA methylation analysis with high-density CpG profiling is a useful approach to comprehensively investigate the role of the host DNA methylome in HR size. Among the total reservoir in infected CD4+ T cells, only a small proportion contains intact provirus (∼1 per million cells) capable of full transcription activation but remain inactivated partially due to heterogenous integration landscape and epigenetic mechanisms.21-23 Most proviruses are defective because of apolipoprotein B mRNA editing enzyme catalytic polypeptide-like 3G (APOBEC3C)-mediated hypermutation,24 large internal deletions, packaging signal (Ψ) deletions, major splice donor mutations and inactivating point mutations.25 Most defective proviruses accumulate swiftly during early HIV-1 infection.24 While previous studies have focused on intact reservoirs, recent research indicates that defective provirus can be activated to produce HIV-1 transcripts when integrated at active transcription sites,25 and it can be recognised by cytotoxic T cells.26 Proviruses escape mutations and remain stable during ART,27 leading to chronic immune activation and inflammation, which are associated with the progression of HIV-related comorbidities.28-30 Thus, it is plausible that host epigenome alterations occur in response to both intact and defective reservoirs after HIV-1 integration. Characterisation of the perturbed host epigenome by intact and defective proviruses may help us discover new targets to eradicate the reservoir.
In this study, we examined the association of DNA methylation features with HR size in CD4+ T cells (HRCD4) from a cohort of WWH who were virally suppressed on ART (N = 427). DNA samples were extracted from isolated CD4+ T cells. Using a methylation capture sequencing (MC-seq) method, we profiled DNA methylation sites across the epigenome and found 245 methylation sites associated with total HRCD4. The genes annotated for significant methylated sites were involved in HIV-1 latency, inflammation and cell differentiation and harboured HIV-1 integration sites. These findings provide new evidence on the potential roles of host epigenetic factors in HR size through type I interferon genes, immune activation and chromosome stability.
2 RESULTS
2.1 Participant characteristics and HIV-1 latent reservoir detection
The study population was derived from the Women's Interagency HIV Study (WIHS) a multi-centre interval cohort study of WWH and a comparison group of women without HIV infection in the United States.31, 32 In 2019, WIHS merged with the Multicenter AIDS Cohort Study (MACS) to form the MACS/WIHS Combined Cohort Study (MWCCS).33 Participants underwent follow-up visits every 6 months, which included the collection of peripheral blood mononuclear cells (PBMC) and demographic, clinical, and ART treatment data. The 427 participants who met the inclusion and exclusion criteria were selected for the current study (see section Methods). Demographic and clinical characteristics are presented in Table 1.
| Variable | Participants (N = 427) |
|---|---|
| Age (mean ± SD) | 47.29 ± 8.22 |
| Sex (F/M) | 427/0 |
| Ancestry (%) | |
| African American | 76.3 |
| European | 12.4 |
| Other | 11 |
| Smoking status (%) | |
| Current | 79.9 |
| Former | 8.9 |
| Never | 11 |
| Alcohol drinks per week (mean ± SD) | 9.3 ± 26.5 |
| On ART + undetected VL (%) | 100 |
| HR (mean ± SD) | |
| Total | 1409 ± 1639 |
| Intact | 279 ± 463.4 |
| 5′-depletion | 600 ± 747.7 |
| 3′-depletion | 530 ± 885.7 |
- Abbreviations: ART, anti-retroviral therapy; HR, HIV-reservoir; VL, viral load.
The average age was 47.3 ± 8.2 years; 76% of the participants self-identified as African American non-Hispanic, 12% as White non-Hispanic and 11% as other races. Most participants (80%) currently smoked tobacco. All participants were on ART and had undetectable viral loads (below the assay lower limit of quantitation) for a minimum of 6 months, with an average duration of 2.1 years, up to the visit at which CD4+ T cells were isolated from PBMCs and were processed to estimate the HRCD4. The genomic DNA extracted from CD4+ T cells was subjected to bisulphite capture sequencing for DNA methylation profiling.
The HRCD4 quantification method for this cohort was detailed previously by Aouizerat et al.34 Briefly, HRCD4 was detected using droplet digital polymerase chain reaction (ddPCR). Intact proviral HIV DNA was measured using a modified intact proviral DNA assay (IPDA). The modified IPDA included the original primer sets targeting the Psi (Y) and env regions of the HIV-1 provirus, and the addition of a primer set targeting a host gene (i.e., CD4+ T cells) to detect and adjust for DNA shearing, which results in underestimates of the HR size. The average HRCD4 size was 1409 ± 1639 copies per million CD4+ T cells. As expected, defective HIV-1 proviruses were more common than intact HRCD4. The average intact HRCD4 was 279 ± 463 copies per million; defective HRCD4 was 531 ± 886 copies per million for APOBEC3G-mediated mutations (3′-defective HRCD4) and 600 ± 748 copies per million for large internal deletions (5′-defective HRCD4).
2.2 Epigenome-wide methylation characterisation of HRCD4
CD4+ T cells epigenome was profiled using bisulphite MC-seq, yielding data on methylation of 3.2 million CpG sites. Quality control (QC) was conducted according to our previous report (see section Methods).35 Only CpG sites with a coverage greater than 10× depth were included. Approximately 2 million CpG sites were analysed after QC. Ancestry and chronological age were included in generalised linear regression models.
We found 245 differentially methylated sites associated with total HRCD4 size (all integrated virus DNA) across the epigenome (false discovery rate, FDR < 0.05) (Figure 1A and Table S1), suggesting that these regions are potentially involved in HIV-1 establishment and/or persistence Among them, 67% (n = 165) were positively associated with HRCD4 size, meaning higher methylation is linked to greater HRCD4 size, while 33% (n = 80) were inversely associated with it (Figure 1B). A substantial proportion of these methylation sites (89%) were annotated within or near genes, with the rest (11%) in intergenic regions. Within gene regions, 52% of CpG sites were in introns, 20% in promoter regions and 11% in exons (Figure 1C). Additionally, 26% of the associated sites mapped to CpG islands, with the rest in non-island regions (open sea 34%, shore 30% and shelf 9%). Notably, although most of the observed associations were positive, 50% of the methylation sites in the promoter regions were negatively associated with total HRCD4.
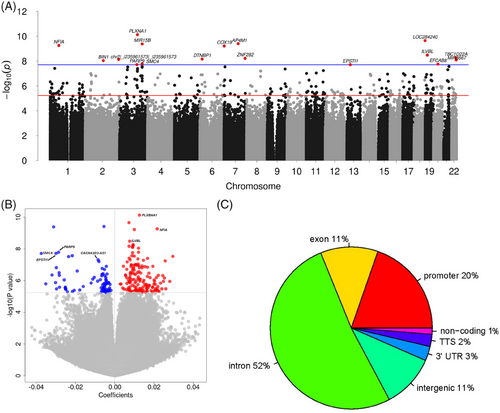
We further examined whether the total HRCD4-associated CpG sites were also significantly associated with intact and defective HRCD4. Among the 245 significant CpG sites for total HRCD4, 38 were nominally associated with intact or defective HRCD4 (p < .05). For example, SMC4 on chromosome 3 at position 160119770 was negatively associated with total (t = −5.79, p = 1.64e−08), intact (t = −4.0, p = 7.63e−05) and 5′-defective (t = −4.78, p = 2.64e−06) HRCD4. PARP9 on chromosome 3 at position 122281940 was also negatively associated with total (t = −5.76, p = 1.92e−08), intact (t = −3.19, p = 1.57e−03), 5′-defective (t = −3.66, p = 2.93e−04) and 3′-defective (t = −4.28, p = 2.39e−05) HRCD4. ISG15 on chromosome 1 at position 949432 was negatively associated with total (t = −5.14, p = 4.66e−07), intact (t = −3.39, p = 7.90e−04) and 5′-defective (t = −4.53, p = 8.24e−06) HRCD4. These genes were previously reported to be involved in interaction with HIV-1 tau protein (SMC4),36 HIV-1 induced apoptosis (PARP)37 and HIV-1 replication (ISG15),38 which may contribute to HIV-1 latency.
Notably, considering the potential impact of menopause on DNA methylation and size, we conducted a secondary analysis to examine differences in HR size and methylation levels across 245 CpG sites between pre- and post-menopausal women, as well as interactions between methylation of 245 sites and HR size by menopausal status. We found no significant differences in HR size between pre- and post-menopausal women. However, 12 out of 245 CpG sites showed nominally significant differences (p < .05) between pre- and post-menopausal women. More interestingly, 14 of 245 CpG sites exhibited interactive effects between menopause status and HR size (Table S2). For example, methylation of ELAVL2 was negatively correlated with HR among pre-menopausal women but positively correlated among post-menopausal women (p = .002) (Figure S1, left). Although there is no direct evidence linking ELAVL2 to menopause, this gene encodes an RNA-binding protein highly expressed in ovaries, testes and neurons. Genetic variants in ELAVL2 have been linked to primary ovarian insufficiency.39 Additionally, methylation of a CpG site on the promoter of LMAN2 (Lectin, Mannose Binding 2) was negatively correlated with HR among pre-menopausal women but positively correlated among post-menopausal women (p = 6.8e−04; Figure S1, right), with a nominally significant association with menopause status (p = .03). LMAN2 encodes a type I transmembrane lectin associated with poor prognosis in breast cancer.40
Similarly, to investigate whether cigarette smoking influences the association between HR and DNA methylation, we examined the interactive effects between HR and current smoking status. We identified 14 nominally significant CpG sites affected by the interaction between smoking and HR size (p < .05) (Table S3). For example, methylation of a CpG site on TNFRS13C was increased among smokers with higher HR size (Figure S2).
2.3 Differentially methylated regions were significantly associated with total HRCD4
To find significant differentially methylated regions (DMR), we conducted an analysis using the ‘bump hunting’ approach (see section Methods). This analysis found DMRs containing at least 2 CpG sites associated with total HRCD4. We found 85 significant DMRs across 22 chromosomes (Table 2). Among them, 28 DMRs were negatively associated with HRCD4 while 57 DMRs were positively associated.
| Chr | Start | End | # CpGs | Gene | t | p | FDR |
|---|---|---|---|---|---|---|---|
| 1 | 61545077 | 61545123 | 2 | NFIA | 5.4 | 1.1e−09 | 9.2e−08 |
| 3 | 160119633 | 160122505 | 12 | MIR16-2, MIR15B, SMC4 | −5.0 | 4.7e−09 | 2.0e−07 |
| 3 | 122281883 | 122281941 | 2 | DTX3L, PARP9 | −5.4 | 3.8e−08 | 1.1e−06 |
| 13 | 43565289 | 43565401 | 3 | EPSTI1 | −4.7 | 5.8e−08 | 1.2e−06 |
| 14 | 74250898 | 74250927 | 2 | ELMSAN1 | 4.8 | 2.9e−07 | 4.1e−06 |
| 3 | 54888035 | 54888057 | 6 | CACNA2D3 | −4.4 | 2.9e−07 | 4.1e−06 |
| 2 | 43398273 | 43398277 | 2 | – | 5.1 | 5.4e−07 | 6.5e−06 |
| 7 | 44924186 | 44924194 | 2 | PURB | −4.5 | 6.7e−07 | 7.1e−06 |
| 1 | 12676155 | 12676183 | 2 | DHRS3 | 5.0 | 9.4e−07 | 7.7e−06 |
| 19 | 41815093 | 41815101 | 3 | CCDC97 | 4.5 | 1.0e−06 | 7.7e−06 |
| 10 | 88852547 | 88852560 | 2 | GLUD1 | 4.6 | 1.1e−06 | 7.7e−06 |
| 1 | 119522623 | 119522631 | 2 | TBX15 | 4.5 | 1.1e−06 | 7.7e−06 |
| 1 | 949394 | 949433 | 3 | ISG15 | −4.6 | 1.2e−06 | 7.7e−06 |
| 11 | 118790174 | 118790223 | 3 | – | 4.6 | 1.4e−06 | 7.7e−06 |
| 1 | 207996299 | 207996461 | 3 | – | 4.8 | 1.4e−06 | 7.7e−06 |
| 17 | 7381527 | 7381577 | 2 | ZBTB4 | 4.5 | 2.4e−06 | 1.3e−05 |
| 21 | 42798945 | 42799143 | 7 | MX1 | −4.4 | 2.7e−06 | 1.3e−05 |
| 15 | 44829519 | 44829525 | 2 | EIF3J, EIF3J-AS1 | −4.5 | 3.0e−06 | 1.4e−05 |
| 1 | 207996636 | 207996676 | 2 | – | 4.7 | 3.3e−06 | 1.4e−05 |
| 9 | 134139856 | 134139880 | 3 | FAM78A | 4.2 | 3.3e−06 | 1.4e−05 |
| 10 | 22612799 | 22613027 | 2 | COMMD3-BMI1, BMI1 | 4.5 | 3.4e−06 | 1.4e−05 |
| 22 | 18635396 | 18635462 | 3 | USP18 | −4.4 | 3.8e−06 | 1.4e−05 |
| 16 | 4714768 | 4714796 | 3 | MGRN1 | 4.5 | 4.1e−06 | 1.4e−05 |
| 2 | 37551058 | 37551083 | 5 | – | 4.6 | 4.1e−06 | 1.4e−05 |
| 11 | 75062095 | 75062115 | 2 | ARRB1 | 4.4 | 6.3e−06 | 2.1e−05 |
| 21 | 42797710 | 42797717 | 2 | MX1 | −4.5 | 7.6e−06 | 2.4e−05 |
| 14 | 50159543 | 50159550 | 2 | KLHDC1 | 4.4 | 8.7e−06 | 2.6e−05 |
| 11 | 121324233 | 121324511 | 3 | SORL1 | 4.3 | 8.7e−06 | 2.6e−05 |
| 19 | 54876395 | 54876448 | 2 | LAIR1 | 4.2 | 9.3e−06 | 2.6e−05 |
| 1 | 12538489 | 12538543 | 4 | VPS13D | 4.4 | 9.3e−06 | 2.6e−05 |
| 1 | 949698 | 949878 | 12 | ISG15 | −4.2 | 1.1e−05 | 2.8e−05 |
| 1 | 949563 | 949599 | 4 | ISG15 | −4.3 | 1.1e−05 | 2.8e−05 |
| 15 | 69707926 | 69707938 | 2 | KIF23 | −4.4 | 1.2e−05 | 3.1e−05 |
| 17 | 47437978 | 47438153 | 3 | ZNF652, LOC102724596 | 4.2 | 1.4e−05 | 3.4e−05 |
| 15 | 55562701 | 55562788 | 4 | RAB27A | −4.1 | 1.7e−05 | 4.0e−05 |
| 22 | 27068060 | 27068092 | 4 | MIAT, MIATNB | 4.2 | 1.8e−05 | 4.2e−05 |
| 4 | 38666920 | 38667432 | 2 | KLF3-AS1, KLF3 | 4.3 | 1.8e−05 | 4.2e−05 |
| 12 | 120700363 | 120700438 | 3 | PXN | 4.0 | 2.4e−05 | 5.2e−05 |
| 9 | 6716463 | 6716482 | 4 | – | −4.2 | 2.5e−05 | 5.2e−05 |
| 17 | 79265630 | 79265645 | 2 | SLC38A10 | 4.1 | 2.5e−05 | 5.2e−05 |
| 4 | 103682822 | 103682824 | 2 | MANBA | 4.2 | 2.5e−05 | 5.2e−05 |
| 21 | 45509115 | 45509120 | 2 | TRAPPC10 | 4.3 | 2.7e−05 | 5.3e−05 |
| 11 | 614844 | 614857 | 3 | IRF7 | −4.1 | 2.8e−05 | 5.4e−05 |
| 1 | 949479 | 949508 | 3 | ISG15 | −4.2 | 2.8e−05 | 5.4e−05 |
| 10 | 90641813 | 90642005 | 3 | STAMBPL1 | −4.2 | 2.9e−05 | 5.5e−05 |
| 14 | 105751631 | 105751638 | 2 | BRF1 | 4.1 | 3.1e−05 | 5.6e−05 |
| 22 | 39637150 | 39637172 | 4 | PDGFB | 4.1 | 3.2e−05 | 5.6e−05 |
| 1 | 226069169 | 226069191 | 2 | TMEM63A | 4.1 | 3.2e−05 | 5.6e−05 |
| 19 | 4380572 | 4380611 | 2 | SH3GL1 | 4.1 | 3.3e−05 | 5.6e−05 |
| 3 | 43385089 | 43385113 | 2 | SNRK | 4.2 | 3.4e−05 | 5.6e−05 |
| 14 | 24631374 | 24631383 | 2 | IRF9 | −4.2 | 3.4e−05 | 5.6e−05 |
| 6 | 137630457 | 137630478 | 2 | – | 4.2 | 3.4e−05 | 5.6e−05 |
| 11 | 614567 | 614763 | 2 | IRF7 | −4.2 | 3.7e−05 | 5.9e−05 |
| 9 | 6716396 | 6716425 | 5 | – | −4.1 | 3.9e−05 | 6.0e−05 |
| 17 | 42462262 | 42462268 | 2 | ITGA2B | 4.1 | 4.4e−05 | 6.7e−05 |
| 20 | 62530409 | 62530421 | 2 | DNAJC5 | 4.1 | 4.7e−05 | 6.8e−05 |
| 2 | 43398215 | 43398245 | 2 | – | 4.1 | 4.7e−05 | 6.8e−05 |
| 2 | 173294414 | 173294494 | 2 | ITGA6 | 4.0 | 4.8e−05 | 6.8e−05 |
| 21 | 45509391 | 45509401 | 3 | TRAPPC10 | 4.1 | 4.8e−05 | 6.8e−05 |
| 22 | 38610341 | 38610350 | 2 | MAFF | 4.1 | 4.9e−05 | 6.8e−05 |
| 9 | 6716209 | 6716228 | 2 | – | −4.1 | 4.9e−05 | 6.8e−05 |
| 11 | 61213606 | 61213621 | 2 | SDHAF2 | 4.1 | 5.2e−05 | 7.0e−05 |
| 11 | 74975246 | 74975248 | 2 | ARRB1 | 4.0 | 5.3e−05 | 7.1e−05 |
| 17 | 7381831 | 7381857 | 2 | ZBTB4 | 4.1 | 5.6e−05 | 7.3e−05 |
| 2 | 71681539 | 71681542 | 2 | DYSF | 4.0 | 6.1e−05 | 7.9e−05 |
| 19 | 19281257 | 19281272 | 2 | MEF2BNB-MEF2B, MEF2B | 4.0 | 6.3e−05 | 8.0e−05 |
| 11 | 109962729 | 109962762 | 2 | ZC3H12C | 4.0 | 6.8e−05 | 8.5e−05 |
| 21 | 45509454 | 45509457 | 2 | TRAPPC10 | 4.0 | 6.8e−05 | 8.5e−05 |
| 14 | 61109826 | 61109857 | 2 | – | 4.0 | 7.1e−05 | 8.7e−05 |
| 4 | 85416937 | 85416953 | 2 | NKX6-1 | 3.9 | 7.9e−05 | 9.5e−05 |
| 16 | 53535169 | 53535242 | 2 | AKTIP | 3.9 | 9.0e−05 | 1.1e−04 |
| 1 | 154318280 | 154318316 | 2 | ATP8B2 | 3.9 | 9.7e−05 | 1.1e−04 |
| 8 | 133098472 | 133098504 | 2 | HHLA1 | −3.9 | 1.1e−04 | 1.2e−04 |
| 17 | 37308830 | 37308847 | 2 | PLXDC1 | 3.9 | 1.1e−04 | 1.3e−04 |
| 9 | 6716240 | 6716271 | 2 | – | −3.9 | 1.2e−04 | 1.3e−04 |
| 6 | 168714672 | 168714675 | 2 | DACT2 | 3.9 | 1.3e−04 | 1.4e−04 |
| 9 | 6716324 | 6716351 | 3 | – | −3.9 | 1.3e−04 | 1.4e−04 |
| 9 | 79791171 | 79791179 | 2 | VPS13A | 3.9 | 1.4e−04 | 1.5e−04 |
| 3 | 122401131 | 122401302 | 3 | PARP14 | −3.8 | 1.4e−04 | 1.5e−04 |
| 5 | 142435222 | 142435443 | 2 | ARHGAP26 | −3.8 | 1.5e−04 | 1.5e−04 |
| 15 | 85177989 | 85178063 | 2 | SCAND2P | 3.8 | 1.5e−04 | 1.5e−04 |
| 1 | 79090602 | 79092806 | 2 | IFI44L | −3.8 | 1.5e−04 | 1.5e−04 |
| 21 | 38065618 | 38065639 | 2 | – | 3.8 | 1.9e−04 | 1.9e−04 |
| 5 | 17219328 | 17219331 | 2 | BASP1 | 3.8 | 1.9e−04 | 1.9e−04 |
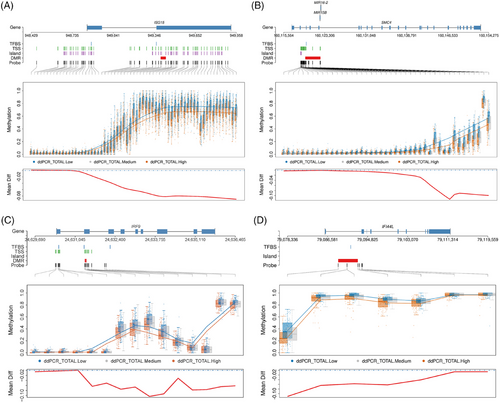
Notably, four DMRs on ISG15, spanning 21 CpG sites, were significant, including one region harbouring 12 CpG sites. All four regions on ISG15 were inversely associated with HRCD4 (Figure 2A). These CpG sites were either located in the TSS region or exons, suggesting that the aberrant methylation of ISG15 may regulate ISG15 expression, which is associated with HR. Another large significant DMR was located on chromosome 3 at SMC4, spanning 2872 base pairs and harbouring 12 CpG sites (Figure 2B). Similarly, this DMR was also inversely associated with HRCD4. Other negatively HRCD4-associated DMR were in genes such as PARP9, MX1, EPSTI1, USP18, IRF9, IFI44L and IRF7 (Figure 2C,D). Together, consistent with the findings in EWAS for individual CpG association, the results from DMR analysis further demonstrate that dysregulation of these genes (e.g. ISG15, SMC4, PARP9, MX1, EPSTI1, USP18, IRF9, IFI44L and IRF7) through methylation may indicate that HIV-1 avoids immune detection and eradication in CD4+ T cells, allowing it to maintain its latent status.
2.4 Total HIV-1 CD4+ T cell reservoir-associated DNA methylation is related to HIV-1 integration
Because half of the HRCD4-associated differentially methylated sites were located in intronic regions, we explored whether our 245 methylation sites were enriched in known HIV-1 genic integration sites by compiling HIV-1 integration sites reported across three recent studies.41-43 We found that a subset of the genes harbouring significant CpG sites were targets of HIV-1 integration. Methylation at most of these integration sites was positively associated with HRCD4 size.
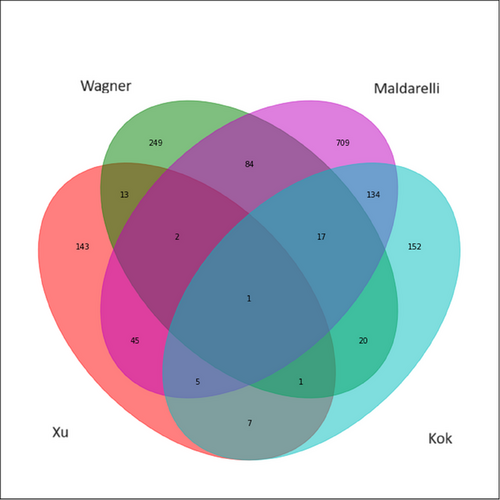
One gene, IMPDH2, was a common HIV-1 integration site between our study and the three previous studies.41-43 A CpG site on chromosome 3 at position 49066890, which was in the promoter region of IMPDH2, was positively associated with HRCD4 size. We also found that 23 HRCD4-associated genes overlapped with HIV-1 integration sites reported by Kok et al.41 Also, a few other genes were shared between our study and the other two previous studies,41, 42 including NFIA, TMTC3, SPPL3, DLEU2, ELMSAN1 and ACSF3. A total of 53 genes overlapped with HIV-1 integration sites reported by Maldarelli et al.43 (e.g., ISG15, TTC19, KCNG1, EIF3J, COX7A2L, TTC19, LAIR1, PARP9, JAK1 and CCDC97) and 17 with sites reported by Wagner et al.42 (e.g., TTBK2, CDK16, ATP5PO and TFDP1) (Figure 3).
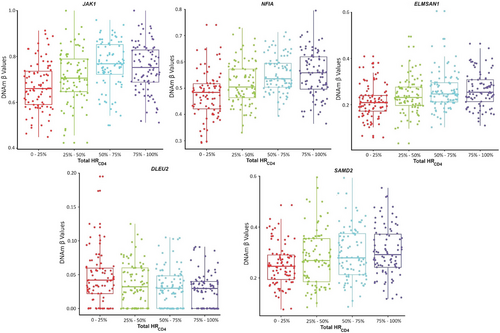
The most frequently reported HIV-1 integration site, BACH2,42, 43 was nominally associated with HRCD4 (p = .001) in our study. Additionally, several other previously reported integration sites displayed consistent patterns of differential methylation, with higher methylation levels in the top 25th percentile of total HRCD4 size compared with the lowest 25th percentile. For example, a CpG site at JAK1 had 15% higher methylation in the top 25th percentile for HRCD4 size, compared with the lowest 25th percentile (chr1 65503557, p = 5.74e−07) (Figure 4A). Similarly, two CpG sites at NFIA showed 7 and 10% higher methylation levels in the top 25th percentile (chr1 61542330, p = .00004; chr1 61545122, p = 5.49e−10) (Figure 4B). Other examples include three CpG sites for ELMSAN1 (chr14 74244367, p = 6.62e−05; chr14 74250897, p = 1.43e−07; chr14 74250926, p = .00004) (Figure 4C), two CpG sites for DLEU2 (chr13 50653658, p = .00001; chr13 506554306, p = 1.88e−06) (Figure 4D) and one CpG site for SMAD2 (chr18 45458622, p = 1.28e−05) (Figure 4E). The results indicate that aberrant DNAm on the genes involved in chromatin remodelling and maintaining genomic stability.
2.5 Total HRCD4-associated CpG sites are enriched in pathways relevant to HIV-1 latency
We performed gene enrichment analysis for the set of genes close to or harbouring 245 aberrant methylation sites using the human MSigDB database (https://www.gsea-msigdb.org/gsea/msigdb/), which includes 33591 genes from nine major collections. In the hallmark gene set, we found significant enrichment for HRCD4-associated genes in the interferon α response (gene ratio: 16/79; padj = 1.50e−10) and interferon γ response (gene ratio: 16/79; padj = 3.45e−06) (Figure 5A,B and Table 3). In the interferon α gene set, nine genes were associated with HRCD4, with eight of these overlapping between the ‘interferon α response’ and ‘interferon γ response’ gene sets. These shared genes were USP18, MX1, ISG15, IRF7, IFIT3, IFI44L, IFI27 and EPST1, all of which reached epigenome-wide significant association with total HRCD4. Four of these genes are involved in the regulation of inflammatory function (i.e., IRF7, IFIT3, IFI44L and IFI27).
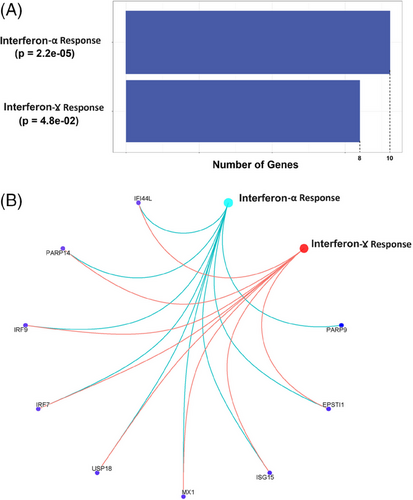
| Pathway | Gene ratio | p | q |
|---|---|---|---|
| Hallmark pathways | |||
| Interferon-α response | 10/97 | 2.2e−05 | 1.0e−03 |
| Interferon-γ response | 8/200 | 4.8e−02 | 7.7e−01 |
| GO molecular function pathways | |||
| Tau protein binding | 5/272 | 1.1e−03 | 3.0e−01 |
| Vinculin binding | 3/272 | 1.2e−03 | 3.0e−01 |
| DNA binding transcription repressor activity | 13/272 | 6.1e−03 | 6.7e−01 |
| DNA binding transcription activator activity | 16/272 | 1.1e−02 | 6.7e−01 |
| DNA binding transcription factor binding | 16/272 | 1.2e−02 | 6.7e−01 |
| Aromatic amino acid transmembrane transporter activity | 2/272 | 1.7e−02 | 6.7e−01 |
| Oligopeptide transmembrane transporter activity | 2/272 | 1.7e−02 | 6.7e−01 |
| Intermediate filament binding | 2/272 | 2.0e−02 | 6.7e−01 |
| Peptide transmembrane transporter activity | 2/272 | 2.0e−02 | 6.7e−01 |
| Amino acid transmembrane transporter activity | 5/272 | 2.0e−02 | 6.7e−01 |
| Transcription factor binding | 18/272 | 2.2e−02 | 6.7e−01 |
| Cell adhesion molecule binding | 17/272 | 2.2e−02 | 6.7e−01 |
| Organic anion transmembrane transporter activity | 8/272 | 2.3e−02 | 6.7e−01 |
| 2-Oxoglutarate-dependent dioxygenase activity | 4/272 | 2.3e−02 | 6.7e−01 |
| Glycosyltransferase activity | 10/272 | 2.3e−02 | 6.7e−01 |
| Β-1-3-Galactosyltransferase activity | 2/272 | 2.6e−02 | 6.7e−01 |
| Proton transmembrane transporter activity | 6/272 | 2.7e−02 | 6.7e−01 |
| Glycosaminoglycan binding | 9/272 | 2.9e−02 | 6.7e−01 |
| Mechanosensitive ion channel activity | 2/272 | 3.0e−02 | 6.7e−01 |
| l-Amino acid transmembrane transporter activity | 4/272 | 3.2e−02 | 6.7e−01 |
| Demethylase Activity | 3/272 | 3.2e−02 | 6.7e−01 |
| Organic Acid Transmembrane Transporter Activity | 7/272 | 3.4e−02 | 6.7e−01 |
| Cadherin binding | 11/272 | 3.4e−02 | 6.7e−01 |
| Insulin-like growth factor receptor binding | 2/272 | 3.4e−02 | 6.7e−01 |
| Heparin binding | 7/272 | 3.6e−02 | 6.7e−01 |
| Core promoter sequence-specific DNA binding | 3/272 | 3.7e−02 | 6.7e−01 |
| G-protein activity | 3/272 | 3.7e−02 | 6.7e−01 |
| Monosaccharide binding | 4/272 | 3.8e−02 | 6.7e−01 |
| Magnesium ion transmembrane transporter activity | 2/272 | 3.8e−02 | 6.7e−01 |
| Protein self-association | 4/272 | 4.2e−02 | 6.7e−01 |
| NAD+ binding | 2/272 | 4.2e−02 | 6.7e−01 |
| Opsonin binding | 2/272 | 4.2e−02 | 6.7e−01 |
| Mannose binding | 2/272 | 4.7e−02 | 6.9e−01 |
| Protein serine threonine tyrosine kinase activity | 3/272 | 4.9e−02 | 6.9e−01 |
Gene set enrichment analysis employing Gene Ontology (GO) molecular function terms resulted in 34 HRCD4-associated gene sets that were nominally significant but did not remain significant after multiple-testing correction. Nevertheless, these gene sets were highly relevant to HIV-1 pathogenesis (Figure 6A,B and Table 3). The top-ranked gene sets included ‘tau protein binding’ (p = .001), ‘vinculin binding’ (p = .001), ‘transcription factor binding’, ‘DNA binding transcription repressor activity’ (p = .006), ‘DNA binding transcription factor binding’ (p = .01) and ‘interferon binding’ (p = .02). Nine genes were enriched in more than one gene set (i.e., SMAD2, FOXK2, FOXP1, IRX3, PURB, SK1, ISL1, NFIA and TFDP1).
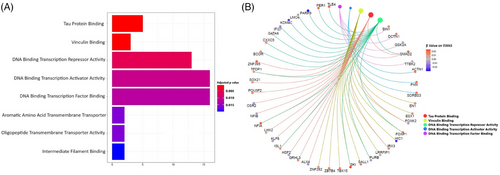
Interestingly, SMAD2 connects the ‘tau-protein binding’ pathway to the ‘DNA binding transcription factor’ and ‘DNA binding transcription activator activity’ pathways. A methylation site at SMAD2 was positively associated with HRCD4 (t = 4.43, p = 1.28e−05). SMAD2 is responsible for transmitting the signal of transforming growth factor (TGF)-beta,44 which plays a crucial role in regulating various cellular processes like cell proliferation, differentiation and apoptosis.
3 DISCUSSION
Here, we report the first epigenome-wide association study on total HRCD4, highlighting significant new insights into DNA methylation mechanisms involved in the HR. Using bisulphite capture sequencing on samples collected from 427 women with virally suppressed HIV-1 infection, we comprehensively characterised the epigenomic landscape of HRCD4 over 2 million CpG sites. We found 245 DNA methylation sites and 87 regions in CD4+ T cells associated with total HRCD4 size. Notably, genes and pathways related to significant CpG sites were involved in the host immune responses, particularly type I interferon genes and JAK1, which regulate HIV-1 replication and integration. We also observed HRCD4-associated aberrant methylation on the genes involved in chromosome stability, which may contribute to HIV-1 integration. Our results, derived from the largest sample size reported to date, reveal specific methylation mechanisms for HIV-1 persistence that may inform the potential target eradication strategies for HIV care.
Consistent with previous findings,25 we found that the majority of the integrated DNA proviruses were defective. Recent studies have reported that both intact and defective integrated HIV-1 DNA proviruses remain transcriptionally active under suppressive ART, contributing to prolonged immune activation45-47 and greater comorbidity among PWH. Epigenetic regulation of host factors plays a critical role in HIV-1 transcriptome activation and silencing. In line with our current understanding of the mechanisms of HIV-1 pathogenesis, we found epigenetic alterations in genes involved in immune defence, interactions with viral proteins and HIV-1 integration, suggesting that aberrant DNA methylation of these genes is linked to HIV-1 persistence.
Notably, we found aberrant DNA methylation of multiple type I interferon genes associated with total HRCD4 size. For example, four significant CpG sites for the interferon-induced protein ISG15 were inversely associated with HRCD4. A 445 base pairs region in ISG15 containing multiple CpG sites was significantly less methylated in the highest total HRCD4 size quartile compared with the lowest quartile. Similarly, three regions in interferon regulatory transcription factor 7 (IRF7) encompassed 20 CpG sites that were less methylated in the highest compared with the lowest HRCD4 quartile. Additionally, a region spanning 4 CpG sites on IRF9 was significantly less methylated in the highest compared with the lowest HRCD4 quartile. Other interferon-induced genes such as IFI44L, MX1, PARP9 and USP18 consistently showed an inverse relationship between CpG methylation and HRCD4 size. Interferon genes are known to function as antiviral signalling molecules, regulate immune function and induce apoptosis. As a result, it is presumed that increased expression of interferon genes due to hypomethylation may enhance restriction factor activity, resulting in limited HIV-1 replication and maintaining HRCD4 in a latent state. Furthermore, interferons also promote increased CD4+ T cell localisation and HIV coreceptor expression, potentially enlarging the reservoir through seeding other cells. Our results support this complex and critical role of interferons in HIV-1 replication and latency.48
Another notable finding is that a hypermethylated CpG site at JAK1 was associated with larger HRCD4 size. JAK1 is a critical mediator of immune activation and is involved in HIV-1 latency. Previous studies have shown that JAK1 interacts with the HIV-1 transcriptome and is essential for its life cycle. HIV-1 envelop surface glycoprotein gp12049, 50 has been found to induce the expression of JAK1 through the Tat-mediated activity of PIK3R1.51 Additionally, other studies have found that reduced expression levels of JAK1 are associated with the inhibition of HIV LTR-beta-gal activity.52 A clinical trial showed that ruxolitinib, a JAK1/2 inhibitor, significantly decreased immune activation markers in PWH and reduced HRs.53 Thus, the aberrant methylation of JAK1 in relation to HRCD4 size in this study further supports the significant role of JAK1 in HIV-1 latency.
HIV-1 genome tends to integrate into highly expressed genes and genes involved in clonal expansion and cell cycle control.41 In this study, several genes involved in HIV-1 integration and latency showed significant association with HRCD4. For example, the methylation of NFIA, a transcription factor highly expressed in HIV-1 latently infected CD4+ T cells,54 was positively associated with HRCD4 size, with the methylation being more pronounced in the highest HRCD4 size quartile.
Aberrant DNA methylation associated with HRCD4 was also discovered in genes responsible for chromosome stability, such as SMC4. SMC4 is part of a family of genes that encode the structural maintenance of chromosome (SMC) proteins, which are parts of the cohesion and condensin complexes essential for chromatid pairing and chromosome segregation during mitosis.55 Dysfunction of SMC proteins may increase vulnerability to HIV-1 integration into the host genome. They also play critical roles in regulating transcription and ATPase domains and are crucial for maintaining chromosome structure and the repair and stability of DNA. SMC4 is also a positive regulator of innate inflammatory immune responses and cell proliferation,56 which are key host factors for HIV-1 persistence. It is also believed that in T cells, SMC4 interacts with HIV-1 Tat protein, which controls HIV-1 DNA replication and nuclear architecture.36 The observed aberrant DNA methylation associated with HRCD4 size in SMC4, further supports its involvement in the establishment and maintenance of HR.
Of note, it is well documented that cigarette smoking has remarkable impacts on DNA methylome.57 We previously reported that cigarette smoking was strongly associated with DNA methylation among PWH.58 In this study, we found significant differential effects of DNA methylation on HR size between current smokers and non-smokers, suggesting that smoking may influence the relationship between DNA methylation and HR size. The causal relationship among smoking, DNA methylation and HR size warrants further investigation in a larger sample population.
We acknowledge some limitations in our study. First, we were unable to find epigenome-wide significant methylation sites specific for intact or defective reservoirs, necessitating more sensitive detection methods. Additionally, sample input requirements and technical challenges of studying HRCD4 in a large sample of PWH precluded the measurement of the functional HRCD4 (e.g., QVOA) in conjunction with IPDA. A precise measurement of HIV-1 latency requires quantifying cellular HIV-1 RNA transcription. Measuring possible HR in a subtype of CD4+ T cells in a large sample is challenging. As a result, confirming the identified methylation sites for HRCD4 using single-cell methylation profiles is needed. In addition, we are unable to rule out the potential confounding effect of cigarette smoking on DNA methylation marks, as most of the participants were smokers. Our cross-sectional study design limits the ability to assess the causal relationship between altered DNA methylation and HR size. Both longitudinal and ex vivo studies are needed to figure out the causality between DNA methylation and reservoir size. Last, this study did not include men. Future research is also needed to replicate these findings in men living with HIV and explore the dynamic interaction between the genes found in this study and the HIV-1 genome in reservoir establishment and maintenance as well as sex difference.
In summary, this comprehensive characterisation of the epigenome concerning HRCD4 sheds light on the epigenetic mechanisms underlying HIV-1 persistence. Most importantly, these findings offer new gene targets such as ISG15, PARP, NFIA and SMC4 for an in-depth exploration of host responses to the HR. These targets could potentially serve as molecular points of interest for the development of novel treatments.
4 METHODS
4.1 Ethics approval and consent to participate
The study was approved by the Committee of Human Research Subject Protection at Yale University and the Institutional Research Board Committee of the Connecticut Veteran Healthcare System. Informed consent was provided by all MWCCS participants via protocols approved by institutional review committees at each affiliated institution.
4.2 Participants and HR detection
Clinical data and specimens used in this study were collected by MWCCS.33 MWCCS is the largest observational cohort of HIV infection in the United States. The inclusion criteria were WWH who had undetectable HIV-1 viral load (below the limit of quantitation for the assay) on ART treatment for at least 6 months.59 MWCCS is a longitudinal cohort study and the cohorts span the changes in the lower limit of detection for the assay, ranging from 400 (the earliest assay) to 20 (the current assay). The different threshold was adjusted in the analytical model below. A total of 427 WWH who met the criteria were selected for the study. The average duration of ART treatment was ∼2.1 years among the participants. CD4+ T cells were isolated by negative bead selection using the DynaBeads Untouched Human CD4 kit. Genomic DNA was then extracted from CD4+ T cells using magnetic bead-based nucleic acid isolation. ddPCR was employed to measure the intact proviral HIV DNA using the modified IPDA assay as described previously by Bruner et al.,60 providing estimates of intact provirus per one million CD4+ T cells. Additional details are discussed elsewhere.34
4.3 Genome-wide DNA MC-seq
Genome-wide DNA methylation was conducted at the Yale Center for Genome Analysis according to the following steps.
4.3.1 Methyl-Seq target enrichment library prep
The concentration of genomic DNA was measured using fluorometry, while DNA quality was evaluated by determining the A260/A280 and A260/A230 ratios through spectrophotometry. DNA integrity and fragment size were confirmed utilizing a microfluidic chip analyzed on an Agilent Bioanalyzer. To prepare the whole genome sequencing libraries with indexed paired ends, the SureSelect XT Methyl-Seq kit (Agilent; part#G9651B) was used according to the manufacturer's instructions. Specifically, DNA samples exceeding 350 ng were enriched for methylation sites using the custom SureSelect Methyl-Seq Capture Library. The PCR amplification of these enriched and bisulphite-converted libraries was performed with custom-made indexed primers provided by IDT (Coralville, Iowa). The resulting dual-indexed libraries were then quantified through quantitative PCR (qPCR) using the Library Quantification Kit from KAPA Biosystems (Part#KK4854). Insert size distribution was assessed with the Caliper LabChip GX system. Samples meeting a threshold concentration of 2 ng/µL proceeded to sequencing.
4.3.2 Flow cell preparation and sequencing
Sample concentrations were adjusted to 10 nM and then loaded onto an Illumina NovaSeq flow cell, achieving a concentration sufficient for 40 million passing filter clusters per sample. Sequencing was performed using 100 bp paired-end reads on an Illumina HiSeq NovaSeq, following Illumina's standard procedures. Data from the sequencing runs were transferred to the high-performance computing cluster at the Yale Center for Genome Analysis. To ensure real-time quality monitoring, a positive control prepared from the bacteriophage Phi X library (provided by Illumina) was spiked into each lane at a concentration of 0.3%. The 10 bp dual index was read during additional sequencing cycles that automatically proceeded after the first read.
4.3.3 Preprocessing and QC
Signal intensities were converted to individual base calls during the sequencing run using the Real Time Analysis software. Sample de-multiplexing and alignment to the human genome were conducted with Illumina's CASAVA 1.8.2 software suite. The sample error rate was maintained below 1%, and the distribution of reads per sample within a lane was required to be within reasonable tolerance. QC of sequencing reads was performed following standard procedures as previously described.61 The quality of sequence data was analysed using FastQC (ver. 0.11.8). Adapter sequences and low-quality fragments at the 5′ and 3′ ends (phred score < 30) were removed using Trim_galore (ver. 0.6.3_dev).
Reads were aligned to the bisulphite-converted human genome (hg19) using the Bismark pipelines (ver. v0.22.1_dev) with default parameters.62 On average, 87% of the sequences per sample were mapped to the genome. Quality-trimmed paired-end reads were transformed into either a bisulphite-converted forward strand (C → T conversion) or a bisulphite-treated reverse strand (G → A conversion of the forward strand). Only CpG sites with a coverage depth greater than 10 were considered for the final analysis to ensure the quality of CpG sites. This resulted in a total of 427 samples and 2 078 054 CpG sites available for epigenome-wide association analysis.
Genes were annotated using the Homer annotatePeaks.pl script, including intergenic, 5′UTR, promoter, exon, intron, 3′UTR, transcription start site (TTS) and non-coding regions. CpG island, shore, shelf and open sea annotations were defined by locally developed bash and R scripts based on the genomic coordinates (hg19) of CpG islands from the UCSC genome browser. CpG shores were defined as regions up to 2 kb from CpG islands, and CpG shelves as regions up to 2 kb from CpG shores.
4.4 Identification of CpG sites associated with HR
To ensure no other cell types were represented in the genomic DNA isolated from CD4+ T cells obtained from magnetic bead separation, we first estimated the PBMC cell type proportion using the Houseman method.63 The distribution of the HIV-1 proviral DNA count (i.e., HR size) was normalised using a log10 transformation. Epigenome-wide association analysis was performed using generalised linear models, in which methylation beta value at each CpG site was modelled as the dependent variable, and total HR size was modelled as the independent variable, while also adjusting for age, self-reported race, cell type proportion and smoking status. We also adjusted the model for the lower detection limit of each HIV-1 RNA viral load assay. The significance level was set at a FDR of 0.05. CpG sites that were evaluated for significant association with total HR were then re-evaluated for association with intact HRCD4, 5′-defective HRCD4 and 3′-defective HRCD4 separately.
4.5 Identification of DNA methylation regions associated with total HR
Leveraging the bump-hunting framework,64 we analysed DNA methylation regions (as opposed to individual CpG sites) that were associated with total HRCD4. Bump hunting provides the advantage of effectively modelling measurement errors, removing batch effects and detecting regions of interest for continuous measures such as HRCD4. We modified the bump hunting pipeline of Jaffe et al.64 to increase the sensitivity for detecting methylation regions. In the regression model, instead of using methylation β value at each CpG site, we used the t-statistic value that was derived from EWAS regressed on the total HRCD4. The p value of each region was then estimated following the method proposed by Lui et al.65 A significant region was defined as having at least two consecutive CpG sites and an adjusted p < .05.
4.6 Gene set enrichment analysis
Genes adjacent to CpG sites were selected for gene set enrichment analysis. A cutoff of p < .05 was used to ensure enough genes were selected for enrichment analysis. We used the Hallmark database from the Molecular Signatures Database (https://www.gsea-msigdb.org/gsea/msigdb/) and the GO database for the enrichment analyses.
AUTHOR CONTRIBUTIONS
K. X. oversaw the study, including DNA methylation sequencing data collection, data processing, statistical analyses and manuscript preparation. X. Z. conducted data processing and analysis and contributed to manuscript preparation. B. E. A. and K. A. handled CD4+ T cell isolation, genomic DNA isolation and HIV-1 reservoir detection. B. C. Q., G. P. P. and D. B. H. participated in analytic strategy and manuscript preparation. D. K. P., H. H. B., C. D. L., E. T. G., M. H. C., N. M. A., M. H. K. and P. C. T. participated in the study's conduct, including protocol development, participant recruitment, follow-up and biospecimen collection. A. V. contributed to manuscript preparation. V. C. M. contributed to the interpretation of the findings and preparation of the manuscript. E. O. J. and B. E. A. contributed to the study design, interpretation of findings and manuscript preparation. All authors had full access to all the data in the study, had read and approved the final manuscript and had accepted responsibility to submit it for publication.
ACKNOWLEDGEMENTS
The authors appreciate the support of the Yale Center of Genomic Analysis. Data for this study were collected by the Women's Interagency HIV Study (WIHS), now the MACS/WIHS Combined Cohort Study (MWCCS). The authors gratefully acknowledge the contributions of the study participants and the dedication of the staff at the MWCCS sites. The contents of this publication are solely the responsibility of the authors and do not represent the official views of the National Institutes of Health (NIH). The project was supported by the National Institute on Drug Abuse [R61DA047011 (Johnson and Aouizerat), R33DA047011 (Johnson and Aouizerat), R01DA052846 (Xu), R01DA061926 (Xu and Aouizerat), R01DA061995 (Xu and Sinha), R01DA047063 (Xu and Aouizerat), R01DA047820 (Xu and Aouizerat), R01DA061926 (Xu and Aouizerat)], the Emory Center for AIDS Research [P30-AI050409 (Marconi)] and the MWCCS (Principal Investigators): Atlanta CRS (Ighovwerha Ofotokun, Anandi Sheth and Gina Wingood), U01-HL146241; Bronx CRS (Kathryn Anastos, David Hanna and Anjali Sharma), U01-HL146204; Brooklyn CRS (Deborah Gustafson and Tracey Wilson), U01-HL146202; Data Analysis and Coordination Center (Gypsyamber D'Souza, Stephen Gange and Elizabeth Topper), U01-HL146193; Chicago-Cook County CRS (Mardge Cohen, Audrey French and Ryan Ross), U01-HL146245; Northern California CRS (Bradley Aouizerat, Jennifer Price and Phyllis Tien), U01-HL146242; Metropolitan Washington CRS (Seble Kassaye and Daniel Merenstein), U01-HL146205; Miami CRS (Maria Alcaide, Margaret Fischl and Deborah Jones), U01-HL146203; UAB-MS CRS (Mirjam-Colette Kempf, James B. Brock, Emily Levitan and Deborah Konkle-Parker), U01-HL146192; UNC CRS (M. Bradley Drummond and Michelle Floris-Moore), U01-HL146194. The MWCCS is funded primarily by the National Heart, Lung, and Blood Institute (NHLBI), with additional co-funding from the Eunice Kennedy Shriver National Institute Of Child Health & Human Development (NICHD), National Institute On Aging (NIA), National Institute Of Dental & Craniofacial Research (NIDCR), National Institute Of Allergy And Infectious Diseases (NIAID), National Institute Of Neurological Disorders And Stroke (NINDS), National Institute Of Mental Health (NIMH), National Institute On Drug Abuse (NIDA), National Institute Of Nursing Research (NINR), National Cancer Institute (NCI), National Institute on Alcohol Abuse and Alcoholism (NIAAA), National Institute on Deafness and Other Communication Disorders (NIDCD), National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK), National Institute on Minority Health and Health Disparities (NIMHD) and in coordination and alignment with the research priorities of the National Institutes of Health, Office of AIDS Research (OAR). MWCCS data collection is also supported by UL1-TR000004 (UCSF CTSA), P30-AI-050409 (Atlanta CFAR), P30-AI-073961 (Miami CFAR), P30-AI-050410 (UNC CFAR), P30-AI-027767 (UAB CFAR), P30-MH-116867 (Miami CHARM), UL1-TR001409 (DC CTSA), KL2-TR001432 (DC CTSA) and TL1-TR001431 (DC CTSA). The funding sources had no role in the design and conduct of the study; the collection, management, analysis or interpretation of the data, the preparation, review or approval of the manuscript; or the decision to submit the manuscript for publication.
CONFLICTS OF INTEREST STATEMENT
V. C. M. has received investigator-initiated research grants (to the institution) and consultation fees (both unrelated to the current work) from Eli Lilly, Bayer, Gilead Sciences and ViiV. The remaining authors declare that they have no competing interests.
Open Research
DATA AVAILABILITY STATEMENT
Summary statistics of the epigenome-wide analysis are presented in the Supporting Information tables. Access to individual-level DNA methylation data from the MACS/WIHS Combined Cohort Study Data (MWCCS) may be obtained upon review and approval of a MWCCS concept sheet. Links and instructions for online concept sheet submission are on the study website (https://statepi.jhsph.edu/mwccs/).




