Spatial distribution of immune cells and their proximity to STING+ cells are associated with survival in glioblastoma
Hanwool Jeon, Hayeong Kang, Jihyun Im and Suin Jo Contributed equally to this work.
Dear Editor,
Glioblastoma (GBM), the most aggressive malignant tumour, is increasingly treated with immunotherapy.1-3 The stimulator of interferon genes (STING) pathway4 is key to tumour immunity and a studied target for immunotherapy.5 This study explores the immune landscape of GBM, focusing on spatial relationships between tumour-associated immune cells (TAICs)6 and STING-expressing cells, uncovering patterns linked to prognosis.
We studied 14 recurrent GBM patients using protein composition and Gene Ontology (GO) analysis and analyzed immune pathways in 69 newly diagnosed GBM patients undergoing standard therapy. Spatial analysis of cells was performed using QuPath, CytoMAP, and R, as illustrated in Figure 1A. From our proteomic analysis, we identified protein groups with marked upregulation in patients with favourable responses. A total of 99 proteins were highly upregulated in the favourable group, while 170 were more pronounced in the unfavourable group (Figure 1B). Clusters of downregulated and upregulated proteins were identified using the Benjamini-Hochberg False Discovery Rate. The expression of TMEM173, the gene encoding STING, was significantly elevated in the favourable group, as measured by protein expression using a mass spectrometer. The results were represented as an abundance ratio, and statistical significance was confirmed using the adjusted p-value. (Figure 1C). GO analysis showed that upregulated proteins in the favourable group were mainly linked to immune response activation. (Figure 1D). Pathways such as ‘regulation of innate immune response’ and ‘phagocytic respiratory burst’ were strongly linked to innate immune activation (Figure 1E). Additionally, pathways like ‘cell surface receptor signalling in immune response’, ‘T cell migration’ and ‘positive regulation of T cell receptor signalling’ were significantly linked to immune response activation (Figure 1F). p-Values were transformed to -log10 for statistical significance, with values above 1.3 considered significant. Table S1 provides the GO categories associated with immune response activation.
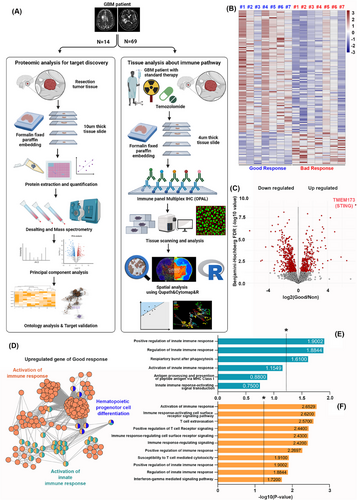
We used multiplex immunohistochemistry (IHC) to analyze immune cell distribution and protein markers in the TME. Markers included CD4+ (helper T cells), CD8+ (cytotoxic T cells), CD11c+ (dendritic cells), TCRγ/δ+ (γ/δ T cells), ATRX+ (tumour cells) and DAPI+ (nuclei), with STING as a primary marker of interest.
We explored correlations between immune cell populations identified by multiplex IHC and patient survival (Figure 2A). Tumour specimens were stained with multiplex immunofluorescence, and a pathologist selected regions of interest (ROIs), which were scanned at high magnification and analyzed with markers including ATRX, CD8, STING, DAPI, CD4, CD11c and TCRγ/δ. (Figure 2B). Multiplex IHC enabled analysis of cell interactions in the tumour microenvironment, quantifying marker expression and its impact on prognosis. Correlation analysis between marker expression and overall survival (OS) revealed a significant positive correlation between STING+ cell count and OS (R = 0.256, p = .036) (Figure 2C). No other markers showed a statistically significant correlation with OS. Linear regression analysis showed an increase in OS with higher STING+ cell counts (β = 0.481, p = .036) (Figure 2D). Table S2 summarizes linear regression results for each marker and OS. When adjusted for progression-free survival (PFS), increased STING+ cell counts were significantly linked to improved OS (β = 0.302, p = .025, R2 = 0.692) (Figure 2G). Table S3 details the results of the analysis correlating cell counts with OS, adjusted for PFS. Comparing patients with high and low STING expression revealed that only the patient with low STING expression experienced recurrence 1.5 years after surgery (Figure 2E). Using QuPath, cell coordinates and phenotypes were mapped for spatial analysis in R. Visualization showed that the patient without GBM recurrence had a higher number of STING-expressing cells (Figure 2F). These results show that multiplex IHC enables visual comparison of TME cell composition differences between patients with favourable and unfavourable outcomes.
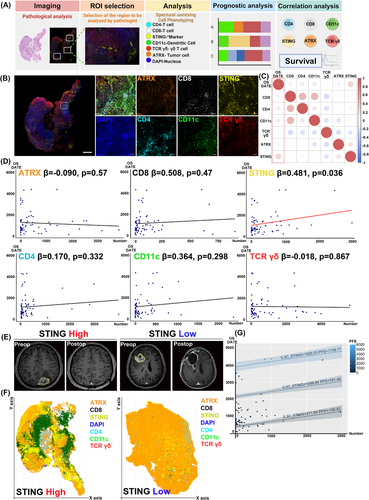
The STING pathway affects the TME by modulating immunosuppressive and immunoproliferative conditions through T cells and dendritic cells, influencing tumour progression based on the context.7 This study analyzed spatial interactions of STING+ cells, which are present in hematopoietic, endothelial, epithelial, and fibroblast cells.8 STING-expressing endothelial cells play a key role in promoting immune responses driven by macrophages and help in T cell recruitment across blood vessels.9 In the brain's microvascular structure, which comprises the blood-brain barrier (BBB), type I interferons secreted by endothelial cells enhance immune responses, impacting tumour immunity.10 This study specifically targets STING+CD4−CD8−CD11c−ATRX−TCRγ/δ− cells, which are likely endothelial cells forming the BBB.
We measured mean distances from STING+ cells to ATRX+ cells, TCRγ/δ+ cells, CD4+ cells, CD8+ cells and CD11c+ cells (Figure 3A). The correlation analysis between STING expression and immune markers in our study was conducted using an unsupervised approach. This method allowed us to identify natural patterns and relationships within the data without introducing potential biases from predefined categories or labels. From the patient group, we selected individuals with favourable or poor OS outcomes and generated positional plots of surgical samples (Figure 3B). ROIs chosen by the pathologist were further examined at high magnification, with cell coordinates derived from these plots. Voronoi diagrams were used for selected ROIs to merge clusters and simplify visual representation (Figure 3B). Patients with high STING expression showed CD4+ cells near STING+ cells and few CD11c+ cells, while low STING expression patients had CD11c+ cells near STING+ cells and scarce CD4+ cells (Figure 3B). Notably, the patient with a close spatial relationship between CD4+ and STING+ cells had a favourable prognosis, with an OS of 3371 days, whereas the patient with CD11c+ cells near STING+ cells had a poor prognosis, with an OS of 108 days.
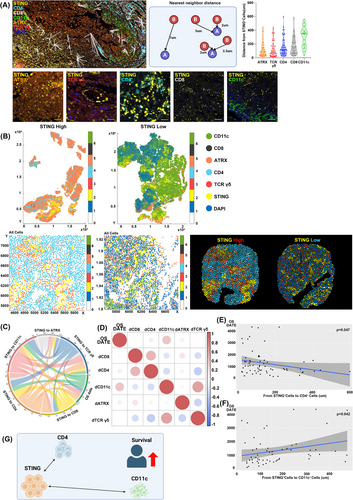
The chord diagram showed a significant link between OS and the mean distances from STING+ cells to CD4+ and CD11c+ cells (Figure 3C). Correlation analysis demonstrated a significant negative correlation between the distance from STING+ cells to CD4+ cells and OS (R = -0.089, p = .047). Conversely, a positive correlation between the distance from STING+ cells to CD11c+ cells and OS was also identified (R = 0.342, p = .042) (Figure 3D). Linear regression confirmed that shorter distances between STING+ and CD4+ cells were linked to lower OS (p = .047) (Figure 3E). Likewise, greater distances between STING+ and CD11c+ cells were associated with increased OS (p = .042) (Figure 3F).
These findings indicate a potential association between the spatial relationships of STING+ cells and nearby immune cells and survival outcomes in GBM patients. In particular, closer proximity between STING+ cells and CD4+ cells showed a trend toward improved survival, while greater distance between STING+ cells and CD11c+ cells appeared to correlate with better outcomes (Figure 3G). Given STING's dual roles in tumour promotion and inhibition, these spatial interactions may contribute to shaping the immune microenvironment in a nuanced manner.
AUTHOR CONTRIBUTIONS
Hanwool Jeon: Conceptualization, methodology, formal analysis, data curation, funding acquisition and writing and editing; Hayeong Kang: Conceptualization, methodology, formal analysis, data curation and writing and editing; Jihyun Im: Validation, visualization, formal analysis, data curation and writing and editing; Suin Jo: Methodology, validation, visualization, data curation and writing and editing; Hyunchul Jung: Resources; Moinay Kim: Resources; Jae Hyun Kim: Resources; Jeong Hoon Kim: Resources; Chang-Ki Hong: Resources; Young-Hoon Kim: Resources; Sang Woo Song: Resources; Jinha Park: Methodology and data curation; Sang-Yeob Kim: Methodology, data curation, supervision and writing and editing, Seungjoo Lee: Conceptualization, methodology, investigation, validation, formal analysis, data curation, resources, supervision, funding acquisition and writing and editing.
ACKNOWLEDGEMENTS
We thank the Clinical Proteomics Core, and the Laboratory of Animal Research at the ConveRgence mEDIcine research center (CREDIT) at Asan Medical Center for their equipment, services, and expertise.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest.
FUNDING INFORMATION
This research was supported by a grant from the Basic Science Research Program, funded by the Korean government's Ministry of Science and ICT (MSIT) (2022R1A2C2011941), Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHID), funded by the Ministry of Health & Welfare, Republic of Korea (grant number: RS-2024-00438911) to Seungjoo Lee. Additionally, support was provided by the Health Fellowship Foundation and the Korean government's Ministry of Science and ICT (2022R1C1C2002698) to Hanwool Jeon.




