Mitochondrial homeostasis orchestrates the fate of beta-cells and the outcomes of islet transplantation
In addition to producing energy, mitochondria are also key regulators of cell survival, death and immune signalling. This is particularly evident in the pancreas, where mitochondrial function governs the fate of both acinar cells and insulin-producing beta-cells, especially under inflammatory or stressed conditions. Over recent years, increasing attention has been directed towards investigating the role of mitochondrial dynamics (fusion, fission, mitophagy and biogenesis) in shaping disease outcomes for metabolic diseases, including pancreatitis, autoimmune diabetes and islet transplantation.1 In this regard, the study by Cobo-Vuilleumier et al. provided a valuable new layer of understanding by showing that the nuclear receptor liver receptor homolog-1 (LRH-1/NR5A2) reprograms both macrophages and dendritic cells towards an immune-tolerant phenotype by modulating mitochondrial functionality.2 This research identified mitochondria as a central checkpoint not only for innate immune activation but also for the preservation of beta-cells. The authors demonstrated that pharmacological activation of LRH-1 in myeloid cells triggered mitohormesis, characterised by a suppression of oxidative phosphorylation, slight enhancement of glycolytic activity, and induction of activating transcription factor 4 (ATF4)–growth differentiation factor 15 (GDF15) signalling. Consequently, pro-inflammatory macrophages and dendritic cells were converted into tolerogenic phenotypes that mitigated local immune aggression against islets in models of autoimmune diabetes. These findings suggest that reprogramming immune cell metabolism via mitochondrial remodelling could serve as a novel strategy for the restoration of islet tolerance in type 1 diabetes (T1D). Importantly, this mitochondrial-driven form of immunomodulation may synergise with other interventions that protect the islets.
Of particular interest is the vulnerability of pancreatic beta-cells to mitochondrial stress. These cells are metabolically demanding and rely heavily on mitochondrial ATP production to couple glucose sensing with insulin secretion. Unlike many other cell types, beta-cells exhibit relatively low levels of endogenous antioxidant enzymes, making them especially prone to oxidative damage.3 In a recently published study, Amo-Shiinoki et al. provided compelling evidence that mitochondrial dysfunction is a central driver of beta-cells dedifferentiation in Wolfram syndrome (WS), which is caused by mutations in the endoplasmic reticulum (ER)-resident protein WFS1.4 The authors demonstrated that Wfs1-null beta-cells undergo dedifferentiation rather than apoptosis, marked by loss of mature beta-cells markers (e.g., MafA) and re-expression of progenitor genes (e.g., Aldh1a3, Neurog3). Notably, these changes were accompanied by significant metabolic remodelling: impaired glycolysis-TCA coupling, reduced ATP production, and increased phosphorylation of pyruvate dehydrogenase, despite preserved mitochondrial oxidative capacity. These alterations reflect a state of functional mitochondrial insufficiency, which critically undermines insulin secretion and beta-cells identity. Importantly, the deletion of the redox regulator thioredoxin-interacting protein (Txnip), which was upregulated by ER stress, restored glycolytic flux, ATP production, and beta-cells maturity, thereby preventing diabetes progression. These findings reinforce the concept that mitochondrial homeostasis is not merely supportive but determinative of beta-cells functionality and survival, and its disruption can lead to dedifferentiation and irreversible β-cell failure, as seen not only in genetic diabetes but also with implications for islet transplantation.
Our group has previously demonstrated that mesenchymal stem cells (MSCs) exert a protective effect on islets, specifically via the modulation of ER stress.5 In our recent study (unpublished), we used a co-transplantation model in which extracellular vesicles (EVs) derived from human umbilical cord mesenchymal stem cells (hucMSC-EVs) were delivered with primary pancreatic islets under the renal capsule of streptozotocin-induced diabetic mice. We found that hucMSC-EVs significantly improved islet survival, fine-tuned communication between the ER and mitochondria, restored mitochondrial ultrastructure and preserved the functionality of beta-cells. In addition, hucMSC-EVs alleviated ER stress markers (C/EBP homologous protein, CHOP; binding immunoglobulin protein, Bip) and reduced apoptosis in beta-cells. Our data support a model in which MSCs act not only as immune modulators but also as mitochondrial protectors to preserve beta-cells function and improve islet engraftment. When combined with insights from Cobo-Vuilleumier et al., it becomes evident that the dual modulation of both immune cell metabolism and beta-cells mitochondrial integrity is essential for optimal outcomes in islet transplantation. These findings are particularly relevant in the clinical setting. Early after islet transplantation, the graft is exposed to hypoxia, nutrient deprivation, ischemia-reperfusion injury and innate immune activation.6, 7 These stressors lead to rapid mitochondrial dysfunction, triggering the apoptosis or necrosis of beta-cells and resulting in the loss of primary grafted islets. Strategies that enhance mitophagy, stabilise ER stress signalling, or deliver mitochondrial support, either pharmacologically or via cell-based therapy such as MSCs, may significantly improve the survival of islets during this critical period (Figure 1).
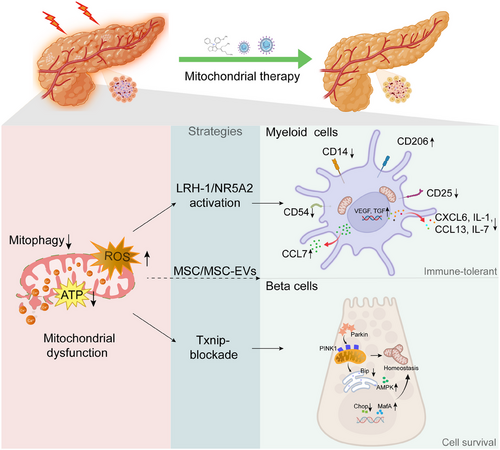
From a translational perspective, targeting mitochondrial pathways offers several advantages. First, mitochondrial regulators, such as LRH-1, can be pharmacologically activated, and agonists are currently under investigation for metabolic diseases.8 Second, MSC-based products (including EVs) have proved to be safe in early-phase clinical trials and could be engineered to deliver mitochondria-targeted cargos.9 Third, mitochondrial health represents a converging point for diverse pathways (e.g., oxidative stress, ER stress and immune signalling), making the mitochondria a highly integrative therapeutic target.10
In conclusion, mitochondrial homeostasis emerges as a unifying mechanism that governs the fate of both pancreatic cells and immune regulators of inflammation. The research by Cobo-Vuilleumier et al. elegantly demonstrates how reprogramming mitochondrial function in immune cells can establish tolerance and protect beta-cells. When considered alongside MSC-mediated mitochondrial rescue strategies, a strong case emerges for integrative approaches targeting mitochondria to improve the outcomes of islet transplantation and halt the progression of pancreatic inflammatory diseases. Future research should continue to elucidate the crosstalk between mitochondrial dynamics, immune signalling, and islet function, with the goal of translating these insights into clinical therapies.
AUTHOR CONTRIBUTIONS
BC Kuang, YY Zhao, Conceptualization, Literature Review, Writing Original Draft; NQ Gong Writing Review & Editing, Supervision.
ACKNOWLEDGEMENTS
The National Natural Science Foundation of China (82370759, 82170772 and 81873623), Major State Basic Research Development Program of China (2013CB530803), Clinical Research Physician Program of Tongji Medical College (2018PT32018).
CONFLICT OF INTEREST STATEMENT
The authors declare no conflicts of interest.
ETHICS STATEMENT
Not applicable.




