AAV gene therapy vectors in the TMJ
Abstract
Objectives
The goal of this project was to evaluate the use of two adeno-associated viral vector serotypes, adeno-associated viral vectors (AAV)-2 and AAV-6, approved for and used for gene therapy in humans, for the delivery of therapeutic genes to the temporomandibular joint (TMJ) and the attendant sensory nerves.
Methods
Young adult wild-type C57BL/6 mice were intra-articularly inoculated with AAV-2 and AAV-6 encoding the reporter gene gfp, the expression of which was assessed in the TMJ as well as along nerves innervating the TMJ.
Results
AAV-2 and AAV-6 serotypes were characterized by varying levels of tissue tropism demonstrating different efficacy of infection for articular chondrocytes, meniscal fibroblasts, and trigeminal neurons. Specifically, AAV-2 infected both neurons and articular chondrocytes/meniscal fibroblasts, whereas AAV-6 showed selectivity primarily for neurons.
Conclusions
The results of this study are clinically significant in the successful application of gene therapy vectors for TMJ disorders, as this new knowledge will allow for appropriate targeting of specific therapeutic genes to selective tissues (neurons vs. chondrocytes/fibroblasts) as needed by using specific viral vector serotypes.
1 INTRODUCTION
Gene therapy is a treatment that involves the administration of specific nucleic acids to modify gene expression in patients. The concept of gene therapy is almost 40 years old, and the first meaningful studies were done around 30 years ago (Rosenberg et al., 1990). Recent advances in transfer vectors have led to the approval of two gene therapies by the Food and Drug Administration (FDA), including Luxturna in 2017 for inherited retinal dystrophy and Zolgensma in 2019 for spinal muscular atrophy. Moreover, there are approximately 900 investigational new drug (IND) applications for gene therapy clinical studies. Many of these gene therapies have been developed based on adeno-associated viral vectors (AAV).
After more than 25 years of development, gene therapy for arthritis has finally entered clinical practice. In South Korea, gene therapy was approved for the treatment of osteoarthritis, and other gene therapeutics are in the pipeline elsewhere (Deviatkin et al., 2020; Evans et al., 2018). Gene therapy has been tested in joint dysfunction and arthritis animal models. Opioid precursor genes, such as enkephalins and β-endorphin or its receptors, have been successfully transferred to afferent sensory neurons via intrathecal administration, as well as direct injections into dorsal root ganglia or the sciatic nerve (Beutler et al., 1995; Braz et al., 2001; Finegold et al., 1999; Goss et al., 2001; Guedon et al., 2015; Ogawa et al., 2018; Pohl & Braz, 2001; Towne et al., 2009; Xu, Gu, Li, et al., 2003, Xu, Gu, Xu, et al., 2003). These studies resulted in enhanced production of opioid peptides or receptors, and a reduction of hyperalgesia in various nociceptive models. However, despite efficacious outcomes, these previous studies depended on direct injections of viral vectors into sensory ganglia or the spinal cord, and neurosurgical procedures that are invasive in nature and associated with possible morbidity. Moreover, intrathecal, intra-ganglionic, or nerve fiber injections do not address the location of pain origin, but rather, randomly cover an expansive area of sensory input.
With the emergence of gene therapy for joint disorders, it is plausible that in the near future similar treatments will become available for temporomandibular joint disorders. In a mouse model of temporomandibular joint (TMJ) inflammation (Lai et al., 2006), we previously developed and tested an experimental feline immunodeficiency viral (FIV) vector for the transfer of the human μ-opioid receptor (Kyrkanides et al., 2007). However, FIV vectors have yet to be approved by the FDA for use in humans. Li et al. (2009) reported effective infection of the TMJ disc, glenoid fossa, and condylar cartilage by AAV2 serotype vector transferring the reporter gene eGFP. However, it is unclear from their work whether AAV2 can successfully infect trigeminal neurons. Based on the work of Xu, Gu, Li, et al. (2003) and Xu, Gu, Xu, et al. (2003), we know that AAV2 is neurotropic when injected directly into ganglia or neuronal bundles, but it is not known whether this vector can infect neurons via their free nerve endings.
The goal of this project was to evaluate the use of two different AAV serotype vectors approved for and already used for gene therapy in humans for the delivery of therapeutic genes to the TMJ, in particular AAV2 and AAV6. To accomplish our goal, we employed different serotypes of the AAV vector, transferring the reporter gene green fluorescent protein (gfp) to the TMJ of mice via intra-articular administration. Our study closes the scientific gap in this area with the generation of new knowledge that will allow the development of gene therapies for TMJ disorders.
2 MATERIALS AND METHODS
2.1 Viral vectors
The adeno-associated viral vectors (AAV) transfer vector for the reporter gene green fluorescent protein (gfp) was constructed by Wang et al. (2003) and packaged for this study by the Core Facility of the National Institute of Drug Abuse in different serotypes (type -2 and -6,) as previously described (Howard et al., 2008), and kindly donated to S. K.
2.2 Vertebrate animals
The protocols, including vertebrate animals, were reviewed and approved by the University of Rochester IACUC (UCAR Protocol # 2003-191). A total of five male and five female wild-type C57BL/6 mice were purchased from JAX (Bar Harbor, ME) and housed in the University of Rochester Vivarium facility for 2 weeks for acclimation. The mice (N = 5) were randomly assigned to two groups, each to test a different AAV serotype (types -2 and -6).
2.2.1 Anesthesia
Ketamine (40 mg/kg) administration via intraperitoneal injections was used for anesthesia.
2.2.2 Virus administration
Mice were injected with 10 µl of an aqueous solution containing 1012 infectious AAV particles using a fine needle (33 GA) under a surgical plane of anesthesia. The needle was inserted into the TMJ from a posterior-inferior direction and solutions were injected into the superior joint space. The mice consistently showed no signs of distress or discomfort. The mice were returned to their cages after viral inoculation.
2.2.3 Hazard control
Personnel observed a BSL-2 level of precaution when injecting viral particles into suitably anesthetized animals, and the skin of the injected animals was treated with alcohol/iodine to inactivate any potential virus on the skin surface before removal from the laminar flow hood. All materials related to the injections were disposed of in biohazard-approved receptacles and autoclaved in accordance with Biosafety Office's recommendation. All genes and vectors used were registered with the Institutional Biosafety Committee. In addition, our laboratory underwent annual inspections by the institutional chemical hygiene officer and complied with NIH and institutional guidelines.
2.2.4 Euthanasia
The mice were euthanized 5 weeks after intra-articular TMJ inoculation by CO2 inhalation followed by decapitation.
2.3 TMJ histopathology
After the mice were euthanized, their heads were harvested, de-fleshed, and immersed in 10% formalin solution for fixation. Subsequently, the specimens were decalcified in ethylenediaminetetraacetic acid solution, processed, and paraffin-embedded. Histology TMJ sections were cut and collected onto glass slides, deparaffinized, and subsequently analyzed as previously described (Lai et al., 2006). Serial parasagittal sections collected every 100 µm covering the entire TMJ condyle were evaluated under ×40 magnification. First, the TMJ sections were stained by H&E. Second, GFP protein was detected by immunohistochemistry using a rabbit anti-gfp polyclonal antibody as previously described (Fiorentino et al., 2008). The sections were then viewed under an Olympus BX51 light microscope for the presence of immune-positive structures. Images were captured using an attached CCD digital camera.
2.4 Brain stem and trigeminal ganglia analyses
After the mice were euthanized, their brain stems and trigeminal ganglia were harvested and fixed by immersion into 10% formalin solution (Fiorentino et al., 2008). In brief, brain stems were sectioned horizontally at 18 µm on a freezing cryostat. Sections every 180 μm collected serially on glass slides covering the entire region of interest (−5 mm to +10 mm relative to obex for the brain stem and the entire trigeminal ganglia) were included from each animal. The sections were then analyzed under an Olympus BX51 fluorescent microscope for the presence of GFP+ cell bodies. Images were acquired using an attached CCD digital camera.
3 RESULTS
3.1 AAV serotype tropism toward TMJ articular tissues
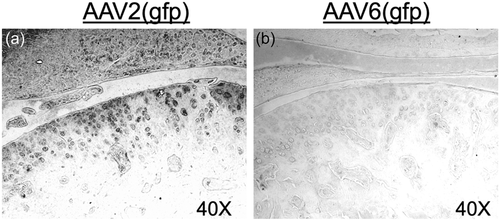
Immunohistochemical analysis of TMJ histology sections for the reporter gene gfp revealed that AAV-2, but not AAV-6, serotype is capable of infecting the articular chondrocytes and meniscal fibroblasts (Figure 1).
3.2 AAV serotype tropism toward nerve fibers in the TMJ
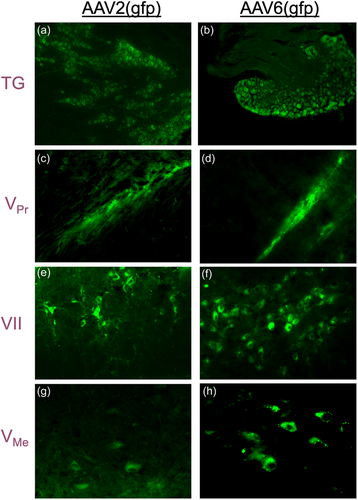
Analysis of gfp expression under a fluorescent microscope revealed that both AAV-2 and AAV-6 serotypes effectively infected trigeminal nerves after intra-articular administration, presumably through uptake by the free nerve endings, with evidence of retrograde transport of the transfer vector to nuclei in the neuronal cell bodies located in the Gasserion ganglion of the trigeminal nerve (TG) and the facial nerve nucleus (VII) for the facial nerve. Fluorescence of GFP was also detected along the trigeminal axis, including neuronal cell bodies located in the trigeminal ganglia and proximally in the principal trigeminal nucleus (VPr) and the mesencephalic trigeminal nucleus (VMe). Remarkably, AAV-2 and AAV-6 efficiently infected both trigeminal and facial nerve nerves (Figure 2), as GFP was found to be expressed in the trigeminal ganglion (TG) as well as the nucleus of the facial nerve (VII).
4 DISCUSSION
Advances in AAV vector safety and efficacy have enabled the FDA to recently approve two gene therapies, including Luxturna in 2017 for inherited retinal dystrophy and Zolgensma in 2019 for spinal muscular atrophy. Moreover, a gene therapy was approved for the treatment of osteoarthritis and has entered clinical practice in South Korea. Predictably, gene therapy for TMJ disorders is in the foreseeable future. To this end, previous work in our laboratory using FIV vectors (Kyrkanides et al., 2007) demonstrated, in a mouse model of TMJ inflammation (Lai et al., 2006), that infection of TMJ tissues and trigeminal nerve endings after intra-articular administration significantly alleviated the attendant nocifensive behavior. Interestingly, we also observed amelioration of the attendant hard tissue pathology (Kyrkanides et al., 2007), which resulted in part from gene therapy inhibition of neurogenic inflammation in the TMJ (Fiorentino et al., 2008). However, FIV vectors are not FDA approved for human use, whereas AAV vectors are. The goal of this research project was to assess the effectiveness of AAV vectors of different serotypes in transferring exogenous genes to the TMJ and the free nerve endings therein.
We employed two different AAV serotypes in our experiments, AAV-2 and AAV-6. Interestingly, our results demonstrate different levels of tropism for different cell types for these vectors. Specifically, we found that AAV-2 can effectively infect articular chondrocytes, meniscal fibroblasts, and trigeminal sensory nerves via intra-articular injection into the TMJ. In contrast, AAV-6 showed a selective preference for neurons, but not for articular chondrocytes or meniscal fibroblasts.
Our results further demonstrate that both AAV-2 and AAV-6 efficiently transduce trigeminal neurons with the reporter gene gfp following intra-articular inoculation, presumably after infecting free nerve endings (Figure 3). Previous studies reported such neurotropism of AAV-6 in rat neurons cultured in vitro (Howard et al., 2008). However, we are the first to describe the ability of viral vectors, including FIV (Kyrkanides et al., 2004) in the past and AAV here, to exhibit retrograde uptake by sensory nerve endings in vivo. In contrast, previous attempts required intra-neuronal or intra-ganglionic administration, which are both clinically challenging approaches. Conversely, intra-articular injections into the TMJ are routinely performed by oral surgeons.
Our results are very significant for the successful clinical application of AAV vectors, as they will allow scientists and clinicians to selectively target therapeutic genes to specific tissues (nerves vs. chondrocytes/fibroblasts) as needed. For example, the transfer of a therapeutic transgene to sensory neurons may be beneficial for treating TMJ disorders, but at the same time may be detrimental if transferred to articular chondrocytes and/or meniscal fibroblasts. Taken together, our findings provide important information for making relevant educated decisions when designing gene therapy strategies for the TMJ. In conclusion, AAV vector tissue tropism is an important characteristic, knowledge of which provides a significant advantage in designing gene therapy strategies for TMJ disorders.
As for the next steps, now that the tropism of the AAV-2 and AAV-6 vectors has been identified, we will clone the human μ-opioid therapeutic gene as previously developed (Kyrkanides et al., 2007) into the AAV vectors and evaluate their therapeutic efficacy in alleviating orofacial nociception after intra-articular administration in the TMJ, and its biodistribution to off-target sites in the mouse (Lai et al., 2006) as previously described (Kyrkanides et al., 2007). These data will subsequently be employed in preparing an IND application with the FDA, which when approved, will allow us to proceed with Phase-I clinical trials in human patients.
AUTHOR CONTRIBUTIONS
Sabine M. Brouxhon, Michael Kerry O'Banion, and Stephanos Kyrkanides participated in the execution of the work, analysis of the data, and composition of the manuscript, whereby Stephanos Kyrkanides served as the lead investigator.
ACKNOWLEDGMENTS
We would like to thank Dr. Ross H. Tallents and Ms. Jen-nie H. Miller for their assistance in animal care, injection of the viral vectors, animal euthanasia, and tissue processing. We would also like to express our gratitude to Dr. Brandon Harvey, Chief, Molecular Mechanisms of Cellular Stress and Inflammation, and Director of the Transgenic Rat Project, who packaged the AAV vectors at the National Institute for Drug Abuse/National Institute of Health. This study was funded in part by NIH grants AR055035 and DE017765 to S. K., as well as start-up funding provided to S. K. from the Eastman Dental Foundation through the University of Rochester Eastman Institute for Oral Health.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
Open Research
DATA AVAILABILITY STATEMENT
Data will be made available to investigators' requests.




