Synaptogenesis and synaptic protein localization in the postnatal development of rod bipolar cell dendrites in mouse retina
Funding information: This work was supported by NRSA F32 EY-024815 to I.A.A, R00 EY-022910 to F.A.D., EY-17101 to R.OL.W., NEI Morphology Resource Core (P30 EY-002162), Research to Prevent Blindness Career Development Award and Unrestricted Grant to the Department, Kirchgessner Foundation, E. Matilda Ziegler Foundation, and Klingenstein-Simons Award.
Abstract
Retinal responses to photons originate in rod photoreceptors and are transmitted to the ganglion cell output of the retina through the primary rod bipolar pathway. At the first synapse of this pathway, input from multiple rods is pooled into individual rod bipolar cells. This architecture is called convergence. Convergence serves to improve sensitivity of rod vision when photons are sparse. Establishment of convergence depends on the development of a proper complement of dendritic tips and transduction proteins in rod bipolar cells. How the dendrites of rod bipolar cells develop and contact the appropriate number of rods is unknown. To answer this question we visualized individual rod bipolar cells in mouse retina during postnatal development and quantified the number of dendritic tips, as well as the expression of transduction proteins within dendrites. Our findings show that the number of dendritic tips in rod bipolar cells increases monotonically during development. The number of tips at P21, P30, and P82 exceeds the previously reported rod convergence ratios, and the majority of these tips are proximal to a presynaptic rod release site, suggesting more rods provide input to a rod bipolar cell. We also show that dendritic transduction cascade members mGluR6 and TRPM1 appear in tips with different timelines. These finding suggest that (a) rod bipolar cell dendrites elaborate without pruning during development, (b) the convergence ratio between rods and rod bipolar cells may be higher than previously reported, and (c) mGluR6 and TRPM1 are trafficked independently during development.
1 INTRODUCTION
The sensitivity of vertebrate vision is made possible by the rod photoreceptors’ ability to detect a single photon and transmit that signal (Baylor, Lamb, & Yau, 1979). In the retina, vision at low light levels is mediated by the primary rod bipolar pathway (Dacheux & Raviola, 1986; Smith, Freed, & Sterling, 1986). In this pathway, absorption of a single photon by rod outer segments is transformed into graded reduction of glutamate release at rod terminals, which is detected by postsynaptic rod bipolar cells. Synapses with rods are established at the dendritic terminals, or tips, of rod bipolar cells (Freed, Smith, & Sterling, 1987). At these tips, glutamate detection begins with the metabotropic glutamate receptor, mGluR6, followed by amplification through a coupled G-protein, and ending downstream through the closure of transient receptor potential melastatin-1 cation channel, TRPM1 (Shen et al., 2009; Morgans, Brown, & Duvoisin, 2010; Nawy & von Gersdorff, 2011). Recent serial electron microscopy (EM) reconstructions of rod-to-rod bipolar cell synapses show ∼25 rods contacting a single rod bipolar cell (Tsukamoto, Morigiwa, Ueda, & Sterling, 2001; Tsukamoto & Omi, 2013). The convergence between multiple rods and a single rod bipolar cell is important for proper retinal function. Convergence depends on the development of a proper complement of dendritic tips and transduction proteins in rod bipolar cells. How this convergence ratio is obtained during development is unknown.
Because of practical limitations, the development of the rod bipolar cells’ dendritic trees and the temporal expression patterns of transduction proteins within them have not yet been examined on the level of individual cells. For instance, rod bipolar cells comprise more than a quarter of all bipolar cells and their density is an estimated ∼15,000/mm2 (Jeon, Strettoi, & Masland, 1998; Wassle, Puller, Muller, & Haverkamp, 2009). In addition to their large population, the dendritic trees of rod bipolar cells overlap with each other (Tsukamoto & Omi, 2013). High density and overlap make it difficult to separate the dendrites from an individual rod bipolar cell. Previously, EM reconstruction has been used to elucidate connection with rods and determine synaptic architecture. However, while precise localization of proteins within fine structures is possible using immuno-gold EM methods, combining immuno-localization and serial EM reconstruction is extremely difficult. To address these issues, we used retinas from Grm6-tdTomato transgenic mice, in which only a subset of all rod bipolar cells expresses the fluorescent protein tdTomato (Kerschensteiner, Morgan, Parker, Lewis, & Wong, 2009). This line enables visualization of the dendritic trees of individual rod bipolar cells without overlap from neighboring cells.
In the present study, we examined the early postnatal development of rod bipolar cell dendritic tips to determine the process by which rod bipolar cells contact the mature number of presynaptic neurons. Specifically, we wanted to determine if rod bipolar cells establish contacts with excess rods and then prune a subset of these connections (i.e., relationship between number of rod contacts over time that is not monotonic), or if rod bipolar cells establish connections with the appropriate number of rods without pruning (i.e., relationship between number of rod contacts over time that is monotonic). We show here that the number of dendritic tips monotonically reaches an asymptote by P30 and does not change significantly through P82. Our account of dendritic tips and their association with rods demonstrates that the convergence between rods and rod bipolar cells may be greater than previously reported. We also investigated the temporal expression patterns of mGluR6 and TRPM1 in dendritic tips. We present evidence suggesting that the appearance of TRPM1 is delayed compared to mGluR6, and that these proteins are trafficked independently.
2 MATERIALS AND METHODS
2.1 Animals
Retinas were taken from the Grm6-TdTomato transgenic mouse line on a C57BL/6J background at different stages of postnatal development (Kerschensteiner et al., 2009). A subpopulation of rod bipolar cells expresses tdTomato at high levels, which allows for their easy identification from the rest of the rod bipolar cell population. To facilitate recognition of individual cells, only retinas with high signal-to-noise ratio of tdTomato expression were selected for imaging. To avoid the spatial gradient of development, we selected bipolar cells within the central 1/3 of the retina.
2.2 Tissue preparation
All animal handling and euthanasia followed guidelines of the Institutional Animal Care and Use Committee at the University of California, San Francisco under approved protocols. Retinas were isolated from enucleated eyes, flat-mounted on filter paper (Millipore) and fixed at room temperature with 4% PFA for 15 min. For immunohistochemistry, retinas were incubated overnight at 4°C in blocking solution (5% Normal donkey serum + 0.5% Triton X-100). Blocking was followed by incubation in primary antibodies for 5 days at 4°C and secondary antibodies for 1 day at 4°C. Washes with PBS followed each antibody incubation. The following primary antibodies were used: sheep anti-mGluR6 (1:200 gift from Catherine Morgans; Morgans, Ren, & Akileswaran, 2006 and Kirill Martemyanov; Cao, Posokhova, & Martemyanov, 2011), mouse anti-TRPM1 (1:50 gift from Theodore Wensel and Melina Agosto; Agosto et al., 2014), rabbit anti-Ribeye (1:200 Synaptic Systems #192103), rabbit anti-PKCα (1:200 Sigma-Aldrich #P4334). The following secondary antibodies were used: donkey anti-sheep Alexa-405, donkey anti-mouse Alexa-405, donkey anti-rabbit Alexa-488, donkey anti-mouse Alexa-488, donkey anti-mouse Alexa-647, donkey anti-sheep Alexa-633, donkey anti-sheep DyLight-405, donkey anti-mouse DyLight405 (all at dilution 1:500 Molecular Probes and Jackson ImmunoResearch).
2.3 Imaging
Retinas were oriented and imaged with the ganglion cell side up. Flat mounted retinas were imaged on a Zeiss LSM 700 confocal microscope with an oil-immersion Zeiss 63X 1.4NA objective. Voxel sizes were either 0.05 µm or 0.1 µm per pixel (x, y-axes) and 0.2 µm or 0.4 µm per pixel (z-axis). Each optical section of a z-stack acquisition was averaged 2–4 times. Super-resolution confocal imaging with deconvolution (Huygens, SVI) was performed on select samples using the Leica TCS SP8 HyVolution 2 and the Zeiss AiryScan LSM 880. Voxel sizes were 0.035 µm per pixel (x, y-axes) and 0.13–0.16 µm/pixel (z-axis).
2.4 Analysis
Images were median filtered by the surrounding 3 pixels and processed with ImageJ and Amira (Mercury Computer Systems). A 3D binary representation of the dendrites and soma of each fluorescently labeled rod bipolar cell was created by selecting a frame-by-frame intensity threshold and manually tracing the tdTomato signal. This process resulted in a “mask” for each cell (Figure 1c). Based on the mask and the z-depth, a dendritic contour heat map of each cell was obtained in Matlab (MathWorks, MA) by assigning different color values to the z positions of a confocal stack (Figure 1e). Subsequently, fluorescence from protein staining in the remaining acquisition channels was multiplied with the masked cell, extracting only the signal overlapping with the mask. This allowed us to isolate mGluR6 and TRPM1 for each individually masked cell.
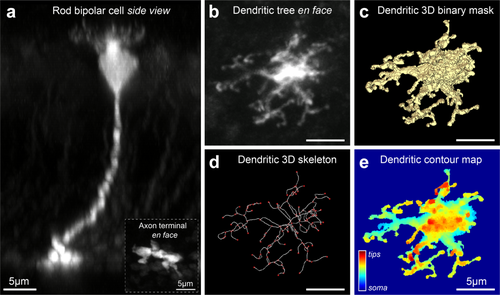
Identification and analysis of dendrites in individual rod bipolar cells. (a) Side view of a rod bipolar cell in the Grm6-tdTomato mouse line at P30. Maximum intensity projection of a confocal image from a flat mount retina. Background fluorescence is from weakly labeled ON bipolar cells. Inset shows the bulbous axon terminal of the cell in the innermost portion of the inner plexiform layer (IPL). (b) En face view of the dendritic tree of the same cell. (c) Three-dimensional binary representation of the bipolar soma and dendrites from the confocal image shown in (b), referred to as ‘mask’ in the main text. (d) Dendritic skeleton generated from the 3D binary representation of the cell in (c). Skeleton consists of nodes (red) and segments (white). (e) Heat map of dendritic tree and soma in which z-position is indicated by color
Dendritic tip counts at each developmental stage were obtained from masked cells in several sequential steps: Step 1. A 3D spatial graph representation (referred to as “skeleton”) of each cell was obtained from the constructed 3D binary representation (referred to as “3D mask”; Figure 1d). The features of the skeleton represented a first approximation of the number of tips for each cell. Step 2. A manual correction of the skeleton tip approximation was performed to remove false positive tips. Step 3. From the cell 3D mask, a manual annotation of obvious tips not identified by step 1 and not representing a false positive from step 2 was performed giving the final tip count for each cell. The same procedure was followed when analyzing tip numbers from super resolution data, DiI filling, and serial EM reconstruction data (generously provided by Christian Behrens and Timm Schubert); see Figure 2f.
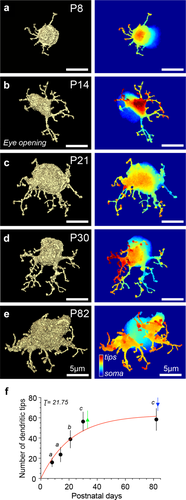
Rod bipolar cell dendrites elaborate during postnatal development. (a–e) En face view of rod bipolar cell 3D binary mask at indicated postnatal days (P) with a corresponding dendritic contour map on the right. Somas in cell mask images appear different sizes because somas have been cut off at different planes to accentuate the dendritic trees. The soma is displayed in the dendritic contour map in cool colors. (f) Dendritic tip counts based on the skeletonization of the cell mask for each age. For filled circles: P8 = 15.9 ± 4.4, n = 9 cells, n = 4 retinas; P14 = 23.6 ± 7.2, n = 14 cells, n = 4 retinas; P21 = 38.8 ± 8.7, n = 10 cells, n = 3 retinas; P30 = 56.3 ± 3.8, n = 13 cells, n = 4 retinas; P82 = 58.3 ± 10.9, n = 12 cells, n = 4 retinas. For filled triangles: P30 = 58.3 ± 8.8, n = 8 cells, n = 1 retina (electron microscopy data, green triangle), P82 = 74 ± 7.1, n = 3 cells, n = 2 retinas (super resolution confocal data, blue triangle). Different letters above time points represent a significant difference in the number of dendritic tips (e.g., a vs. b); same letters above time points represent no significant difference in the number of dendritic tips (e.g., a vs. a); mean ± SD, two-tailed post-hoc Tukey test, p < .0001 for all comparisons). Red line is a fit of the data with a saturating exponential function; time constant τ is in postnatal days. Filled triangles data excluded from the fit and offset for clarity
To obtain total mGluR6 puncta counts for each cell, we used the isolated mGluR6 staining profile for each masked cell (referred to as “masked mGluR6”; Figure 3a). To account for the possibility that mGluR6 puncta change sizes through development, we selected a circular region of interest (ROI) of fixed diameter to mark a typical punctum of mGluR6 staining at each age (Figure 3b). The same ROI diameter (d) was then used to mark all of the remaining mGluR6 puncta for that cell and remained constant for all cells of the same age (P8 d = 550 nm, P14 d = 550 nm, P21 d = 650 nm, P30 d = 600 nm, P82 d = 750 nm). In cases where the masked mGluR6 signal did not have the typical punctate appearance (e.g., a cluster of staining or an elongated branch), the ROIs in a cluster with touching, but not overlapping borders, were each considered to represent a single count for mGluR6 when at least half of the ROI circle had mGluR6.
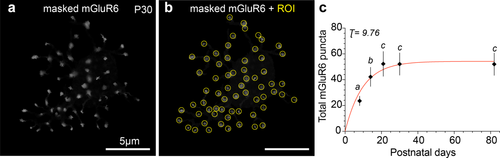
Number of mGluR6 puncta per rod bipolar cell increases during postnatal development. (a) Maximum intensity projection of mGluR6 puncta associated with a masked rod bipolar cell at P30; cell mask is not shown. (b) Each mGluR6 punctum of the masked cell was accounted for by a region of interest (ROI) whose size is representative for each age, for P30 ROI diameter = 600 nm (see Methods). (c) Total mGluR6 puncta/cell at each developmental stage: P8 = 23.6 ± 3.8, n = 9 cells, n = 4 retinas; P14 = 42.2 ± 7.5, n = 14 cells, n = 4 retinas; P21 = 52.3 ± 9.7, n = 10 cells, n = 3 retinas; P30 = 52.2 ± 8.7, n = 13 cells, n = 4 retinas; P82 = 52 ± 8.7, n = 12 cells, n = 4 retinas. Different letters above time points represent a significant difference in the number of mGluR6 puncta; same letters above time points represent no significant difference in the number of mGluR6 puncta (mean ± SD, two-tailed post-hoc Tukey test, p < .0001 for P8 vs. all, p < .05 for P14 vs. P21, P30, P82). Red line is a fit of the data with a saturating exponential function; time constant τ is in postnatal days
To determine the developmental co-expression of mGluR6 and TRPM1 in the dendritic tips, we used the following steps for each cell: Step 1. Figure 4a–e outlines the procedure for obtaining the background noise for each stain and applying it to the masked mGluR6 and TRPM1 profiles. Step 2. Figure 4f–j outlines the procedure for determining the mGluR6 and TRPM1 intensity profiles within individual tips. Step 3. The highest intensity pixel value for each mGluR6 and TRPM1 peak was compared to its respective baseline, obtained from Step 1. Peak values higher than 2 standard deviations (SD) of respective baseline were considered to represent tips positive for mGluR6 and TRPM1. Comparisons between peak mGluR6 and TRPM1 intensity to baseline were performed in Matlab.
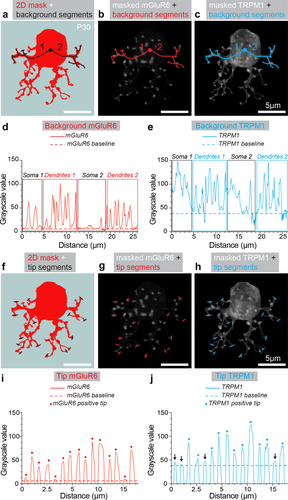
Determination of background noise, threshold, and tip localization for mGluR6 and TRPM1. (a) Example of a cell in which the 3D binary cell mask is collapsed into 2D (red). To determine the level of background noise for each protein, 2D masks were used to trace background segment ROIs from the center of the cell (black circle) to a termination within two or more dendritic arms (black lines 1 & 2). (b) Following the step in (a), background segments (red) were transposed onto the maximum intensity projection of the masked mGluR6 signal. (c) The same procedure was performed for masked TRPM1 (blue). (d) Intensity profile of background ROI segments for mGluR6 taken from (b) and concatenated along the x-axis. The regions of the segments that fall within the soma and the dendrites are outlined with rectangles. The dashed red line represents the mGluR6 background threshold, which was the average of the lowest 25% intensity values of mGluR6 in this cell. (e) Intensity profile of background ROI segments for TRPM1 taken from (c) and concatenated along the x-axis. The dashed blue line represents the TRPM1 background threshold selected for this cell obtained in the same way as (d). (f) To determine the staining of mGluR6 and TRPM1 within a dendritic ending, tip segment ROIs were traced over the 2D mask of the cell. Tip segments were drawn over the area where a dendritic process terminates (black lines drawn over 15 tips, which represent 25% of total tips analyzed for this cell). To ensure unbiased selection of dendritic tips, the tip segments were drawn at the same time as the background segments (in a) and without any reference to the masked mGluR6 or TRPM1. (g) Tip segments (red) transposed onto the maximum intensity projection of the masked mGluR6 signal. (h) Tip segments (blue) transposed onto the maximum intensity projection of the masked TRPM1. (i) Intensity profile of the tip ROI segments for mGluR6 taken from (g) and concatenated along the x-axis. Each peak represents the maximum intensity for mGluR6 within one tip. Dashed red line is the background threshold for mGluR6, as obtained from (d). Peak intensity values 2 standard deviations (SD) over the baseline are denoted with a red dot. (j) Intensity profile of the tip ROI segments for TRPM1 taken from (e) and concatenated along the x-axis. Dashed blue line is background threshold from (e) and peaks of 2SD over baseline are denoted with a blue dot. Arrows indicate tips without TRPM1 detected
To determine the efficiency of rod sampling by each rod bipolar cell, we overlaid the masked mGluR6 (yellow), all mGluR6 for the entire image (green), and the rod bipolar cell 3D mask (Figure 9a). Green mGluR6 puncta falling in part or entirely over a dendritic portion of the masked cell, displaying <50% yellow signal, were considered to represent a “missed” rod input (Figure 9a). The ratio of total mGluR6 for a cell to total mGluR6 plus “missed” mGluR6 was used to construct the graph in Figure 9c.
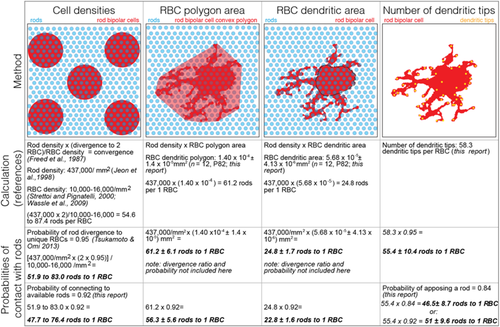
In Table 1, we estimated the rod bipolar cell dendritic area in two ways: (a) by calculating the area of the dendritic field taken from a 2D maximum projection of the dendritic tree with the soma removed (lower estimate), or (b) by calculating the area of a convex polygon, whose anchor points are the farthest reaching dendrites (higher estimate).
2.5 Statistics and fits
ANOVA with post-hoc Tukey's test were performed using Prism (GraphPad, CA). Data in Figures 2f, 3c and 6j were fit with a saturating exponential function of the form Y = P* (1 − exp (−K*x)), where Y is the number of tips, P is the plateau, K is the rate constant in reciprocal units to x, and x is postnatal days. We assumed a plateau of 80.
3 RESULTS
3.1 Identification and analysis of individual rod bipolar cells
We identified isolated rod bipolar cells in Grm6-tdTomato transgenic mice. A confocal z-stack reconstruction of a typical postnatal age (P)30 cell in cross-sectional view is shown in Figure 1a. Individual rod bipolar cells were identified morphologically by their bulbous axonal endings in the inner most region of the inner plexiform layer (IPL; Figure 1a, inset) and their highly-branched dendritic trees in the outer plexiform layer (Figure 1b). For each cell, a three-dimensional binary representation (3D mask, Figure 1c) was created. To illustrate depth in contour maps, dendritic tips appear in warmer colors while the soma normally appears in cooler colors (Figure 1e). From the mask, a thinning of the label to a string of connected pixels (3D skeleton, Figure 1d) allowed us to trace the processes to their termination and identify structures as dendritic tips. We classified a tip as the termination of a dendritic process. Dendritic tips occur at varying z positions relative to the soma, likely reflecting the breadth of the OPL where rod bipolar cells make contact with rod spherules.
3.2 The number of rod bipolar cell dendritic tips increases from P8 to P30
To quantify the development of dendritic processes, we analyzed rod bipolar cells at five postnatal ages. Examples of masked rod bipolar cells at P8, P14, P21, P30, and P82 are presented in Figure 2a–e. We confirmed the identity of each cell as a rod bipolar by tracing the axon stalk to the axon terminal in the innermost region of the IPL. Cells at P8 were the most difficult to identify because neurite extensions are still present around the axon terminal (Morgan, Dhingra, Vardi, & Wong, 2006). For that reason, we used the location of the axon terminal within the IPL as the main criterion to choose cells at P8. Each cell mask (Figure 2a–e, left panel) has a corresponding contour map (Figure 2a–e, right panel). For clarity of representation, most of the cell body in the cell masks is cut off, but can be seen in its entirety in the corresponding contour map on the right. Throughout development, dendritic trees become more complex and overall cell size increases. Using the cell mask and skeleton generated for each rod bipolar cell, we determined the number of dendritic tips during postnatal development (Figure 2f). The number of dendritic tips increased until P30 and did not significantly change at P82. This suggests that rod bipolar cells develop the appropriate number of tips and possibly contact the appropriate number of rods by P30. We fitted these data with a saturating exponential function (red smooth line in Figure 2f) and obtained a time constant of τ = 21.8 days, which is the amount of time required to reach ∼63% of the maximum number of tips. The developmental trajectory indicates that rod bipolar cells do not undergo a pruning process.
The number of tips we observed at P21, P30, and P82 is significantly higher than the convergence ratios previously reported for rod bipolar cells in mice, that is, 22–25 rods to 1 rod bipolar cell (Tsukamoto et al., 2001; Tsukamoto & Omi, 2013). To check whether confocal resolution limitations and tdTomato expression could introduce artifacts, we confirmed these counts by other methods. We obtained images of P82 rod bipolar cells using super resolution confocal microscopy, which improves resolution by 1.5-fold. The mean ± SD from tip counts of three cells was 74 ± 7.1 (blue triangle, Figure 2f), a value that was higher but not significantly different when compared to P82 cells (58.3 ± 10.9) imaged with conventional confocal microscopy. Furthermore, we obtained examples of rod bipolar cells at P30 from an EM reconstruction dataset that had been published previously and performed an identical masking and tip analysis on eight cells (Helmstaedter et al., 2013; Behrens, Schubert, Haverkamp, Euler, & Berens, 2016). The mean ± SD for these counts was 58.3 ± 8.8 (green triangle, Figure 2f) a value that was not significantly different from what we had obtained at P30 with our confocal data (56.3 ± 3.8). In addition, preliminary results from DiI filling of mature rod bipolar cells (P76) reveal tip counts of 59.3 ± 9.3 per cell (n = 3 cells, n = 2 retinas; mean ± SD, data not shown). Taken together, these data enumerate 56–74 mature rod bipolar cell dendritic tips. The number of tips could represent an approximation of the number of rods converging onto a rod bipolar cell. We will return to this point in the Discussion.
3.3 Total number of mGluR6 puncta in rod bipolar cells increases from P8 to P21
To determine the developmental presence of the glutamate receptor within the dendritic tips of the rod bipolar cell, we next quantified the number of mGluR6 puncta in individual rod bipolar cells at each developmental stage. From each cell mask, we extracted the mGluR6 signal associated exclusively with that particular cell (Figure 3a). Each punctum of mGluR6 expression was counted by placing a ROI over the masked mGluR6 signal (Figure 3b, see Materials and Methods). Total mGluR6 puncta/cell increased significantly from P8 to P21. Differences between mGluR6 puncta counts were significantly different for P8 versus all later ages and for P14 versus all later ages. The number of mGluR6 puncta/cell appears to reach an asymptote by P21, as differences between counts for ages of P21 and greater were not significantly different from each other (Figure 3c). We fit the mGluR6 data with a saturating exponential and found that mGluR6 puncta/cell increased with a τ = 9.8 days. Thus, mGluR6 appears in the dendritic tips before the mature number of dendritic tips is established.
3.4 Almost half of rod bipolar cell dendritic tips have mGluR6 and TRPM1 at eye opening
Previous studies have shown that absence of mGluR6 in rod bipolar cell dendritic tips completely abolishes rod bipolar signaling (Masu et al., 1995; Dryja et al., 2005). The transduction channel TRPM1 is similarly important for proper rod bipolar cell function, since its absence leads to loss of the scotopic light response (Morgans et al., 2009; Koike et al., 2010). Given the central role of these two proteins in rod bipolar cell function, we next set out to determine the developmental distribution of TRPM1, as well as the correlation between the presence of mGluR6 and TRPM1 during development. TRPM1 expression is not restricted to dendritic tips, but is also observed at high levels throughout the dendritic arms and the rod bipolar cell soma. The ubiquity of TRPM1 made it difficult to determine if TRPM1 staining was part of the tip or part of the preceding dendritic arm. Therefore, we could not unambiguously count TRPM1 puncta, as we had done with mGluR6. To quantify TRPM1, we devised a different approach, which is outlined in Figure 4. We used this approach for the quantifications in Figures 5 and 6. Examples of cells stained for mGluR6 and TRPM1 at each age are shown in Figure 5a–e. The intensity profiles of mGluR6 and TRPM1 within selected dendritic tips are to the right of each image. For clarity, only intensity profiles of 20–25% of the total tips for each age are displayed; similar results were obtained when all visible tips from the 2D mask were analyzed. Using the criteria outlined above, we observed that at P8, 45.5% of rod bipolar dendritic tips have mGluR6 and TRPM1 present. This number increased to 58.1% by eye opening at P14, a time when the mGluR6/TRPM1-dependent scotopic b-wave is present. Tips with both proteins reached 70.3% at P21 and this percentage did not change significantly through P82 (Figure 5f, red bars). We also examined tips for the absence of either protein without reference to the other. As tips with both proteins increased steadily, tips without either protein decreased with age (Figure 5f, white and grey bars). At P8, 31% lacked mGluR6 and 43% lacked TRPM1. At P14 and eye opening, 17.8% of tips did not have mGluR6 compared to 33% of tips negative for TRPM1. Through the rest of the examined ages there was a pattern of fewer tips lacking mGluR6 compared to TRPM1, however only comparisons between P8 and P21/P82 were significantly different (p < .01). These results suggest that throughout development rod bipolar cells have tips without a complete complement of transduction proteins.
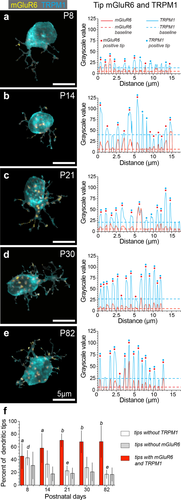
Expression of mGluR6 and TRPM1 in rod bipolar cell dendrites increases with age. (a–e) Examples of mGluR6 and TRPM1 expression in rod bipolar cells at P8, P14, P21, P30, and P82. The mGluR6 and TRPM1 signal are overlaid in all images. (Right) Intensity profiles of tip line segments for each cell (20–25% of total tips analyzed) concatenated along the x-axis, obtained as in Figure 4. Peaks are maximum intensity values for mGluR6 (red) and TRPM1 (blue) within a tip. Dashed red and blue lines represent the baseline threshold for each signal. Peaks with values greater than 2SD of respective baseline are marked with a red and/or blue dot. (f) Summary for all tips analyzed at the different ages. Percentage of tips with both mGluR6 and TPRM1 (red bars, mean ± SD) were 45.5% ± 19.9 at P8, n = 9 cells, n = 4 retinas; 58.1% ± 23.1 at P14, n = 14 cells, n = 4 retinas; 70.3% ± 10.4 at P21, n = 9 cells, n = 3 retinas; 67.9% ± 18 at P30, n = 12 cells, n = 4 retinas; and 74.2% ± 10.2 at P82, n = 9 cells, n = 4 retinas. For tips with mGluR6 and TRPM1, P8 versus P14 was not significant, P8 versus P21, P30, P82 had p < .05, two-tailed post-hoc Tukey test; different letter above red bars indicates significance for the measure between time points, same letter or no letter indicates no significance. Percentage of tips without mGluR6 (gray bars) were 30.1% ± 21.3 at P8, 17.8% ± 11.3 at P14, 18.6% ± 8.6 at P21, 21% ± 14.4 at P30, and 16.9% ± 10.7 at P82. There was no significant difference for all comparisons between ages for tips without mGluR6. Percentage of tips without TRPM1 (white bars) were 42% ± 11.7 at P8, 33% ± 21.4 at P14, 22.7% ± 7.9 at P21, 27.6% ± 16.7 at P30, and 17.2% ± 6.9 at P82. Comparisons for P8 versus P21 (p < .05) and P8 versus P82 (p < .01) for percentage of tips without TRPM1 were significant, there was no significant difference for all other comparisons between ages for tips without TPRM1 (two-tailed post-hoc Tukey test)

mGluR6 and TRPM1 independently trafficked to rod bipolar cell dendritic tips. (a) Example of rod bipolar cell at P82 stained for mGluR6 and TRPM1 and displaying a tip (solid red outline within boxed region and dashed white outlines with arrows in b and c) without either mGluR6 (b) or TPRM1 (c). (d) Rod bipolar cell at P82 and a tip (solid red outline within boxed region and dashed white outlines with arrows in e and f) with mGluR6 (e) and without TRPM1 (f). (g) Rod bipolar cell at P82 and a tip (solid red outline within boxed region and dashed with outlines in h and i) without mGluR6 (h) and with TRPM1 (i). Saturating exponential fits and time constants for percentage of tips with mGluR6 and TRPM1 (black smooth line, black open circles, τ = 7.4 days), percentage of tips with TRPM1 (blue smooth line, blue open circles, τ = 7.5 days), and percentage of tips with mGluR6 (red smooth line, red open circles, τ = 4.0 days). Tips with mGluR6 were observed earlier compared to tips with TRPM1
3.5 mGluR6 and TRPM1 are trafficked independently to tips and mGluR6 appears first
Next we wondered whether postsynaptic proteins were trafficked together or independently. We observed tips that lacked both TRPM1 and mGluR6 (Figure 6a–c). We also observed tips with mGluR6 only (Figure 6d–f), as well as tips with TRPM1 only (Figure 6g–i). To determine if TRPM1 and mGluR6 arrive at dendritic tips simultaneously during development, we determined the percentage of tips that were positive for either or both proteins across ages (Figure 6j). Using the same fit parameters as before, we obtained a τ = 4.0 days for mGluR6-positive tips, τ = 7.5 days for TRPM1-positive tips, and τ = 7.4 days for mGluR6 + TRPM1-positive tips. These results demonstrate that during early postnatal development mGluR6 and TRPM1 are trafficked to the tips independently of each other and that mGluR6 appears in tips prior to TRPM1.
3.6 Rods connect to two separate rod bipolar cells with postsynaptic proteins in close apposition to the rod ribbon
In addition to the convergence between rods and rod bipolar cells, each rod diverges to approximately 2 rod bipolar cells (Tsukamoto & Omi, 2013). Existing anatomical evidence for rod divergence comes from EM studies. We used super resolution confocal microscopy to examine rod divergence to rod bipolar cells. Staining for both mGluR6 and TRPM1 resembles a coffee bean-shaped doublet. The mGluR6 and TRPM1 “doublets” usually overlap with each other. To determine if both puncta of an mGluR6/TRPM1 doublet are filled by a rod bipolar cell process, we traced rod bipolar cell dendrites from masked cells to their termination at an mGluR6/TRPM1 doublet (Figure 7a–c). An example of divergence of the rod signal would manifest as one rod bipolar cell process associated with one of the doublet mGluR6 and TRPM1 puncta. We observed the occupation of only one mGluR6 and one TRPM1 punctum by individual rod bipolar cell processes (Figure 7c). This arrangement was also evident in some of the conventional confocal data (not shown). In the opposite case, a rod bipolar cell process that fills both of the doublet puncta would indicate a rod bipolar cell that receives two inputs from the same rod. We rarely observed this scenario in the super resolution data (data not shown). The rarity of bifurcating dendrites is in agreement with EM reconstruction data from the Tsukamoto and colleagues. To confirm the divergence of the rod signal to two separate rod bipolar cells, we next wanted to determine if we could identify instances of a tdTomato labeled process in one of our masked cells that overlapped with a marker for all rod bipolar cells. To that end, we stained P82 retina with protein kinase C alpha (PKCα). We observed that the tdTomato signal of our cell of interest always overlapped with PKCα and PKCα-positive cells surrounded our masked cell (Figure 7d–f). Next, we examined the dendritic processes in detail (Figure 7g). One such example is shown in Figure 7h, where a tdTomato-positive process overlaps with PKCα and one of the mGluR6 puncta. Figure 7h shows a second process that does not overlap with tdTomato signal but overlaps with the other mGluR6 punctum. The two dendritic processes are also apposed to a rod ribbon, as shown by the horseshoe staining pattern of Ribeye in the bottom two panels of Figure 7h. Taken together, these data indicate that divergence of rods to different rod bipolar cells is prevalent.
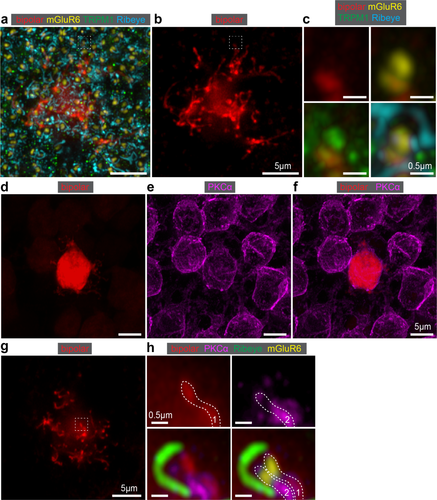
Localization of rod bipolar cell postsynaptic proteins and demonstration of rod divergence. (a) Deconvolved confocal image of P82 rod bipolar cell expressing tdTomato and stained for mGluR6, TRPM1, and Ribeye. (b) The same rod bipolar cell without synaptic protein staining and with the soma cutoff to highlight the dendritic tips. (c) Magnification of a single dendritic process (white dashed box in a and b) showing the tdTomato expression (top left), tdTomato and mGluR6 (top right), tdTomato and TRPM1 (bottom left), and tdTomato, mGluR6, TRPM1, and Ribeye (bottom right). Note that the rod bipolar cell process coincides with one punctum of the mGluR6/TPRM1 ‘doublet’. (d) Confocal image of a tdTomato-expressing P82 rod bipolar cell. (e) PKCα staining showing all rod bipolar cells. (f) Overlay of the tdTomato and PKCα. (g) Cell from D at the level of dendritic tips. (h) Magnification of one dendritic tip (white dashed box in g) showing the tdTomato expression (top left); PKCα (top right); tdTomato, PKCα, and Ribeye (bottom left); and tdTomato, PKCα, Ribeye, and mGluR6 (bottom right). Example of divergence of the rod (Ribeye) to two different rod bipolar cell processes (white dashed outlines 1 and 2). Only one tdTomato rod bipolar cell process coincides with one of the PKCα-positive dendrites and one of the mGluR6 ‘doublet’ puncta
3.7 The majority of identified rod bipolar cell dendritic tips appose a rod ribbon
We next wanted to determine how often a tip identified and counted by our criteria also apposes a rod ribbon. This necessitated tracing the dendritic processes from a masked cell and determining proximity to Ribeye staining (i.e., rod ribbon; Figure 8a). We utilized the super resolution confocal dataset to determine if Ribeye staining is apposed to a dendritic tip in three dimensions. Using the cell mask, we traced individual dendritic processes to each identified dendritic tip (Figure 8b). The width of the synaptic cleft between rods and rod bipolar cells is ∼100–200 nm in electron micrograph data (Sterling, Freed, & Smith, 1988; Rao-Mirotznik, Harkins, Buchsbaum, & Sterling, 1995). To account for methodological and resolution differences between the electron and confocal microscopy, we chose 850 nm as a conservative cutoff to indicate association between the dendritic tip and rod ribbon. For the two cells we analyzed, the average distances between the tip and middle of the ribbon were 447 ± 237 and 378 ± 236 nm. We observed that for one rod bipolar cell, 59 out of 69 tips were with a ribbon and for another rod bipolar cell, 64 of 79 tips were associated with a ribbon. On average, 16.5% of tips we count may be independent of a rod contact. This number is close to the percentage of tips we obtained from our confocal data for tips without mGluR6 (16.9%) and without TRPM1 (17.2%) at that age (see Figure 5f).
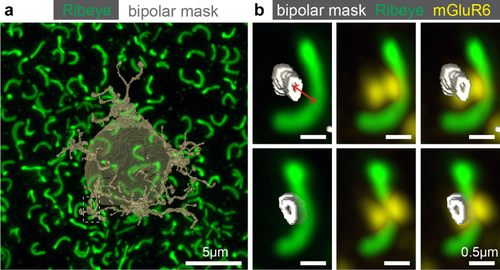
Apposition of rod terminals and rod bipolar cell dendritic tips. (a) Transparent 3D mask from a rod bipolar cell with all Ribeye within the field of view (P82). (b) A single dendritic tip traced to a ribbon and overlaid with mGluR6 signal. The tip occupies one of the two mGluR6 puncta. Red line represents the shortest distance (∼480 nm) from the center of the masked tip to the center of the Ribeye staining used to determine apposition of rod bipolar cell tips and rod terminals. (Bottom) The same tip, ribbon and mGluR6 are rotated ∼90° to highlight the position of the dendritic tip under the ribbon
3.8 Rod bipolar cells contact the majority of available rods
Finding a minority of mature dendritic tips independent of a rod ribbon, led us to wonder if these “orphan” dendrites were residual from a developmental competition. Dendrites from neighboring rod bipolar cells might be competing for rod input during postnatal development thus resulting in “orphan” tips. To answer this question, we compared (a) the mGluR6 extrinsic to the cell of interest within the field of view (green, Figure 9a), (b) the masked mGluR6 intrinsic to the cell of interest (yellow, Figure 9a), and (c) the 3D mask of the cell (transparent dark grey, Figure 9b, see Materials and Methods for details of analysis). Using mGluR6 as a proxy for rod location, we observed that the rod bipolar cell samples nearly all rods within reach, with average sampling between P30 and P82 of 92% (Figure 9c). This analysis was difficult to perform on the P8–P21 ages, for which only single points are displayed on the same graph. We could only identify an average of 2 “missed” rod inputs per cell across ages, as judged indirectly by extrinsic mGluR6 over the cell's dendrites. Thus, these results show that the rod bipolar cell dendritic field samples the majority of available rods in the mature retina and that the “orphan” tips are unlikely to be remnants of developmental competition for rods.
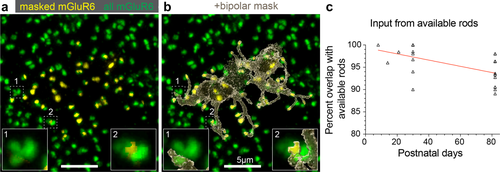
Rod bipolar cells connect to the majority of available rods. (a) Masked (yellow) and total (green) mGluR6 for a P82 rod bipolar cell. Boxes 1 and 2 show instances of possible missed rod input, as judged by mGluR6 unoccupied by the mask. (b) Transparent rod bipolar cell mask overlaid with the masked and total mGluR6 with same boxes highlighted as in (a). (c) Mature rod bipolar cells rarely missed available rod input. An average of 2 ‘missed’ rod inputs per cell (open triangles) were found at P30 and P82. Only single points analyzed at the earlier ages (P8–21)
For the mature rod bipolar cells, we made an independent measure of rods available to each rod bipolar cell. We measured the polygon of the dendritic mask, excluding the soma and multiplied this area by a previously reported rod density (Jeon et al., 1998, Table 1, column 2), This measure estimated 61.2 ± 6.1 rods available to the rod bipolar cell at P82, which is on par with the number of dendritic tips at this age (however, see Discussion and Table 1 for further calculations). Thus, these results suggest that the rod bipolar cell has sufficient dendritic tips to sample all available rods within its dendritic field.
4 DISCUSSION
In this study, we found evidence for synaptogenesis without pruning in rod bipolar cell dendrites. The lack of pruning is similar to the development of the cone-to-type 6 ON cone bipolar cell synapse, and is dissimilar to the development of the cone-to-types 7 and 8 ON cone bipolar cell synapses, as well as to development of the neuromuscular junction and climbing fiber inputs to Purkinje cells in the cerebellum (Rakic, Bourgeois, Eckenhoff, Zecevic, & Goldman-rakic, 1986; Bleckert & Wong, 2011; Dunn & Wong, 2012). Alvarez and Sabatini suggest that neurons that prune use activity to eliminate synapses. In contrast, neurons that exhibit synaptogenesis without pruning during development use activity to form or stabilize synapses (Alvarez & Sabatini, 2007). Such a framework is consistent with our observations that rod bipolar cells establish tips and synaptic proteins after rods become light responsive.
4.1 Number of dendritic tips differs from reported convergence ratios and consistent with predicted convergence ratios
Using confocal reconstructions of fluorescently labeled rod bipolar cells, we find that mature (P82) rod bipolar cells have 58.3 ± 10.9 dendritic tips. In addition, we confirmed these tip numbers by counting tips on P82 cells from a super resolution confocal data set, resulting in 74 ± 7.1 tips, and P30 cells from an EM reconstruction dataset, resulting in 58.3 ± 8.8 tips. Similarly, Keeley and colleagues performed DiI-labeling of rod bipolar cells (P72 ± 14) and counted approximately 42 dendritic tips in the wild type retina (Keeley & Reese, 2010). In the present study, it was not possible to determine the association of rod bipolar cell tips with Ribeye from the conventional confocal dataset. However, we were able to quantify this relationship in a super resolution dataset at P82 (74 ± 7.1 tips), from which we determined that 83.5% of identified tips connect to a rod. This represents approximately 62 tips in a rod bipolar cell associated with a rod.
The number of dendritic tips reported here exceeds that of rod convergence in prior reports (25 rods: 1 rod bipolar cell) (Tsukamoto & Omi, 2013). This convergence ratio was obtained by serial EM to reconstruct a small portion of mouse retina and focused on identifying synapses between rod spherules and rod bipolar cell dendritic tips. Tsukamoto and colleagues did not report the presence of dendritic tips that were not associated with rods. Tsukamoto et al. did report the presence of a “Y-shaped bifurcation of the [rod bipolar]-invaginating dendrite within the rod spherule”, which comprised 3.4% of the rod bipolar cell population in their sample. Interestingly, the second tip in those rod bipolar cells was not immediately apposed to a ribbon. In our data, the additional tips do not appear to be part of a similar bifurcation. The majority of extra tips we observe appears to represent sites of rod contact, while the minority appears to be orphaned of a rod connection. “Orphan” tips may represent cone contact, horizontal cell contact, or may be completely independent of presynaptic partners. If the dendritic tips we counted are not engaged in synaptic contacts, then it is possible that these dendrites have escaped analysis of EM data. Thus, additional rod bipolar cell tips may be a common finding across methods. For example, recent larger-scale serial EM reconstructions have noted rod bipolar cell contacts with cones and report higher rod bipolar cell tip numbers (∼35), when ribbons are not used for reference (Behrens et al., 2016). Such results are consistent with physiological measures of rod bipolar cells that receive direct input from cones (Pang et al., 2010). A subset of rod bipolar cell dendritic tips likely associate with cone pedicles, but a quantification of the rod bipolar cell association with cones and horizontal cells remains to be done. The physiological function of all dendritic tips has yet to be determined.
We have preliminary evidence suggesting that a subset of these tips does not contact photoreceptors. We used the super-resolution data to determine if any of the mGluR6 negative tips (Figure 5f) were apposed to a rod synaptic ribbon. Preliminary analysis of this data indicates that 25% of mGluR6 negative tips were not apposed to Ribeye staining in one cell (data not shown). This represents 4.8% of all tips for this cell. However, with fixed tissue we cannot determine if tips that lack mGluR6 and are independent of a rod ribbon, are either (a) dynamically looking for rod contacts, and adjusting mGluR6 expression accordingly, or (b) are permanently nonfunctional dendrites.
Despite the discrepancy between our tip count and the reported rod-to-rod bipolar cell convergence ratio, we find a close match between our tip counts associated with rods and the two estimates of convergence ratios that follow. First, in cat retina, the ratio
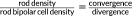 has been described (Freed et al., 1987). Starting with a divergence of 1 rod to 2 rod bipolar cells (Figure 7h), we use the above formula to estimate the number of rods available to each rod bipolar cell. The ratio between twice an average rod density of 437,000 rods/mm2 (Jeon et al., 1998) and an average rod bipolar cell density of 10,000–16,000 rod bipolar cells/mm2 (accounting for eccentricity differences; Strettoi & Pignatelli, 2000; Wassle et al., 2009) provides an estimate of the rods available to a rod bipolar cell (R). The 2013 study by Tsukamoto and Omi (2013) reports ∼95% probability that a rod contacts two different rod bipolar cells and here we report that there is a ∼92% probability that a rod bipolar will contact a rod within its reach (Figure 9). Taking these two probabilities into account, we estimate the rod-to-rod bipolar cell convergence to be: R(2*0.95)(0.92) = 47.7 to 76.4 rods to 1 rod bipolar cell (Table 1, column 1). Second, the rod density and the rod bipolar cell dendritic polygon area provide an estimate of 56.3 ± 5.6 rods to 1 rod bipolar cell (taking into account the rod connection probability; Table 1, column 2). These two independent estimates of rod convergence to rod bipolar cells are similar. If we instead use a lower estimate of the rod bipolar cell dendritic area and rod density, we estimate 22.8 ± 1.6 rods to 1 rod bipolar cell (taking into account the rod connection probability; Table 1, column 3). The lower and upper estimates for rod convergence match the previously and currently reported convergence ratios. In our analysis of tips, we have a direct measure of the percent of tips apposed to a rod ribbon for a small dataset: ∼84%. If we account for ribbon apposition probability (84%) and the probability that a rod bipolar will contact a rod within its reach (92%), we obtain two estimates of convergence based on tip counts (Table 1, column 4). Thus, though our dendritic tip counts exceed the reported convergence ratios between rods and rod bipolar cells, other independent estimates also suggest that the convergence ratio could be higher than previously reported.
has been described (Freed et al., 1987). Starting with a divergence of 1 rod to 2 rod bipolar cells (Figure 7h), we use the above formula to estimate the number of rods available to each rod bipolar cell. The ratio between twice an average rod density of 437,000 rods/mm2 (Jeon et al., 1998) and an average rod bipolar cell density of 10,000–16,000 rod bipolar cells/mm2 (accounting for eccentricity differences; Strettoi & Pignatelli, 2000; Wassle et al., 2009) provides an estimate of the rods available to a rod bipolar cell (R). The 2013 study by Tsukamoto and Omi (2013) reports ∼95% probability that a rod contacts two different rod bipolar cells and here we report that there is a ∼92% probability that a rod bipolar will contact a rod within its reach (Figure 9). Taking these two probabilities into account, we estimate the rod-to-rod bipolar cell convergence to be: R(2*0.95)(0.92) = 47.7 to 76.4 rods to 1 rod bipolar cell (Table 1, column 1). Second, the rod density and the rod bipolar cell dendritic polygon area provide an estimate of 56.3 ± 5.6 rods to 1 rod bipolar cell (taking into account the rod connection probability; Table 1, column 2). These two independent estimates of rod convergence to rod bipolar cells are similar. If we instead use a lower estimate of the rod bipolar cell dendritic area and rod density, we estimate 22.8 ± 1.6 rods to 1 rod bipolar cell (taking into account the rod connection probability; Table 1, column 3). The lower and upper estimates for rod convergence match the previously and currently reported convergence ratios. In our analysis of tips, we have a direct measure of the percent of tips apposed to a rod ribbon for a small dataset: ∼84%. If we account for ribbon apposition probability (84%) and the probability that a rod bipolar will contact a rod within its reach (92%), we obtain two estimates of convergence based on tip counts (Table 1, column 4). Thus, though our dendritic tip counts exceed the reported convergence ratios between rods and rod bipolar cells, other independent estimates also suggest that the convergence ratio could be higher than previously reported.
4.2 Relationship between morphology and function
Our data show that at P8 less than half of rod bipolar tips have both mGluR6 and TRPM1—two proteins essential for the light response of rod bipolar cells (Morgans et al., 2010). Around the time of eye opening, P14, we observe that the number of tips with both proteins is 59%, a period when the scotopic ERG b-wave is already present in mice (Bakall et al., 2003). In rats, a small b-wave is present around P14, which increases ∼4-fold by P30 and declines modestly at P40 (el Azazi & Wachtmeister, 1992). Interestingly, we observe a similar pattern in the number of dendritic tips—tips increase significantly until P30 and decrease modestly, but not significantly, by P82. We cannot exclude from our fixed tissue data that there is a spike in tip numbers between P30 and P82 that we cannot detect (Figure 2f). Still, at the peak, only 74% of rod bipolar dendritic tips have mGluR6 and TRPM1. The remaining 26% of tips have either one or none of the proteins, which indicates that these tips do not contribute to the b-wave. However, it is also possible that these tips have sufficient levels of both proteins, which are below our detection threshold. For example, knocking out the G-protein subunit Gγ13, results in slightly reduced levels of mGluR6 and TRPM1 expression in rod bipolar cell tips, but the scotopic b-wave amplitude is still at least 50% that of the wild type (Ramakrishnan et al., 2014).
4.3 Spatial sampling efficiency
This study allowed us to observe that a rod bipolar cell collects input from nearly all rods within its reach. We found few missed mGluR6 puncta within the dendritic reach of each cell. Recent work has elucidated that the rod-associated extracellular protein Elfn1 binds mGluR6 and is necessary for synaptogenesis (Cao et al., 2015). We report an early rise in mGluR6 puncta which may be explained by the importance of mGluR6 expression by rod bipolar cells for the correct wiring of the primary rod bipolar pathway (Cao et al., 2015). If there is competition between rod bipolar cells for rod contact, then dendritic tips that fail to express mGluR6 may fail to make rod contact. Such results suggest that, given the lack of pruning observed during development, rod bipolar cells in the vicinity do not compete for rod input. Rather, the density of rod bipolar cells and their number of synaptic sites allows a match between all the rods available and rod bipolar cell dendritic fields. The possibility remains that the excess tips we observed are potential synaptic sites that did not find apposition with rods, and therefore did not acquire the appropriate synaptic proteins.
ACKNOWLEDGMENTS
We would like to thank Peter Sterling and Theodore Wensel for critically reviewing the manuscript; Rachel Wong for supporting pilot experiments; Rachel Care and David Copenhagen for helpful suggestions and discussions; Grace Yang for experimental support; Suling Wang of NEI Morphology Resource Core for help with masking; Drs. Timm Schubert and Christian Behrens for generously sharing their EM reconstructions of rod bipolar cells; Patrick Keeley and Benjamin Reese for advice about DiI filling; and David Castaneda of Leica and Christopher Rieken of Zeiss for technical assistance.
AUTHORS CONTRIBUTIONS
IAA and FAD contributed to experimental design, data acquisition, analysis and writing. WW contributed to data analysis.
CONFLICT OF INTEREST
The authors declare no conflicts of interest.