Nitrogen-Doped Carbon Nanotubes/Polyethersulfone Blend Membranes for Removing Emerging Micropollutants
Abstract
Emerging micropollutants (EMPs) are ubiquitous in water systems and have become a worldwide issue of emerging environmental concern. This paper reports on the fabrication of nitrogen-doped carbon nanotubes/polyethersulfone (N-CNT/PES) blend membranes that have the potential to remove EMPs such as carbamazepine, galaxolide, caffeine, tonalide, technical 4-nonylphenol, and bisphenol A from water. The target compounds were extracted and enriched by solid-phase extraction and analyzed using comprehensive two-dimensional gas chromatography coupled-to-time-of-flight mass spectrometry. The N-CNT/PES blend membranes were synthesized using the modified phase-inversion method and characterized for a variety of physicochemical properties. The permeation properties of the membranes were determined by computing rejection and pure water flux. The 0.01 wt% N-CNT/PES blend membrane were observed to have highest surface area (94.3 ± 0.60 m2/g) and porosity (0.37 ± 0.03 cm3/g). The water flux of the membranes was enhanced by the addition of N-CNTs to the pristine PES membranes. The contact angles for the N-CNT/PES blend membranes gradually declined from 78.39° for the PES membrane to 62.7°, 58.9°, and 58.75° for the 0.01, 0.03, 0.05 wt% N-CNT/PES blend membranes, respectively. The N-CNT/PES blend membranes were highly proficient in removing the selected EMPs with the highest and lowest removal efficiencies being 99.20–99.92% (for galaxolide) and 84.61–87.21% (for caffeine), respectively. The results show the superior capability of the N-CNT/PES blend membranes in removing EMPs from water.
Abbreviations
-
- AHTN
-
- tonalide
-
- BET
-
- Brunauer–Emmett–Teller
-
- BPA
-
- bisphenol A
-
- BSA
-
- bovine serum albumin
-
- CAF
-
- caffeine
-
- CBZ
-
- carbamazepine
-
- EMP
-
- emerging micropollutant
-
- FRR
-
- flux recovery ratio
-
- FTIR
-
- Fourier transform infrared
-
- GC × GC-TOFMS
-
- comprehensive two-dimensional GC coupled to time-of-flight mass spectrometry
-
- HHCB
-
- galaxolide
-
- IEP
-
- isoelectric point
-
- MDH
-
- Minnesota Department of Health
-
- MS
-
- mass spectrometry
-
- N-CNT/PES
-
- nitrogen-doped carbon nanotubes/polyethersulfone
-
- NF
-
- nanofiltration
-
- NMP
-
- 1-methyl-2-pyrrolidone
-
- NP
-
- 4-nonylphenol
-
- PBS
-
- phosphate-buffered saline
-
- PEG
-
- polyethylene glycol
-
- PES
-
- polyethersulfone
-
- RO
-
- reverse osmosis
-
- SDG
-
- sustainable development goal
-
- SPE
-
- solid-phase extraction
-
- SWCNT
-
- single-walled carbon nanotube
-
- TGA
-
- thermogravimetric analysis
-
- WWTP
-
- wastewater treatment plant
1 Introduction
Good quality water is essential to sustain human well-being, livelihoods, and a healthy environment in order to achieve the post-2015 United Nations Sustainable Development Goals (SDGs). The SDG Goal 6, Target 6.3, calls for improvement in the quality of water through reduction of pollution as well as minimizing the discharge of hazardous chemicals and materials among others 1. However, the ubiquitous presence of emerging micropollutants (EMPs) in water poses a potentially new and serious challenge to reaching the objectives of the 2030 Agenda for Sustainable Development and achieving the SDG Goal 6, Target 6.3, namely to “by 2030, improve water quality by reducing pollution, eliminating dumping and minimizing release of hazardous chemicals and materials, halving the proportion of untreated wastewater, and substantially increasing recycling and safe reuse globally” 2-6. The EMPs include a wide range of unregulated natural and man-made chemical species such as pesticides, surfactants, pharmaceuticals, solvents, personal-care products, and various industrial/household additives as well as many other emerging compounds 2, 4, 6. The amount of EMPS in the environment is still expected to increase in the future due to the growing population and high dependence of modern societies on chemicals, especially pharmaceuticals and personal-care products.
The release of inadequately treated wastewater treatment plant (WWTP) effluents into surface water is considered to be the main cause of the presence of EMPs in our environment as conventional WWTPs are not equipped to effectively remove EMPs and they are thus unable to meet the required standards for effluent discharge into the environment 7. The presence of these EMPs in water bodies has been identified as a potential threat to environmental and human health and thus presents a new global water quality challenge. Exposure to low levels of EMPs has been reported to be linked to toxic biological effects including estrogenicity, mutagenicity, and genotoxicity 8-11. The existing conventional bio-physicochemical adsorption systems and water and wastewater treatment processes, such as reverse osmosis (RO) membranes, nanofiltration (NF) membranes, activated sludge, oxidation, activated carbon, biological trickling filters, lime softening, flocculation and coagulation, are not capable of completely degrading or removing these EMPs from wastewater 7, 11-13. For example, typical widely used coagulants such as aluminium sulphate, ferric chloride, or lime are reported to remove less than 20% of EMPs from water 11.
Although chlorination offers residual protection against pathogenic and bacterial re-growth, it results in unpleasant odours and tastes in addition to producing a variety of disinfection by-products (DBPs) in drinking water 14-16. The use of ozonation has received less attention as an alternative disinfection option because it is more costly and has a short lifetime. Adams et al. 17 reported that ion-exchange processes and ultraviolet (UV) photolysis are not viable options for the removal of EMPs despite being advanced water treatment techniques. The majority of the EMPs are thus able to pass through conventional treatment systems and remain soluble in the effluent and end up in aquatic environments owing to their occurrence at trace concentrations in the pg/L to μg/L range, very small sizes, polarity, persistence, and/or their continuous introduction 6, 7, 11-13, 18, 19.
Pressure-driven membrane filtration techniques such as RO, NF, ultrafiltration, and microfiltration are deemed to be some of the new and most effective treatment techniques 17, 20. These techniques have the capability to remove large amounts of organic pollutants from water 21. The application of membrane technology for water and wastewater treatment is cost-effective and technically viable and is considered to be a better alternative than the traditional treatment processes since high removal efficiency of organic pollutants is achieved, and the effluent discharge quality meets the water quality standards applicable to the receiving water body 22.
In addition, the ease of operation of membrane-based separation techniques offers a distinct advantage over conventional separation technologies such as physical separation and chemical oxidation 23. Reverse osmosis is comparatively more efficient in the removal of organic micropollutants than NF. Nevertheless, RO tends to consume more energy, which renders it less attractive than NF; for NF, the removal of organic micropollutants occurs by means of four separate mechanisms, which include solution-diffusion, convection (sieving), charge repulsion, and dielectric exclusion 24. Even though RO and NF based membrane processes are very efficient in removing large amounts of organic pollutants from water, their performance in the removal of EMPs is limited. Consequently, there is a need to develop advanced and reliable water and wastewater treatment techniques and materials in order to ensure effective removal of EMPs which are commonly present in waters at trace concentrations 6, 18, 19, 25.
Nanotechnology has the potential to provide technical solutions to water quality and quantity challenges 25. Nanomaterials such as carbon nanotubes (CNTs) and dendrimers have made major contributions to the advancement of more effective treatment processes among the advanced water treatment technologies 26. The incorporation of oxygen-functionalized carbon nanotubes in polymer membranes has been shown to have the capacity to reduce fouling through modification of the hydrophilic properties of the membranes as well as provision of electric charges to the membranes 27, 28. Specifically, blending organic polymers such as polyethersulfone (PES) with inorganic nanoparticles such as silver nitrate (AgNO3), zirconium oxide (ZrO2), zinc oxide (ZnO), GeO2, SiO2, aluminium oxide (Al2O3), titanium dioxide (TiO2), and magnesium oxide (MgO) has been reported to improve the hydrophilicity of the hydrophobic commercial membranes 23, 27-34. Daraei et al. 35 reported that the introduction of low amounts of polymer-modified CNTs into the membrane matrix has the potential to further enhance the characteristics of membranes due to increased compatibility with membrane bulk polymer as well as a higher number of functional groups compared to acid-functionalized CNTs. The electrostatic repulsion between the surface of the membrane and some EMPs is enhanced by the increase in the membrane's zeta potential with low amounts of functionalized CNTs in the polymer matrix 36. The EMPs would thus be retained as no particle deposition on the membrane surface occurs and therefore membrane pore clogging is reduced or prevented 36.
A study by Kaminska et al. 37 revealed that the incorporation of small quantities of single-walled carbon nanotubes (SWCNT) in PES membranes improved adsorption and removal of endocrine disrupting micropollutants (technical 4-nonylphenol, NP, and bisphenol A, BPA) from synthetic wastewater. Fabrication of nitrogen-doped carbon nanotubes/polyethersulfone (N-CNT/PES) blend membranes provides a unique opportunity to eliminate these recalcitrant compounds, that is, EMPs, from drinking water systems 11, 23, 36-41. Earlier studies by Daraei et al. 35, Choi et al. 38, and Vijaya Lakshmi et al. 40 showed that N-CNT/PES blend membranes tend to have improved water flux, porosity, hydrophilicity, roughness, mechanical, and rejection properties, subsequently enhancing membrane fouling resistance. A study conducted by Phao et al. 41 synthesized and assessed N-CNT/PES blend membranes for suitability in drinking water treatment by measuring the pure water flux and rejections of polyethylene glycol (PEG) as a model pollutant in synthetic water. The majority of studies on performance of N-CNT/PES blend membranes have also focused on model solutions of magnesium chloride salt rejection.
Although there have been numerous studies worldwide on the occurrence, distribution, fate, effects of EMPs as well as assessments of their removal possibilities 3-13, 18, 19, 25, there is a lack of available data on the removal of EMPs from real water samples using N-CNT/PES blend membranes. Some interventions to remove EMPs from water would include, but are not limited to, upgrading of existing water and wastewater treatment plants in order to cater for the removal of some, if not all, EMPs as well as the fabrication of N-CNT/PES blend membranes. The novelty of this study lies in evaluating the characteristics and efficiency of the fabricated N-CNT/PES blend membranes in removing selected EMPs (i.e, carbamazepine (CBZ), galaxolide (HHCB), caffeine (CAF), tonalide (AHTN), NP, and BPA) from real water and wastewater samples collected from selected monitoring sites in Mpumalanga and Northwest Provinces in South Africa.
2 Materials and methods
2.1 Materials
Polyethersulfone was obtained from Solvay Advanced Polymers, Johannesburg, South Africa (MW: 58 000 g/mol). The following reagents were sourced from Sigma–Aldrich, Johannesburg, South Africa: Nitric acid (analytical grade), 1-methyl-2-pyrrolidone (analytical grade), pyridine (analytical grade), acetonitrile, dichloromethane, and methanol (CHROMASOLV® gradient grade for HPLC), ferrocene (analytical grade), phosphate-buffered saline tablets and bovine serum albumin (BSA). The EMP standards, namely CBZ, AHTN, HHCB, CAF, NP, and BPA, were supplied by Sigma–Aldrich. All chemicals were used without further purification.
2.2 Water sample collection
Water samples were collected monthly between June 2014 and February 2015 from drinking water and wastewater sources in Mpumalanga and North West Provinces using standard sampling procedures. Using 1 L glass bottles, water samples were collected as grab samples directly from the sampling sites; these bottles were then stored in a dark cool place until analysis. For the analysis of EMPs (CBZ, AHTN, HHCB, CAF, NP, and BPA) sample bottles were rinsed twice with source water from the sampling site before obtaining the final sample.
2.3 Preparation of samples and extraction of EMPs
The 1000 mg/L stock solutions for CBZ, AHTN, HHCB, NP, and BPA were prepared by weighing 1 mg of each into a vial (1.5 mL) and dissolving the sample in methanol (HPLC grade) (1 mL) under vortex and ultrasonication for 10 min. In addition, 1000 mg/L stock solutions for CAF were prepared by weighing 1 mg of CAF into a 1.5 mL vial followed by dissolving the sample in dichloromethane (HPLC grade) (1 mL) under vortex and ultrasonication for 10 min. The stock solutions were stored in a refrigerator at <4°C. From each of the stock solutions, 100 μL was pipetted and placed into a 1.5 mL vial and made up to the 1 mL mark with methanol (HPLC grade) to prepare a 100 mg/L mixed standard solution from which working standards of 10, 20, 30, 40, and 50 mg/L were prepared for the preparation of the calibration curve.
Solid-phase extraction (SPE) was used to pre-concentrate, clean, and extract the analytes in water samples. The Supelco Visiprep™ large volume sampler was used to load the water samples onto the pre-conditioned SPE cartridges (Waters Oasis® HLB) (i.e, the water-wettable, reversed-phase sorbent) (500 mg, 6 cc). Methanol (10 mL) and 5 mL deionized water were used to pre-condition the SPE cartridges. Water samples (10 mL), containing the target analytes, were passed through the SPE cartridge at a flow rate of 1 mL/min followed by washing with deionized water (5 mL). The SPE cartridges were then dried by vacuum to eliminate the excess water before eluting the compounds with 5 mL of acetonitrile. Nitrogen gas was used to evaporate the extracted solution to dryness on a water bath at 30°C and the residue was reconstituted with 1 mL acetonitrile for GC × GC-TOFMS analysis 42.
2.4 GC × GC-TOFMS analyses
The SPE extracted samples were analyzed using 4D GC × GC-TOFMS (LECO, St. Joseph, MI, USA), which is equipped with a thermal modulator as well as with a split/splitless injector. A low-polarity phase Rxi-5SilMS column was employed for the first dimension analysis (1D GC). A polar Rxi-17SilMS was used for the second dimension analysis (2D GC). A constant linear velocity of 1.9 mL/min was employed for the carrier gas (helium). Using an autosampler (Agilent 7890A series), splitless injection mode was used with the vaporized sample moving through the injection port liner. The transfer line to source of the TOFMS detector was run at 250°C. A source temperature of 230°C and a filament bias voltage of −70 eV were used. The MS mass range was 45–550 atomic mass units, amu, with the data acquisition rate at 20 spectra/s, while the detector voltage was 1750 V. The inlet temperature was set at 200°C using 40°C as a modulator offset temperature, and a purge time of 60 s. The positive ion mode with an ionization voltage of 70 eV was used to run the MS. Prior to injection, the syringe was cleaned five times with n-hexane and once with the sample. An external standard mixture was measured after each batch of five samples to verify instrument measurement performance. The LECO ChromaTOF® software was used to process and consecutively visualize the data on 2D and 3D chromatograms.
2.5 Fabrication of N-CNT/PES blend membranes
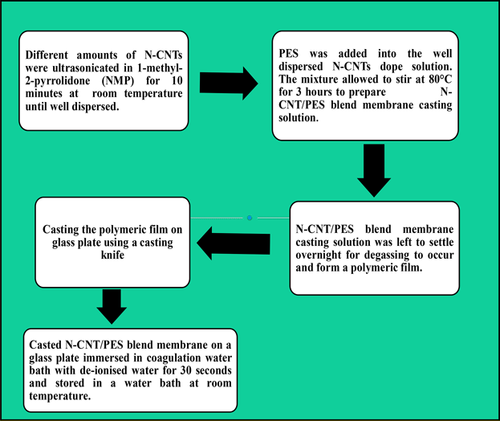
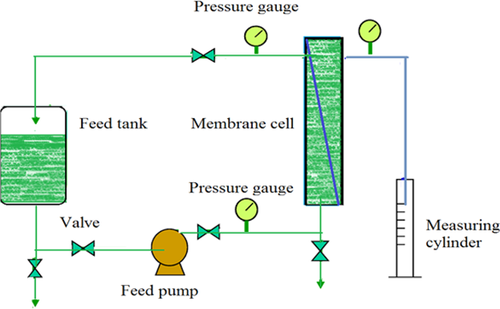
The N-CNTs were synthesized by a floating catalyst chemical vapour deposition method at 850°C using ferrocene (FeC10H10) as catalyst, acetonitrile as a nitrogen source, acetylene as carbon precursor and argon as the carrier gas 41, 43, 44. The synthesized N-CNTs were functionalized by adding them to a solution of 35% HNO3, and stirring under reflux for 4 h at 110°C. The phase-inversion method was used to synthesize the N-CNT/PES blend membranes (Fig. 1). Initially, the N-CNTs were dispersed in 1-methyl-2-pyrrolidone (NMP) by ultrasonication to form a homogeneous mixture. Thereafter, PES was dissolved in the N-CNT/NMP solution by continuous stirring and heating at 80°C for 3 h. The resultant solution was then left overnight in order to remove air bubbles and for the product to settle. Casting of the N-CNT/PES polymer solution was done on a glass plate using a casting knife with a knife gap of 200 μm. The wet polymer solution coated on a glass plate was air-dried for 40 s before immersing the glass plate in a coagulation bath containing deionized water where phase inversion occurred followed by detachment of the film from the glass plate surface. The fabricated membranes were then kept in deionized water pending characterization and usage.
2.6 Characterization of N-CNT/PES blend membranes
2.6.1 Determination of pore size and surface area of the pristine PES membrane, as-grown and functionalized N-CNTs and N-CNT/PES blend membranes
To determine the pore size and surface area of the pristine PES membrane, as-grown and functionalized N-CNTs as well as N-CNT/PES blend membranes, the Brunauer–Emmett–Teller (BET) and Barrett–Joyner–Halenda methods were utilized. The BET method utilizes nitrogen adsorption at liquid nitrogen temperature (77 K) for the determination of surface area, and the surface area studies were conducted using a Micromeritics ASAP 2020 surface area and porosity analyzer (Atlanta, Georgia, USA). Specifically, prior to degassing, about 200 mg of each sample (pristine PES membranes, as-grown and functionalized N-CNTs as well as of the N-CNT/PES blend membranes) was weighed out for degassing and loaded into the sample tube attached to the degas port of the adsorption apparatus and degassed for 3 h at a temperature below their decomposition temperatures.
2.6.2 Evaluation of hydrophilic properties of N-CNT/PES blend membranes
Water contact angle measurements were used to evaluate the wettability and hydrophilic properties of the pristine PES membrane and the N-CNT/PES blend membranes. The samples of the pristine PES membrane as well as the N-CNT/PES blend membranes were placed on a planar glass plate of the contact angle goniometer (OCA 15, Data Physics, Filderstadt, Germany). Using a syringe tip placed at a distance of about 0.5 cm above the surface, deionized water (1 μL) was deposited on the top surface of the pristine PES membrane as well as the N-CNT/PES blend membranes. As part of quality control, a total of five drops of deionized water were randomly deposited on the top surface of the pristine PES membrane as well as the N-CNT/PES blend membranes to obtain reliable average contact angle measurements. The N-CNT/PES blend membrane was considered to be hydrophilic, with improved wettability, when the value of the average contact angle measurements was <90°.
2.6.3 Identification of functional groups and chemical bonding
The Spectrum 100 Fourier transform infrared (FTIR) spectrometer by Perkin Elmer, Massachusetts, USA, equipped with a horizontal universal attenuated total reflectance device, was employed to determine the functional groups that were present on the N-CNT/PES blend membranes and chemical bonding between the N-CNT and PES. Characteristic peaks of the FTIR spectra were recorded in the range of 500–4000 cm−1.
2.6.4 Streaming potential measurements of the PES membranes and N-CNT/PES blend membranes
 (1)
(1)2.6.5 Determination of tensile strength of PES membranes and N-CNT/PES blend membranes
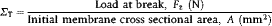 (2)
(2) (3)
(3) (4)
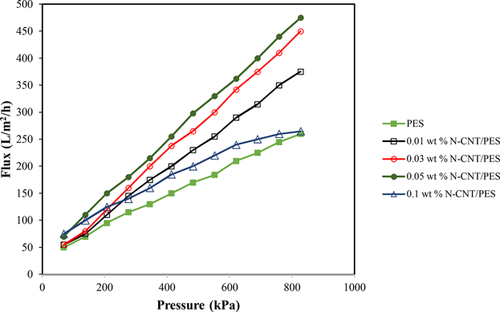
(4)2.7 Removal performance of N-CNT/PES blend membranes
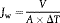 (5)
(5)
 (6)
(6)The concentration of CBZ, AHTN, HHCB, CAF, NP, and BPA in both the feed and permeate was determined using GC × GC-TOFMS after pre-concentration by using SPE. The Kruskal–Wallis test was used to determine temporal variations in levels of the analytes in water.
2.8 Assessment of membrane fouling
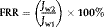 (7)
(7)Each of the PES, 0.01, 0.03, 0.05 and 0.1 wt% N-CNT/PES blend membranes (3 × 3 cm) was submerged into a 10 mL vial containing BSA/PBS (1 g/L, pH 7.4) solution in order to perform the static adsorption-fouling experiment. This was followed by incubating the vials in a water bath for 12 h at 25°C in order to reach equilibrium. The amount of BSA adsorbed on each of the PES, 0.01, 0.03, 0.05, and 0.1 wt% N-CNT/PES blend membranes was computed by comparing the UV-vis absorption intensity variation at 280 nm.
3 Results and discussion
3.1 Characteristics of fabricated PES membrane and N-CNT/PES blend membranes
3.1.1 Surface area and pore volume of PES membrane and N-CNT/PES blend membranes
The BET pore volume and surface area of the pristine PES membrane, as-grown and functionalized N-CNTs and 0.01, 0.03, 0.05, and 0.1 wt% N-CNT/PES blend membrane are given in Tab. 1. The pristine PES membrane registered the lowest pore volume and surface area (Tab. 1). An increase in both the surface area and porosity of the N-CNT/PES blend membranes was observed with increasing amounts of N-CNT in the matrix (Tab. 1). The mean surface area increased from 3.6 m2/g for the pristine PES membrane to 93.9, 94.1, 94.2, and 94.3 m2/g for the 0.01, 0.03, 0.05, and 0.1 wt% N-CNT/PES blend membranes, respectively. The observed increase in both the surface area and porosity of the N-CNT/PES blend membranes after addition of the acid treated N-CNTs was attributed to the removal and decrease in the amount of residual low-surface-area catalyst (FeC10H10) which led to the larger number of active sites available. The increase in the mean pore volume as well as surface area of the N-CNT/PES blend membranes when a small amount of the N-CNTs was added to the membrane was considered desirable for the application of the N-CNT/PES blend membranes in the removal of EMPs from water.
| Membrane | Mean surface area (m2/g) | Mean pore volume (cm3/g) |
|---|---|---|
| Pristine PES | 3.6 ± 0.14 | 0.01 ± 0.001 |
| As-grown N-CNTs | 49.1 ± 0.51 | 0.18 ± 0.02 |
| Functionalized N-CNTs | 93.6 ± 0.62 | 0.33 ± 0.03 |
| 0.01 wt% N-CNT/PES | 93.9 ± 1.1 | 0.34 ± 0.04 |
| 0.03 wt% N-CNT/PES | 94.1 ± 0.35 | 0.35 ± 0.01 |
| 0.05 wt% N-CNT/PES | 94.2 ± 0.30 | 0.36 ± 0.01 |
| 0.1 wt% N-CNT/PES | 94.3 ± 0.60 | 0.37 ± 0.03 |
3.1.2 Effect of pH on ξ of PES membranes and N-CNT/PES blend membranes
The effect of pH on ξ of PES membranes and N-CNT/PES blend membranes is presented in Fig. 3. It was observed that ξ of the PES membrane was less positive at lower pH and more positive at higher pH, with an isoelectric point (IEP) at pH 8. The higher ξ values of the PES membrane at a higher pH shows that the surfaces of the PES membranes were more negative due to the nature of the sulphonic group (OSO), which dissociates markedly at high pH values 45. The observed positive charge on the PES membrane surface could be attributed to positive ion adsorption in the course of measuring the streaming potential 37. In contrast, ξ of the N-CNT/PES blend membranes was observed to be more positive at lower pH and more negative at higher pH with IEPs at pH 7.0, 6.8, 6.0, and 5.8 for the 0.01, 0.03, 0.05, and 0.1 wt% N-CNT/PES blend membranes, respectively. It was also observed that the N-CNT/PES blend membranes attained more negative charge with the increase in N-CNT loading within the polymer matrix (Fig. 3). This finding is in line with the chemical nature of carbon surfaces which are known to be negatively charged in addition to N-CNTs being further converted into carboxylic acid groups (COOH) 46.

3.1.3 Hydrophilic properties of PES membranes N-CNT/PES blend membranes
The functional groups that were introduced onto the surface of acid-treated N-CNTs were identified by FTIR spectroscopy. The FTIR spectra of the PES membrane and 0.01, 0.03, 0.05, and 0.1 wt% N-CNT/PES blend membranes are presented in Fig. 4. Very intense spectral peaks were observed for both the PES membranes and N-CNT/PES blend membranes at 1240 and 1318 cm−1, representing the COC expanse (ether group) as well as the OSO stretch (sulphonic group), respectively 47. Unlike the spectral peak of the PES membranes, the spectral peaks for the 0.01, 0.03, 0.05, and 0.1 wt% N-CNT/PES blend membranes registered additional absorption peaks at 1450–1550, 1583, 1680, and 2983 cm−1, indicating the presence of CC, COO, CO, and CH functional groups, respectively 47. This reaffirmed the introduction of the functional groups onto the N-CNTs, which have the virtue of providing more chemical adsorption sites on the surface of the N-CNT/PES blend membranes and improving the hydrophilic characteristics of the PES blend membranes.
The hydrophilicity of N-CNT/PES blend membrane surfaces was further characterized by contact angle measurements. The pristine PES membrane registered the highest contact angle (78.39 ± 1.6°). The mean contact angle values of the N-CNT/PES blend membranes gradually declined from 62.70 ± 1.3° to 58.90 ± 1.1° to 58.75 ± 1.2° for the 0.01, 0.03, and 0.05 wt% N-CNT/PES blend membranes, respectively (Tab. 2). A decrease in water contact angles was observed; these smaller contact angles signified that the surfaces of the fabricated N-CNT/PES blend membranes were more hydrophilic. Nevertheless, it was observed that the mean contact angle values for the 0.1 wt% N-CNT/PES blend membranes increased to 63.20 ± 0.95° (Tab. 2). This sudden increase in the water contact angle observed in the 0.1 wt% N-CNT/PES blend membranes may be attributed to the aggregation of nanoparticles as the density of N-CNTs increases in the matrix, which may have caused some flaws in the membrane pore structure resulting in the modulation of the effects of modification on the membrane 48. In addition, the increase in the observed water contact angle in the 0.1 wt% N-CNT/PES could also be attributed to the increase in van der Waals forces between the N-CNT/PES blend membranes and the N-CNTs with the increase in N-CNT loading in the structure of the membrane. Similar trends were observed by Phao et al. 41. The increase in hydrophilicity is vital to reduce fouling of the fabricated N-CNT/PES blend membranes because the majority of foulants are characteristically hydrophobic 27. The improvement in hydrophilicity resulting from the functional groups on N-CNTs as well as the increase in top-layer pore sizes causes an increase in the exchange rate between non-solvent and the solvent during the phase inversion 27. The increased pore size resulted in an increase in water permeation across the N-CNT/PES blend membranes 41.
| Membrane | Mean water contact angle (°) |
|---|---|
| Pristine PES | 78.39 ± 1.6 |
| 0.01 wt% N-CNT/PES | 62.70 ± 1.3 |
| 0.03 wt% N-CNT/PES | 58.90 ± 1.1 |
| 0.05 wt% N-CNT/PES | 58.75 ± 1.2 |
| 0.1 wt% N-CNT/PES | 63.20 ± 0.95 |
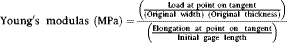
3.1.4 Tensile strength and mechanical attributes of PES membranes and N-CNT/PES blend membranes
Table 3 shows the elongation after fracture, the Young's modulus, maximum load at break, and ultimate tensile strength of the pristine membranes and the N-CNT/PES blend membranes. It was observed that the addition of N-CNTs led to an improvement in the mechanical properties of PES membrane. The ultimate tensile strength increased from 3.12 MPa for the PES membrane to 3.97, 4.10, 4.23, and 4.25 MPa, for the 0.01, 0.03, 0.5, and 0.1 wt% N-CNT/PES blend membranes, respectively (Tab. 3). Σ was also found to increase from 118 MPa for the PES membrane to 128, 132, 138, and 139 MPa, for the 0.01, 0.03, 0.5, and 0.1 wt% N-CNT/PES blend membranes, respectively (Tab. 3). Nevertheless, the elongation after fracture of N-CNT/PES blend membranes declined significantly with increasing amounts of N-CNT content. The increase in both the Young's modulus and ultimate tensile strength indicates that the N-CNTs were well-dispersed within the N-CNT/PES blend membrane matrix. This signified that the well-dispersed N-CNTs formed good bridges and stronger hydrogen bonds between particles within the fabricated membrane matrix thereby reducing stress and strain within the fabricated nanocomposite polymer matrices.
| Membrane | Cross-sectional area (mm2) | Elongation after fracture (%) | Maximum load at break (N) | Ultimate tensile strength (MPa) | E (MPa) |
|---|---|---|---|---|---|
| PES | 4.00 | 28 | 12.46 | 3.12 | 118 |
| 0.01 wt% N-CNT/PES | 4.00 | 22 | 15.89 | 3.97 | 128 |
| 0.03 wt% N-CNT/PES | 4.00 | 20 | 16.38 | 4.10 | 132 |
| 0.05 wt% N-CNT/PES | 4.00 | 16 | 16.91 | 4.23 | 138 |
| 0.1 wt% N-CNT/PES | 4.00 | 12 | 16.99 | 4.25 | 139 |
3.2 Occurrence and removal of EMPs using N-CNT/PES blend membranes
3.2.1 Occurrence of EMPs in water
The mean concentrations of EMPs in water are presented in Tab. 4. Despite an overall increase in the mean monthly concentrations of all analytes in water at the sampling sites from June 2014 through to February 2015, changes were not statistically significant (p > 0.05) at the 95% confidence level based on the results of the Kruskal–Wallis test. However, it was observed that the concentrations of AHTN, CBZ, HHCB, CAF, NP, and BPA in water varied spatially, with BPA being the most widely distributed EMP (Tab. 4). The Minnesota Department of Health, MDH, developed a guidance value of 20 μg/L for BPA (www.health.state.mn.us/cec) 49. In this study, all samples were found to have mean BPA concentrations below the lowest MDH guidance value of 20 μg/L BPA 49 (Tab. 4). Nevertheless, higher levels of BPA in some samples such as spring 1—Sisukumile Secondary School and Lower Lochiel Community present a potential everyday risk to communities. These increased BPA levels can be attributed to the location and open nature of the spring. The spring is centrally located and therefore the spring water is most often used by the community; however, a large amount of litter in the form of plastic products, food-can liners, and paper was observed in and around the spring. The school (Sisukumile Secondary School) is located near the spring and there are some agricultural activities and outside pit latrines in the area.
| Analyte | Sampling site | Mean concentration in the feed (ng/L) | Mean concentration in the permeate (ng/L) | Removal efficiency (%) |
|---|---|---|---|---|
| BPA | Treated water, Eerstehoek WTP | 6.07 ± 0.3 | 0.109 ± 0.021 | 98.20 |
| Raw water, Eerstehoek WTP | 23.96 ± 0.2 | 0.362 ± 0.015 | 98.49 | |
| Effluent, Eerstehoek WWTP | 21.34 ± 0.5 | 0.363 ± 0.030 | 98.30 | |
| Mkomazane River | 14.09 ± 0.0 | 0.247 ± 0.024 | 98.25 | |
| Spring 1, Sisukumile | 181.26 ± 3.9 | 2.556 ± 0.018 | 98.59 | |
| Spring 2, Upper Lochiel Community | 0.00 ± 0.0 | 0.00 ± 0.0 | ||
| Masakhane Primary School | 0.00 ± 0.0 | 0.00 ± 0.0 | ||
| Lipoponyane River | 14.09 ± 0.7 | 0.225 ± 0.023 | 98.40 | |
| Sisukumile water tank (JoJo) | 35.94 ± 3.5 | 0.521 ± 0.035 | 98.55 | |
| CAF | Treated water, Eerstehoek WTP | 8.33 ± 0.25 | 0.898 ± 0.061 | 85.20 |
| Raw water, Eerstehoek WTP | 8.9 ± 0.46 | 1.3 ± 0.46 | 85.39 | |
| Effluent, Eerstehoek WWTP | 9.32 ± 0.04 | 1.434 ± 0.32 | 84.61 | |
| Mkomazane River | 11.76 ± 1.5 | 1.501 ± 0.48 | 87.24 | |
| Spring 1, Sisukumile | 0.00 ± 0.0 | 0.00 ± 0.0 | ||
| Spring 2, Upper Lochiel Community | 0.00 ± 0.0 | 0.00 ± 0.0 | ||
| Masakhane Primary School | 0.00 ± 0.0 | 0.00 ± 0.0 | ||
| Lipoponyane River | 10.69 ± 2.00 | 1.367 ± 0.37 | 87.21 | |
| Sisukumile water tank (JoJo) | 0.00 ± 0.0 | 0.00 ± 0.0 | ||
| CBZ | Treated water, Eerstehoek WTP | 0.00 ± 0.0 | 0.00 ± 0.0 | |
| Raw water, Eerstehoek WTP | 0.00 ± 0.0 | 0.00 ± 0.0 | ||
| Effluent, Eerstehoek WWTP | 58.09 ± 0.22 | 6.407 ± 0.73 | 88.97 | |
| Mkomazane River | 34.97 ± 0.80 | 3.976 ± 0.81 | 88.63 | |
| Spring 1, Sisukumile | 0.00 ± 0.0 | 0.00 ± 0.0 | ||
| Spring 2, Upper Lochiel Community | 0.00 ± 0.0 | 0.00 ± 0.0 | ||
| Masakhane Primary School | 0.00 ± 0.0 | 0.00 ± 0.0 | ||
| Lipoponyane River | 27.01 ± 1.1 | 3.214 ± 0.82 | 88.10 | |
| Sisukumile water tank (JoJo) | 0.00 ± 0.0 | 0.00 ± 0.0 | ||
| HHCB | Treated water, Eerstehoek WTP | 0.00 ± 0.0 | 0.00 ± 0.0 | |
| Raw water, Eerstehoek WTP | 0.00 ± 0.0 | 0.00 ± 0.0 | ||
| Effluent, Eerstehoek WWTP | 24.79 ± 0.29 | 0.171 ± 0.053 | 99.31 | |
| Mkomazane River | 11.21 ± 0.25 | 0.090 ± 0.003 | 99.20 | |
| Spring 1, Sisukumile | 3476.86 ± 14.3 | 2.781 ± 0.71 | 99.92 | |
| Spring 2, Upper Lochiel Community | 0.00 ± 0.0 | 0.00 ± 0.0 | ||
| Masakhane Primary School | 0.00 ± 0.0 | 0.00 ± 0.0 | ||
| Lipoponyane River | 9.4 ± 1.4 | 0.071 ± 0.001 | 99.24 | |
| Sisukumile water tank (JoJo) | 0.00 ± 0.0 | 0.00 ± 0.0 | ||
| AHTN | Treated water, Eerstehoek WTP | 0.00 ± 0.0 | 0.00 ± 0.0 | |
| Raw water, Eerstehoek WTP | 0.00 ± 0.0 | 0.00 ± 0.0 | ||
| Effluent, Eerstehoek WWTP | 25.25 ± 0.12 | 0.366 ±0.05 | 98.55 | |
| Mkomazane River | 16.51 ± 3.1 | 0.248 ±0.03 | 98.50 | |
| Spring 1, Sisukumile | 153.91 ± 2.9 | 1.770 ± 0.38 | 98.85 | |
| Spring 2, Upper Lochiel Community | 0.00 ± 0.0 | 0.00 ± 0.0 | ||
| Masakhane Primary School | 0.00 ± 0.0 | 0.00 ± 0.0 | ||
| Lipoponyane River | 14.5 ± 1.5 | 0.173 ± 0.025 | 98.81 | |
| Sisukumile water tank (JoJo) | 23.44 ± 5.6 | 0.274 ± 0.039 | 98.83 | |
| NP | Treated water, Eerstehoek WTP | 0.00 ± 0.0 | 0.00 ± 0.0 | |
| Raw water, Eerstehoek WTP | 0.00 ± 0.0 | 0.00 ± 0.0 | ||
| Effluent, Eerstehoek WWTP | 20.8 ± 0.5 | 0.385 ± 0.050 | 98.15 | |
| Mkomazane River | 16.2 ± 1.3 | 0.308 ± 0.024 | 98.10 | |
| Spring 1, Sisukumile | 0.00 ± 0.0 | 0.00 ± 0.0 | ||
| Spring 2, Upper Lochiel Community | 0.00 ± 0.0 | 0.00 ± 0.0 | ||
| Masakhane Primary School | 0.00 ± 0.0 | 0.00 ± 0.0 | ||
| Lipoponyane River | 0.00 ± 0.0 | 0.00 ± 0.0 | ||
| Sisukumile water tank (JoJo) | 0.00 ± 0.0 | 0.00 ± 0.0 |
The mean CAF concentrations ranged from 0 to 11 ± 1.5 ng/L (Tab. 4), with the highest mean concentration registered in the Mkomazane River, into which the treated effluent from the Eerstehoek (Elukwatini) WWTP is discharged. The CBZ levels were highest in the Eerstehoek WWTP effluent (Tab. 4). It was observed that NP was present only in the Eerstehoek WWTP effluent (mean concentration = 20.8 ± 0.5 ng/L) and Mkomazane River (mean concentration = 16.2 ± 1.3 ng/L). It is worth noting that NP is a constituent of detergents and an anti-oxidant and it was therefore considered that municipal wastewater would be the main source of NP in water. The mean HHCB levels were highest in spring 1—Sisukumile Secondary School and Lower Lochiel Community with the mean concentration ranging from 0.00 to 3 477 ± 14.3 ng/L (Tab. 4). Similarly, the AHTN levels were highest in spring 1—Sisukumile Secondary School and Lower Lochiel Community (Tab. 4). Spring 1 was of greatest concern as it is the major source of drinking water, and water used for other domestic purposes (i.e, cooking and washing) for the Sisukumile Secondary School and Lower Lochiel Community. These high levels can be attributed to the location and open nature of the spring. Much litter in the form of plastic products in the vicinity of the spring was observed, and the spring water is also used for laundry and livestock watering.
It is worth noting that there are no reported guidance values for CAF, NP, CBZ, HHCB, and AHTN in water and wastewater. It was, however, clear that some communities, especially those living in Mpumalanga Province, are at risk of the ecotoxicological effects of the identified EMPs since all the EMPs were present at all the sampling sites even though found to be at different concentrations. Hence there is a need to remove the EMPs from these waters. Apart from the occurrence of EMPs, the other major water quality problems in the study area are mainly the widespread occurrence of the faecal coliforms and nitrates, which might not necessarily affect the removal of the EMPs. The water at all sampling sites showed generally low levels of mineralization with electrical conductivity values ranging from 28.02 to 760.03 ± 6.38 μS/cm.
3.2.2 Application of the N-CNT/PES blend membranes in the treatment of real water and wastewater samples
The mean concentration of BPA in the permeate ranged from 0.00 ± 0.0 to 2.556 ± 0.018 ng/L registering a removal efficiency ranging from 98.20–98.59% (Tab. 4). The mean concentration of CAF in the permeate ranged from 0 to 1.501 ± 0.48 ng/L with the removal efficiency ranging from 84.61–87.24% (Tab. 4). The mean concentration of CBZ in the permeate ranged from 0 to 6.407 ± 0.73 ng/L with the removal efficiency ranging from 88.10–88.97%. The mean concentration of HHCB in the permeate ranged from 0 to 2.781 ± 0.71 ng/L with the removal efficiency ranging from 99.20–99.92%. The mean concentration of AHTN in the permeate ranged from 0 to 1770 ± 0.38 ng/L with removal efficiency ranging from 98.50–98.85%. The mean concentration of NP in the permeate ranged from 0 to 0.385 ± 0.0.050 ng/L with removal efficiency ranging from 98.50–98.85%. Although it was generally observed that the removal efficiency of all analytes was higher for samples with higher analyte concentration levels (Tab. 4), no statistically significant variations in the removal efficiency of the EMPs were observed with increasing salinity of the water samples (p > 0.05) at the 95% confidence level.
However, the removal of EMPs from real water and wastewater by the N-CNT/PES blend membranes was observed in the following order: HHCB > NP > AHTN > BPA > CBZ > CAF (Tab. 4 and Fig. 5). The removal of the EMPs followed the order of the decrease in hydrophobicity based on the reported pKow, which describes the compounds’ affinity for sorption as well as aggregation. The highest removal efficiency for HHCB can thus be attributed to the fact that it has the highest hydrophobicity and sorption potential, and therefore could be easily rejected by the N-CNT/PES blend membranes. The pKow values for HHCB, NP, AHTN, BPA, CBZ, CAF are 5.90, 5.76, 5.70, 3.32, 2.45, and −0.07, respectively. The EMPs with pKow > 2 are hydrophobic and tend to have higher sorption and aggregation potential, the major mechanisms responsible for the retention of EMPs.

It is worth noting that, except for the findings of Radjenović et al. 50, Gur-Reznik et al. 51, and Kimura et al. 52 who reported a maximum removal efficiency of 100% for CBZ, the maximum removal efficiencies of the N-CNT/PES blend membranes for the EMPs from real water and wastewater samples recorded in this study were higher than those reported by, among others, Nagy et al. 53, Knappe et al. 54, Westerhoff et al. 55, Margot et al. 56, Klontza et al. 57, Clara et al. 58, Hernández-Leal et al. 59, and Gerrity et al. 60 (Tab. 5). The superior performance of the N-CNT/PES blend membranes over other water treatment techniques and materials can be attributed to the observed increase in the Σ, ultimate tensile strength, hydrophilicity, surface area, water flux as well as improved ξ of the fabricated membrane which led to higher rejection of EMPs.
3.2.3 Effect of N-CNT loading and the chemistry of analytes on mean removal efficiency of EMPs by PES membranes and N-CNT/PES blend membranes
The mean removal efficiencies of PES membranes and N-CNT/PES blend membranes for the selected EMPs are given in Fig. 5. From the data given in Fig. 5, it is clear that the N-CNT/PES blend membranes exhibited much higher removal efficiencies than the pristine PES membranes. Furthermore, it is worth noting that there was an improvement in the removal efficiencies of the N-CNT/PES blend membranes with increasing amounts of N-CNT in the matrix. This observation suggests that the increasing amounts of the N-CNT in the polymer matrix thus led to a steady increase in the efficiency of the N-CNT/PES blend membrane to remove EMPs in accordance with the increasing hydrophilicity of the membranes. Although the hydrophilicity of the 0.1 wt% N-CNT/PES blend membranes was lower than the rest of the fabricated N-CNT/PES membranes, it was still higher than that of the PES membrane. This led to the 0.1 wt% N-CNT/PES showing higher removal efficiency than its pristine PES membrane counterpart owing to its higher mean surface area than the pristine PES membrane reported in Tab. 1. The increase in the surface area of the 0.1 wt% N-CNT/PES blend membranes resulted in an increase in the number of sorption sites in the N-CNT/PES blend membrane matrix which increased its removal efficiency.
3.2.4 Effect of pressure applied on water flux of the PES membrane and N-CNT/PES blend membranes
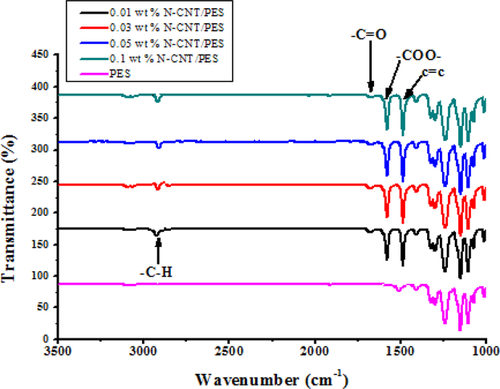
The pure water fluxes as a function of transmembrane pressure for the pristine PES membrane, as well as for the 0.01, 0.03, 0.05, and 0.1 wt% N-CNT/PES blend membranes are given in Fig. 6. The mean water flux of fabricated N-CNT/PES blend membranes was generally greater than that of the PES membrane and all membranes registered the highest flux at a pressure of 827.37 kPa (Fig. 6). There was also a marked increase in water flux as the amount of N-CNT loading increased from the 0.01 to 0.05 wt% N-CNT/PES blend membranes with increases in the amount of pressure applied. This suggested that the increase in transmembrane pressure led to an increase in water permeation and decreased permeation of EMPs thereby leading to improved separation and subsequent removal of the EMPs. This observation was in line with the increase in modification properties with an increase in N-CNT loading observed in the 0.01–0.05 wt% N-CNT/PES blend membranes. However, the 0.1 wt% N-CNT membranes registered the lowest water flux values compared to the rest of the N-CNT/PES blend membranes. The observed decrease in water flux for the 0.1 wt% N-CNT/PES blend membranes could be due to the observed decrease in hydrophilicity of the membrane owing to aggregation of N-CNT particles at the 0.1 wt% loading.
3.2.5 Antifouling performance of the membranes
The %FRR values for the PES membrane and for the 0.01, 0.03, 0.05, and 0.1 wt% N-CNT/PES blend membranes are presented in Tab. 6. Higher %FRR values reflected lower persistent BSA adsorption to the PES membrane, and the 0.01, 0.03, 0.05, and 0.1 wt% N-CNT/PES blend membranes during the filtration process. The recorded mean %FRR values were 42.8% for the PES membrane, and 44.4, 54.7, 60.4, and 59.2% for the 0.01, 0.03, 0.05, and 0.1 wt% N-CNT/PES blend membranes, respectively. The results revealed that higher membrane fouling occurred on the PES membrane surface compared to the surfaces of each of the 0.01, 0.03, 0.05, and 0.1 wt% N-CNT/PES blend membranes. The FRR values then decreased from 60.4% for the 0.05 wt% N-CNT/PES blend membrane to 59.2% for the 0.1 wt% N-CNT/PES blend membrane, possibly as a result of pore blockage with increasing amounts of N-CNTs in the matrix. The results confirmed that the fabricated N-CNT/PES blend membranes were more hydrophilic than the bare PES membrane. The BSA molecules were thus readily adsorbed onto the PES membrane and could not be eliminated by simply washing the membrane with water. Generally, the incorporation of N-CNTs enhanced the antifouling performance of the N-CNT/PES blend membranes owing to the improved membrane hydrophilicity. The higher %FRR values for all the modified PES membranes compared to that of the bare PES membrane suggested that the modification of PES membranes with N-CNTs significantly improved the fouling resistance of the N-CNT/PES blend membranes.
| Membrane | FFR (%) | Adsorbed BSA (μg/cm2) |
|---|---|---|
| PES | 42.8 | 49.1 |
| 0.01 wt% N-CNT/PES | 44.4 | 45.2 |
| 0.03 wt% N-CNT/PES | 54.7 | 40.9 |
| 0.05 wt% N-CNT/PES | 60.4 | 36.2 |
| 0.1 wt% N-CNT/PES | 59.2 | 40.1 |
The antifouling performance of the PES membrane and of the 0.01, 0.03, 0.05, and 0.1 wt% N-CNT/PES blend membranes was also investigated using the BSA static adsorption fouling test. The results of the BSA static adsorption fouling test are presented in Tab. 6. The average amounts of BSA adsorbed (μg/cm2) were 49.1 μg/cm2 for the PES membrane, and 45.2, 40.9, 36.2, and 40.1 μg/cm2 for the 0.01, 0.03, 0.05, and 0.1 wt% N-CNT/PES blend membranes, respectively. These results also confirm that the N-CNT/PES blend membranes are more resistant to BSA adsorption than bare PES membranes. Incorporation of N-CNTs led to the observed decrease in BSA adsorption on the surface of the N-CNT/PES blend membranes.
4 Conclusions and recommendations
Effective N-CNT/PES blend membrane systems were designed and fabricated during this study for the remediation of drinking water contaminated with EMPs, that is, BPA, NP, CAF, HHCB, AHTN, and CBZ. It was found that the performance of the N-CNT/PES blend membranes was largely affected by a wide range of membrane properties (surface area, ξ, hydrophilicity, Σ, ultimate tensile strength, amount of N-CNT loading) as well as the properties of EMPs (hydrophilicity and pKow values) and environmental conditions (applied pressure). The surface area, ξ, ultimate tensile strength, Σ and hydrophilicity (up to 0.05 wt% N-CNT/PES) increased upon adding N-CNT in small increments.
Improved performance of the N-CNT/PES blend membranes in removing EMPs was observed with an increase in the applied transmembrane pressure as well as with increasing amounts of N-CNT in the matrix. This is because an increase in transmembrane pressure leads to an increase in water permeation and a decrease in permeation of EMPS, thereby leading to improved separation and subsequent removal of the EMPs. The EMPs were observed to be removed from real water and wastewater samples by the N-CNT/PES blend membranes in the following order: HHCB > NP > AHTN > BPA > CBZ > CAF. The removal of the EMPs followed the order of the decrease in hydrophobicity based on the reported pKow values. The findings clearly show that N-CNTs have the potential to enhance the performance of PES membranes in the removal of EMPs through modification of the mechanical and chemical properties. The findings of this study also show that modification of PES membranes with N-CNTs enhanced the fouling resistance of the N-CNT/PES blend membranes owing to the observed higher%FRR values and lower BSA adsorption for the modified PES membrane surfaces than those of the bare PES membrane surfaces. It is recommended that further research should be done on the treatability of the membrane concentrate stream generated during membrane filtration for the removal of EMPs from water.
Acknowledgments
The authors are grateful to the University of South Africa for funding and to Mzuzu University for granting study leave to Elijah Wanda. The authors also wish to extend their gratitude to Dr. Hlengilizwe Nyoni and Miss Nozipho Gumbi for the support rendered during this study.
The authors have declared no conflict of interest




