Lantern-Like Diplatinum(III)-Catalyzed Redox-Free Borylation or Silylation of Alkynes
Chuntao Wang
Key Lab of Functional Molecular Engineering of Guangdong Province, School of Chemistry and Chemical Engineering, South China University of Technology, Guangzhou, Guangdong, 510640 China
Search for more papers by this authorXianyang Long
Key Lab of Functional Molecular Engineering of Guangdong Province, School of Chemistry and Chemical Engineering, South China University of Technology, Guangzhou, Guangdong, 510640 China
Search for more papers by this authorCorresponding Author
Tongxiang Cao
Key Lab of Functional Molecular Engineering of Guangdong Province, School of Chemistry and Chemical Engineering, South China University of Technology, Guangzhou, Guangdong, 510640 China
E-mail: [email protected]; [email protected]Search for more papers by this authorCorresponding Author
Shifa Zhu
Key Lab of Functional Molecular Engineering of Guangdong Province, School of Chemistry and Chemical Engineering, South China University of Technology, Guangzhou, Guangdong, 510640 China
School of Chemistry and Chemical Engineering, Zhejiang Sci-Tech University, Hangzhou, Zhejiang, 310018 China
E-mail: [email protected]; [email protected]Search for more papers by this authorChuntao Wang
Key Lab of Functional Molecular Engineering of Guangdong Province, School of Chemistry and Chemical Engineering, South China University of Technology, Guangzhou, Guangdong, 510640 China
Search for more papers by this authorXianyang Long
Key Lab of Functional Molecular Engineering of Guangdong Province, School of Chemistry and Chemical Engineering, South China University of Technology, Guangzhou, Guangdong, 510640 China
Search for more papers by this authorCorresponding Author
Tongxiang Cao
Key Lab of Functional Molecular Engineering of Guangdong Province, School of Chemistry and Chemical Engineering, South China University of Technology, Guangzhou, Guangdong, 510640 China
E-mail: [email protected]; [email protected]Search for more papers by this authorCorresponding Author
Shifa Zhu
Key Lab of Functional Molecular Engineering of Guangdong Province, School of Chemistry and Chemical Engineering, South China University of Technology, Guangzhou, Guangdong, 510640 China
School of Chemistry and Chemical Engineering, Zhejiang Sci-Tech University, Hangzhou, Zhejiang, 310018 China
E-mail: [email protected]; [email protected]Search for more papers by this authorComprehensive Summary
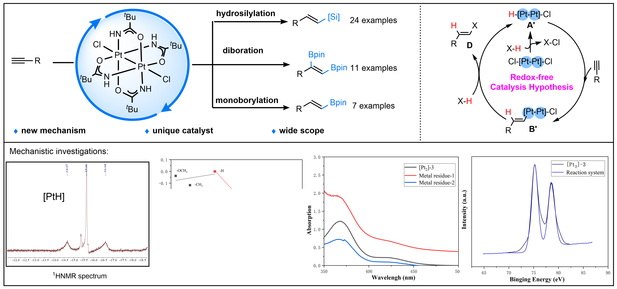
Binuclear platinum(III) complexes were known for their high index of antitumor activity and a lower associated nephrotoxicity. However, the chemistry and reactivity of binuclear platinum(III) compounds have not yet been explored to the same extent as those of platinum(II) and platinum(IV) species. Here, we reported the first binuclear platinum-catalyzed hydrosilylation, monoborylation and diboration reaction of alkynes with excellent selectivity and yield. Moreover, the mechanistic investigation by control experiments, kinetic isotope effect (KIE) study, Hammett plots, NMR spectra, UV−vis spectra, and X-ray photoelectron spectroscopy (XPS) analysis reveal that the Pt(III)2-catalyzed reactions pass through a σ-bond metathesis process rather than the two-electron redox processes of the mononuclear platinum catalysis. Moreover, there are two different rate-determining steps, in which the migratory insertion step dominates the rate of electron deficient substates and σ-bond metathesis process dominates electron rich counterparts, respectively.
Supporting Information
| Filename | Description |
|---|---|
| cjoc202400896-sup-0001-supinfo.pdfPDF document, 6.2 MB |
Appendix S1: Supporting Information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1(a) Uyeda, C.; Farley, C. M. Dinickel active sites supported by redox- active ligands. Acc. Chem. Res. 2021, 54, 3710–3719;
(b) Powers, I. G.; Uyeda, C. Metal–metal bonds in catalysis. ACS Catal. 2017, 7, 936–958;
(c) Cooper, B. G.; Napoline, J. W.; Thomas, C. M. Catalytic applications of early/late heterobimetallic complexes. Catal. Rev. 2012, 54, 1–40;
(d) Cotton, F. A. Discovering and understanding multiple metal-to-metal bonds. Acc. Chem. Res. 1978, 11, 225–232;
(e) Braunstein, P.; Rose, J., 7 - catalysis and related reactions with compounds containing heteronuclear metalmetal bonds. In Comprehensive Organometallic Chemistry ii, Eds.: E. W. Abel; F. G. A. Stone; G. Wilkinson, Elsevier, Oxford, 1995, pp. 351–385;
10.1016/B978-008046519-7.00091-5 Google Scholar(f) Gade, L. H. Highly polar metal–metal bonds in “early–late” heterodimetallic complexes. Angew. Chem. Int. Ed. 2000, 39, 2658–2678;10.1002/1521-3773(20000804)39:15<2658::AID-ANIE2658>3.0.CO;2-C CAS PubMed Web of Science® Google Scholar(g) Cotton, F. A. Discovering and understanding multiple metal-to-metal bonds. Acc. Chem. Res. 1978, 11, 225–232; (h) Mankad, N. P. Selectivity effects inbimetalliccatalysis. Chem. Eur. J. 2016, 22, 5822–5829; (i) Multiple Bonds between Metal Atoms, 3rd edn., Eds: F. A. Cotton; C. A. Murillo; R. A. Walton, Springer Science and Business Media, New York, 2005;10.1007/b136230 Google Scholar(j) Muetterties, E. L.; Krause, M. J. Catalysis by molecular metal clusters. Angew. Chem. Int. Ed. 1983, 22, 135–148.
- 2(a) Brantley, C. L.; Coombs, T. C. Intermolecular pauson–khand reactions of N-substituted maleimides. Tetrahedron Lett. 2017, 58, 4519–4524; (b) Manton, J. C.; Cerpentier, F. J. R.; Harvey, E. C.; Clark, I. P.; Greetham, G. M.; Long, C.; Pryce, M. T. Photochemical or electrochemical bond breaking – exploring the chemistry of (μ2-alkyne)Co2(Co)6 complexes using time-resolved infrared spectroscopy, spectro-electrochemical and density functional methods. Dalton Trans. 2019, 48, 14642–14652; (c) Jeong, N. 5.24 the pauson–khand reaction. In Comprehensive Organic Synthesis, 2nd edn., Ed.: P. Knochel, Elsevier, Amsterdam, 2014, pp. 1106–1178; (d) Dong, J.; Yuan, X.-A.; Yan, Z.; Mu, L.; Ma, J.; Zhu, C.; Xie, J. Manganese-catalysed divergent silylation of alkenes. Nat. Chem. 2021, 13, 182–190; (e) Powers, D. C.; Ritter, T. A transition state analogue for the oxidation of binuclear Palladium(II) to binuclear Palladium(III) complexes. Organometallics 2013, 32, 2042–2045; (f) Powers, D. C.; Ritter, T. Bimetallic redox synergy in oxidative palladium catalysis. Acc. Chem. Res. 2012, 45, 840–850; (g) Toullec, P. Y.; Michelet, V. Cycloisomerization of 1,n-enynes via carbophilic activation. Top. Curr. Chem. 2011, 302, 31–80; (h) Doyle, M. P.; Forbes, D. C. Recent advances in asymmetric catalytic metal carbene transformations. Chem. Rev. 1998, 98, 911–936; (i) Davies, H. M. L.; Lian, Y. The combined C–H functionalization/cope rearrangement: Discovery and applications in organic synthesis. Acc. Chem. Res. 2012, 45, 923–935; (j) Zhu, D.; Chen, L.; Fan, H.; Yao, Q.; Zhu, S. Recent progress on donor and donor-donor carbenes. Chem. Soc. Rev. 2020, 49, 908–950; (k) Cheng, Q. Q.; Deng, Y.; Lankelma, M.; Doyle, M. P. Cycloaddition reactions of enoldiazo compounds. Chem. Soc. Rev. 2017, 46, 5425–5443; (l) Lin, Y.-S.; Takeda, S.; Matsumoto, K. Consecutive double nucleophilic attacks on an olefin promoted by a Platinum(III) dimeric complex. Organometallics 1999, 18, 4897–4899; (m) Matsumoto, K.; Nagai, Y.; Matsunami, J.; Mizuno, K.; Abe, T.; Somazawa, R.; Kinoshita, J.; Shimura, H. A synthetic route to alkyl−PtIII dinuclear complexes from olefins and its implication on the olefin oxidation catalyzed by amidate-bridged PtIII dinuclear complexes. J. Am. Chem. Soc. 1998, 120, 2900–2907; (n) Nagashima, J.; Shimazaki, K.; Sekiya, H.; Iwatsuki, S.; Ishihara, K.; Matsumoto, K. Formation mechanism of 2-methyl-2- buten-1,4-diol and 2-methyl-3-buten-1,2-diol from 2-methyl-1,3- butadiene on a head-to-head pivalamidato-bridged cis-diammineplatinum(III) binuclear complex. Dalton Trans. 2011, 40, 6998–7007; (o) Ochiai, M.; Fukui, K.; Iwatsuki, S.; Ishihara, K.; Matsumoto, K. Synthesis of aryl-platinum dinuclear complexes via ortho C−H bond activation of phenol and transmetalation of arylboronic acid. Organometallics 2005, 24, 5528–5536; (p) Ochiai, M.; Lin, Y.-S.; Yamada, J.; Misawa, H.; Arai, S.; Matsumoto, K. Reactions of a Platinum(III) dimeric complex with alkynes in water: Novel approach to α-aminoketone, α-iminoketone, and α,β-diimine via ketonyl−Pt(III) dinuclear complexes. J. Am. Chem. Soc. 2004, 126, 2536–2545; (q) Andjaba, J. M.; Rybak, C. J.; Wang, Z.; Ling, J.; Mei, J.; Uyeda, C. Catalytic synthesis of conjugated azopolymers from aromatic diazides. J. Am. Chem. Soc. 2021, 143, 3975–3982; (r) Zhou, Y.-Y.; Uyeda, C. Catalytic reductive [4 + 1]-cycloadditions of vinylidenes and dienes. Science 2019, 363, 857–862; (s) Tang, R.; Wan, Q.; Lam, T.-L.; To, W.-P.; Low, K.-H.; Tang, Z.; Du, L.; Lu, W.; Che, C.-M. Copper(I)-based metal-metal-to- ligand charge transfer excited state with halogen-atom transfer photo-reactivity and photocatalysis. Chem 2024, 10, 2807–2828; (t) Wen, S.; Zhang, C.; Liu, L.-J.; Wang, Z.; Sun, D.; He, J. Stable and recyclable copper nanoclusters with exposed active sites for broad-scope protosilylation in open air. Angew. Chem. Int. Ed. 2024, e202416851.
- 3 Wu, R.; Zhu, D.; Zhu, S. Dirhodium: Carbene transformations and beyond. Org. Chem. Front. 2023, 10, 2849–2878.
- 4 Davies, H. M. L.; Liao, K. Dirhodium tetracarboxylates as catalysts for selective intermolecular C–H functionalization. Nat. Rev. Chem. 2019, 3, 347–360.
- 5(a) Shi, Q.; Liao, Z.; Liu, Z.; Wen, J.; Li, C.; He, J.; Deng, J.; Cen, S.; Cao, T.; Zhou, J.; Zhu, S. Divergent synthesis of benzazepines and bridged polycycloalkanones via dearomative rearrangement. Nat. Commun. 2022, 13, 4402; (b) Wu, R.; Chen, K.; Ma, J.; Yu, Z.-X.; Zhu, S. Synergy of activating substrate and introducing C-H···O interaction to achieve Rh2(II)-catalyzed asymmetric cycloisomerization of 1,n-enynes. Sci. China Chem. 2020, 63, 1230–1239; (c) Wang, C.; Wu, R.; Chen, K.; Zhu, S. Enantioselective synthesis of biscyclopropanes using alkynes as dicarbene equivalents. Angew. Chem. Int. Ed. 2023, 62, e202305864; (d) Wang, C.; Zhu, D.; Wu, R.; Zhu, S. Dirhodium-catalyzed enantioselective synthesis of difluoromethylated cyclopropanes via enyne cycloisomerization. Adv. Sci. 2023, 11, 2306404; (e) Zhu, D.; Ma, J.; Luo, K.; Fu, H.; Zhang, L.; Zhu, S. Enantioselective intramolecular C−H insertion of donor and donor/donor carbenes by a nondiazo approach. Angew. Chem. Int. Ed. 2016, 55, 8452–8456; (f) Wu, R.; Lu, J.; Cao, T.; Ma, J.; Chen, K.; Zhu, S. Enantioselective Rh(II)-catalyzed desymmetric cycloisomerization of diynes: Constructing furan-fused dihydropiperidines with an alkyne-substituted aza-quaternary stereocenter. J. Am. Chem. Soc. 2021, 143, 14916–14925; (g) Wu, R.; Chen, Y.; Zhu, S. Rh(II)-catalyzed enynal cycloisomerization for the generation of vinyl carbene: Divergent access to polycyclic heterocycles. ACS Catal. 2023, 13, 132–140; (h) Qiu, S.; Gao, X.; Zhu, S. Dirhodium(II)-catalysed cycloisomerization of azaenyne: Rapid assembly of centrally and axially chiral isoindazole frameworks. Chem. Sci. 2021, 12, 13730–13736; (i) Zhu, D.; Cao, T.; Chen, K.; Zhu, S. Rh2(II)-catalyzed enantioselective intramolecular büchner reaction and aromatic substitution of donor–donor carbenes. Chem. Sci. 2022, 13, 1992–2000; (j) Wang, C.; Zhu, D.; Wu, R.; Zhu, S. Dirhodium-catalyzed enantioselective synthesis of difluoromethylated cyclopropanes via enyne cycloisomerization. Adv. Sci. 2024, 11, 2306404.
- 6 Matsumoto, K.; Sakai, K. Structures and reactivities of platinum-blues and the related amidate-bridged PlatinumIII compounds. In Advances in Inorganic Chemistry, Vol. 49, Ed.: A. G. Sykes, Academic Press, 1999, pp. 375–427.
- 7(a) Boisvert, L.; Goldberg, K. I. Reactions of late transition metal complexes with molecular oxygen. Acc. Chem. Res. 2012, 45, 899–910; (b) Chianese, A. R.; Lee, S. J.; Gagné, M. R. Electrophilic activation of alkenes by Platinum(II): So much more than a slow version of Palladium(II). Angew. Chem. Int. Ed. 2007, 46, 4042–4059; (c) Huo, S. Platinum in chemistry: An adventure from phosphorescent materials to catalytic C−H functionalization. Chem. Rec. 2018, 18, 1583–1595; (d) Sakai, K.; Ozawa, H. Homogeneous catalysis of platinum(II) complexes in photochemical hydrogen production from water. Coord. Chem. Rev. 2007, 251, 2753–2766.
- 8(a) Lin, Y.-S.; Misawa, H.; Yamada, J.; Matsumoto, K. Synthesis of ketonylplatinum(III) dinuclear complexes: Observation of the competitive radical vs electrophilic displacement in Pt(III)-promoted C−H bond activation of ketones. J. Am. Chem. Soc. 2001, 123, 569–575; (b) Matsumoto, K.; Arai, S.; Ochiai, M.; Chen, W.; Nakata, A.; Nakai, H.; Kinoshita, S. Synthesis of the pivalamidate-bridged pentanuclear Platinum(II,III) linear complexes with Pt···Pt interactions. Inorg. Chem. 2005, 44, 8552–8560.
- 9 Dolmella, A.; Intini, F. P.; Pacifico, C.; Padovano, G.; Natile, G. Structural characterization of the ‘lantern-shaped’ Platinum(III) complex [Pt2Cl2{N(H)C(But)O}4]. Polyhedron 2002, 21, 275–280.
- 10(a) Xu, X.; Gao, A.; Chen, W.; Xu, X.; Li, J.; Cui, C. Lanthanum ate amide-catalyzed regio- and stereoselective hydrosilylation of internal alkynes. ACS Catal. 2023, 13, 3743–3748; (b) Ye, F.; Xu, Z.; Xu, L.-W. The discovery of multifunctional chiral P ligands for the catalytic construction of quaternary carbon/silicon and multiple stereogenic centers. Acc. Chem. Res. 2021, 54, 452–470; (c) de Almeida, L. D.; Wang, H.; Junge, K.; Cui, X.; Beller, M. Recent advances in catalytic hydrosilylations: Developments beyond traditional platinum catalysts. Angew. Chem. Int. Ed. 2021, 60, 550–565; (d) Chen, W.; Jiang, C.; Zhang, J.; Xu, J.; Xu, L.; Xu, X.; Li, J.; Cui, C. Rare-earth-catalyzed selective 1,4-hydrosilylation of branched 1,3-enynes giving tetrasubstituted silylallenes. J. Am. Chem. Soc. 2021, 143, 12913–12918.
- 11 Parson, T. G.; Butikofer, J. L.; Houlis, J. F.; Roddick, D. M. Organometallics in superacidic media: Characterization of remarkably stable platinum–methyl bonds in HF/SbF5 solution. Organometallics 2017, 36, 136–141.
- 12(a) Nadeau, B. E.; Beattie, D. D.; Lui, E. K. J.; Tewkesbury, M.; Love, J. A.; Schafer, L. L. Electronic directing group modification for improved Ni(II)-mediated C(sp3)–H activation: A hammett investigation of 8-aminoquinoline. Organometallics 2023, 42, 2326–2334; (b) Guo, H.; Zhang, S.; Yu, X.; Feng, X.; Yamamoto, Y.; Bao, M. [3 + 2] cycloaddition of α-aryl-α-diazoacetates with terminal alkynes via the cooperative catalysis of palladium and acid. ACS Catal. 2021, 11, 10789–10795; (c) Hansch, C.; Leo, A.; Taft, R. W. A survey of hammett substituent constants and resonance and field parameters. Chem. Rev. 1991, 91, 165–195.
- 13(a) Nazari, M.; Shahsavari, H. R. Strong red emissions induced by Pt–Pt interactions in binuclear cycloplatinated(II) complexes containing bridging diphosphines. Appl. Organometal Chem. 2019, 33, e5020; (b) Kishi, M.; Terada, T.; Kuraishi, Y.; Sugaya, T.; Iwatsuki, S.; Ishihara, K.; Matsumoto, K. Axial ligand effect on the reaction mechanism of head-to-head pivalamidato-bridged Pt(III) binuclear complex containing an equatorial bromide ligand with acetone. Inorg. Chim. Acta 2015, 433, 45–51; (c) Saeki, N.; Nakamura, N.; Ishibashi, T.; Arime, M.; Sekiya, H.; Ishihara, K.; Matsumoto, K. Mechanism of ketone and alcohol formations from alkenes and alkynes on the head-to-head 2-pyridonato-bridged cis-diammineplatinum(III) dinuclear complex. J. Am. Chem. Soc. 2003, 125, 3605–3616




