Ytterbium-Catalyzed Tandem Diels–Alder/Claisen Rearrangement/Decarboxylation of Hetero-Allenes for the Synthesis of Diarylmethanes
Bin Chen
Jiangsu Key Laboratory of Advanced Catalytic Materials & Technology, School of Petrochemical Engineering, Changzhou University, Changzhou, Jiangsu, 213164 China
Search for more papers by this authorShan Zhong
Jiangsu Key Laboratory of Advanced Catalytic Materials & Technology, School of Petrochemical Engineering, Changzhou University, Changzhou, Jiangsu, 213164 China
Search for more papers by this authorHuilin Zhan
Jiangsu Key Laboratory of Advanced Catalytic Materials & Technology, School of Petrochemical Engineering, Changzhou University, Changzhou, Jiangsu, 213164 China
Search for more papers by this authorZhangyu Han
Jiangsu Key Laboratory of Advanced Catalytic Materials & Technology, School of Petrochemical Engineering, Changzhou University, Changzhou, Jiangsu, 213164 China
Search for more papers by this authorJianwei Sun
Department of Chemistry, The Hong Kong University of Science and Technology, Clear Water Bay, Kowloon, Hong Kong, SAR, 999077 China
Search for more papers by this authorCorresponding Author
Hai Huang
Jiangsu Key Laboratory of Advanced Catalytic Materials & Technology, School of Petrochemical Engineering, Changzhou University, Changzhou, Jiangsu, 213164 China
E-mail: [email protected]Search for more papers by this authorBin Chen
Jiangsu Key Laboratory of Advanced Catalytic Materials & Technology, School of Petrochemical Engineering, Changzhou University, Changzhou, Jiangsu, 213164 China
Search for more papers by this authorShan Zhong
Jiangsu Key Laboratory of Advanced Catalytic Materials & Technology, School of Petrochemical Engineering, Changzhou University, Changzhou, Jiangsu, 213164 China
Search for more papers by this authorHuilin Zhan
Jiangsu Key Laboratory of Advanced Catalytic Materials & Technology, School of Petrochemical Engineering, Changzhou University, Changzhou, Jiangsu, 213164 China
Search for more papers by this authorZhangyu Han
Jiangsu Key Laboratory of Advanced Catalytic Materials & Technology, School of Petrochemical Engineering, Changzhou University, Changzhou, Jiangsu, 213164 China
Search for more papers by this authorJianwei Sun
Department of Chemistry, The Hong Kong University of Science and Technology, Clear Water Bay, Kowloon, Hong Kong, SAR, 999077 China
Search for more papers by this authorCorresponding Author
Hai Huang
Jiangsu Key Laboratory of Advanced Catalytic Materials & Technology, School of Petrochemical Engineering, Changzhou University, Changzhou, Jiangsu, 213164 China
E-mail: [email protected]Search for more papers by this authorComprehensive Summary
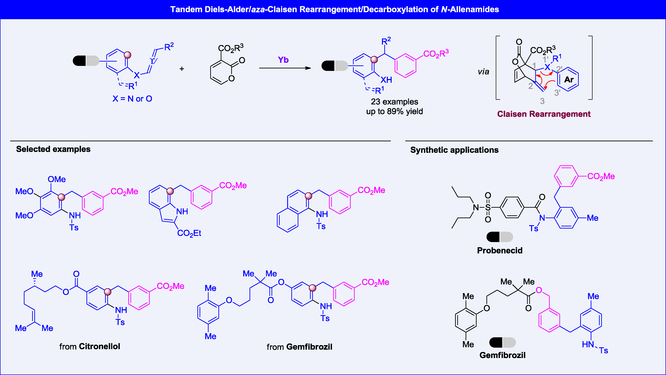
A tandem Diels–Alder reaction/Claisen rearrangement/decarboxylation strategy of N-allenamides (or aryloxyallenes) with 3-alkoxycarbonyl-2-pyrones has been developed for the efficient synthesis of diarylmethanes with moderate to good yields. The reaction exhibits good functional group tolerance and can be applied to late-stage modifications of known drug molecules. Mechanistic studies indicate that the ester group at the 3-position of 2-pyrones is essential, and the initial Diels–Alder reaction between the 2-pyrones and the proximal C=C bond of the N-allenamides (or aryloxyallenes) is crucial for the success of the reaction.
Supporting Information
| Filename | Description |
|---|---|
| cjoc202400874-sup-0001-supinfo.pdfPDF document, 2.7 MB |
Appendix S1: Supporting Information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1(a) Ziarani, G. M.; Moradi, R.; Zandiyeh, M.; Lashgari, N. 4-Hydroxy-6-methyl-2-pyrone: A Versatile Synthon in the Synthesis of Heterocyclic Scaffolds via Multicomponent Reactions. Heterocycles 2018, 96, 381–424; (b) Prendergast, A. M.; McGlacken, G. P. Transition Metal Mediated C-H Activation of 2-Pyrones, 2-Pyridones, 2-Coumarins and 2-Quinolones. Eur. J. Org. Chem. 2018, 6068–6082; (c) Ahmad, T.; Rasheed, T.; Hussain, M.; Rizwan, K. Emergence of 2-Pyrone and Its Derivatives, from Synthesis to Biological Perspective: An Overview and Current Status. Topics Cur. Chem. 2021, 379, 38.
- 2(a) Xu, M.; Cai, Q. Progress of catalytic asymmetric Diels-Alder reactions of 2-pyrones. Chin. J. Org. Chem. 2022, 42, 698–713 (in Chinese); (b) Cai, Q. The [4+2]-Cycloaddition of 2-Pyrone in Total Synthesis. Chin. J. Chem. 2019, 37, 946–976; (c) Huang, G.; Kouklovsky, C.; Torre, A. Inverse-Electron-Demand Diels-Alder Reactions of 2-Pyrones: Bridged Lactones and Beyond. Chem. Eur. J. 2021, 27, 4760–4788.
- 3 He, J.-X.; Si, X.-G.; Lu, Q.-T.; Zhang, Q.-W.; Cai, Q. An Enantioselective Approach to Heteroatom-Containing Bicyclic Derivatives via Inverse-Electron-Demand Diels-Alder Reactions. Chin. J. Chem. 2023, 41, 21–26.
- 4 Si, X.-G.; Zhang, Z.-M.; Zheng, C.-G.; Li, Z.-T.; Cai, Q. Enantioselective Synthesis of cis-Decalin Derivatives by the Inverse-Electron-Demand Diels-Alder Reaction of 2-Pyrones. Angew. Chem. Int. Ed. 2020, 59, 18412–18417.
- 5 Shin, H.-S. Jung, Y.-G. Cho, H.-K. Park, Y.-G. Cho, C.-G. Total Synthesis of (±)-Lycorine from the Endo-Cycloadduct of 3,5-Dibromo-2-pyrone and (E)-β-Borylstyrene. Org. Lett. 2014, 16, 5718–5720.
- 6(a) Huang, G.; Guillot, R.; Kouklovsky, C.; Maryasin, B.; Torre, A. Diastereo- and Enantioselective Inverse-Electron-Demand Diels–Alder Cycloaddition between 2-Pyrones and Acyclic Enol Ethers. Angew. Chem. Int. Ed. 2022, 61, e202208185; (b) Zhang, Z.-M.; Zhang, J.; Cai, Q. Enantioselective and Collective Total Synthesis of Pentacyclic 19-nor-clerodanes. Chem. Sci. 2023, 14, 12598–12605; (c) Xu, M.-M.; Yang, L.; Tan, K.; Chen, X.; Lu, Q.-T.; Houk, K. N.; Cai, Q. An enantioselective ambimodal cross-Diels-Alder reaction and applications in synthesis. Nat. Catal. 2021, 4, 892–900; (d) Yu, H.-B.; Chen, Y.-G.; Tian, Y.; Xie, M.-S.; Guo, H.-M. Nickel(II)-Catalyzed Asymmetric Inverse-Electron-Demand Diels-Alder Reaction of 2-Pyrones with Styrenes and Indenes. ACS Catal. 2024, 14, 8930–8938; (e) Huang, G.; Laporte, A.; Pages, L.; Kouklovsky, C.; Torre, A. Total Synthesis of Lucidumone: Attempted Shortcuts, Dead Ends and Lessons Learnt. Synthesis 2024, 56, 1585–1592.
- 7(a) Delaney, P. M.; Browne, D. L.; Adams, H.; Plant, A.; Harrity, J. P. A. A 2-Pyrone Cycloaddition Route to Functionalized Aromatic Boronic Esters. Tetrahedron 2008 64, 866–873; (b) Xu, M.-M.; You, X.-Y.; Zhang, Y.-Z.; Lu, Y.; Tan, K.; Yang, L.; Cai, Q. Enantioselective Synthesis of Axially Chiral Biaryls by Diels-Alder/Retro-Diels-Alder Reaction of 2-Pyrones with Alkynes. J. Am. Chem. Soc. 2021, 143, 8993–9001; (c) Huang, B.; Xing, D.; Jiang, H.; Huang, L. Lewis Acid-Catalyzed Formal [4 + 2] Reaction of Alkynyl Sulfides and 2-Pyrones to Access Polysubstituted Aryl Sulfides. J. Org. Chem. 2024, 89, 7280–7285; (d) Loupy, A.; Maurel, F.; Sabatie-Gogova, A. Improvements in Diels-Alder Cycloadditions with Some Acetylenic Compounds under Solvent- free Microwave-assisted Conditions: Experimental Results and Theoretical Approaches. Tetrahedron 2004, 60, 1683–1691; (e) Subramanian, L. R. Product Subclass 2: Arene Dicarboxylic Acid Esters. Sci. Synth. 2006, 20b, 947–955.
- 8For a selected review article: Hourtoule, M.; Miesch, L. Construction of C-N and C-O Bonds Based on N-Allenamide Functionalization. Org. Biomol. Chem. 2022, 20, 9069–9084.
- 9(a) Pirovano, V.; Decataldo, L.; Rossi, E.; Vicente, R. Gold-catalyzed Synthesis of Tetrahydrocarbazole Derivatives through an Intermolecular Cycloaddition of Vinyl Indoles and N-Allenamides. Chem. Commun. 2013, 49, 3594–3596; (b) Suárez-Pantiga, S.; Hernández-Díaz, C.; Rubio, E.; González, J. Intermolecular [2+2] Reaction of N-Allenylsulfonamides with Vinylarenes: Enantioselective Gold(I)- Catalyzed Synthesis of Cyclobutane Derivatives. Angew. Chem. Int. Ed. 2012, 51, 11552–11555; (c) Varela, I.; Faustino, H.; Díez, E.; Iglesias-Sigüenza, J.; Grande-Carmona, F.; Fernández, R.; Lassaletta, J. M.; Mascareñas, J. L.; López, F. Gold(I)-Catalyzed Enantioselective [2+2+2] Cycloadditions: An Expedient Entry to Enantioenriched Tetrahydropyran Scaffolds. ACS Catal. 2017, 7, 2397–2402; (d) He, J.; Yang, L.; Zhang, X.; Xu, W.; Wang, H.; Lang, M.; Wang, J.; Peng, S. Stereodivergent Syntheses of N-Heterocycles by Catalyst-Controlled Reaction of Imidazolidines with Allenes. ACS Catal. 2022, 12, 14647–14653; (e) Wang, C.; Xu, G.; Shao, Y.; Tang, S.; Sun, J. Gold-Catalyzed Intermolecular Formal [4 + 2 + 2]-Cycloaddition of Anthranils with Allenamides. Org. Lett. 2020, 22, 5990–5994; (f) Marcote, D. C.; Varela, I.; Fernández-Casado, J.; Mascareñas, J. L.; López, F. Gold(I)-Catalyzed Enantioselective Annulations between Allenes and Alkene-Tethered Oxime Ethers: A Straight Entry to Highly Substituted Piperidines and aza-Bridged Medium-Sized Carbocycles. J. Am. Chem. Soc. 2018, 140, 16821–16833; (g) Xu, B.; Zhang, Z.-M.; Han, J.; Gu, G.; Zhang, J. Enantioselectivity Tunable Gold-Catalyzed Intermolecular [3+2] Cycloaddition of N-Allenamides with Nitrones. Chin. J. Chem. 2022, 40, 1407–1412; (h) Chakrabarty, I.; Inamdar, S. M.; Akram, M. O.; Gade, A. B.; Banerjee, S.; Bera, S.; Patil, N. T. [3+2]-Annulation of Platinum- bound Azomethine Ylides with Distal CQC Bonds of N-Allenamides. Chem. Commun. 2017, 53, 196–199; (i) Faustino, H.; Varela, I.; Mascareñas, J. L.; López, F. Gold(I)-catalyzed [2 + 2 + 2] Cycloaddition of Allenamides, Alkenes and Aldehydes: a Straightforward Approach to Tetrahydropyrans. Chem. Sci. 2015, 6, 2903–2908; (j) Li, G.-H.; Zhou, W.; Li, X.-X.; Bi, Q.-W.; Wang, Z.; Zhao, Z.-G.; Hu, W.-X.; Chen, Z. Gold Catalyzed Enantioselective Intermolecular [3+2] Dipolar Cycloaddition of N-Allenyl Amides with Nitrones. Chem. Commun. 2013, 49, 4770–4772.
- 10(a) Wang, Y.; Zhang, P.; Qian, D.; Zhang, J. Highly Regio-, Diastereo-, and Enantioselective Gold(I)-Catalyzed Intermolecular Annulations with N-Allenamides at the Proximal C=C Bond. Angew. Chem. Int. Ed. 2015, 54, 14849–14852. (b) Lin, T.-Y.; Zhu, C.-Z.; Zhang, P.; Wang, Y.; Wu, H.-H.; Feng, J.-J.; Zhang, J. Regiodivergent Intermolecular [3+2] Cycloadditions of Vinyl Aziridines and Allenes: Stereospecific Synthesis of Chiral Pyrrolidines. Angew. Chem. Int. Ed. 2016, 55, 10844–10848; (c) Pandit, Y. B.; Liu, R.-S. Dynamic Kinetic Resolution in Gold-Catalyzed (4 + 2)-Annulations between Alkynyl Benzaldehydes and Allenamides to Yield Enantioenriched All-Carbon Diarylalkylmethane Derivatives. Org. Lett. 2022, 24, 548–553.
- 11 Zhang, P.-C.; Han, J.; Zhang, J. Pd/PC-Phos-Catalyzed Enantioselective Intermolecular Denitrogenative Cyclization of Benzotriazoles with Allenes and N-Allenamides. Angew. Chem. Int. Ed. 2019, 58, 11444–11448.
- 12 Liu, R.-R.; Hu, J.-P.; Hong, J.-J.; Lu, C.-J.; Gao, J.-R.; Jia, Y.-X. Enantioselective [2 + 2] Cycloaddition of N-Allenamides with Cyclic N-Sulfonylketimines: Access to Polysubstituted Azetidines Bearing Quaternary Stereocenters. Chem. Sci. 2017, 8, 2811–2815.
- 13 Zhong, X.; Tan, J.; Qiao, J.; Zhou, Y.; Lv, C.; Su, Z.; Dong, S.; Feng, X. Catalytic Asymmetric Synthesis of Spirocyclobutyl Oxindoles and beyond via [2+2] Cycloaddition and Sequential Transformations. Chem. Sci. 2021, 12, 9991–9997.
- 14(a) Liu, S.; Qian, H.; Zhang, T.; Xie, H.; Han, Z.; Guo, W.; Huang, H.; Sun, J. Mild Intermolecular Synthesis of a Cyclopropane-Incorporated Tricyclic Skeleton: Unusual Reactivity of Isobenzopyryliums. Angew. Chem. Int. Ed. 2021, 60, 21272–21276;
(b) Zhu, H.; Xu, L.; Zhu, B.; Liao, M.; Li, J.; Han, Z.; Sun, J.; Huang, H. Copper-catalyzed Enantioselective Formal [4+1] and [3+3] Cycloaddition of Ethynylethylene Carbonates. Org. Lett. 2023, 25, 9213–9218;
(c) Chen, L.; Xie, H.; Xue, Y.; Han, Z.; Sun, J.; Huang, H. Palladium-catalyzed [4+2] and [6+2] Dipolar Cycloadditions for the Construction of Benzo[d]isothiazole 1,1-Dioxide Fused 1,3-Oxazinanes and 1,3-Oxazocanes. Chin. J. Chem. 2024, 42, 829–834;
(d) Li, J.; Fang, M.; Liao, M.; Xie, H.; Dong, X.-Q.; Han, Z.; Sun, J.; Huang, H. Synthesis of Medium-sized Heterocycles from Oxetanes Based on an Allylic Amination/Ring-opening Strategy. Chem. Commun. 2023, 59, 14467–14470;
(e) Yang, Z.; Bao, Y.; Huang, J.; Han, Z.; Sun, J.; Huang, H. Tandem Allylic Amination/oxa-Michael addition of Vinyl Methylene Cyclic Carbonates via Palladium-Organo Relay Catalysis. Org. Lett. 2023, 25, 2624–2629;
10.1021/acs.orglett.3c02014 Google Scholar(f) Xie, H.; Chen, L.; Han, Z.; Yang, Z.; Sun, J.; Huang, H. Pd-Catalyzed Ligand-directed Divergent Cycloaddition of Cyclic 1-Azadienes with Oxo-1,4-dipoles. Org. Lett. 2023, 25, 5011–5016; (g) Yang, Z.; Xie, H.; Tang, L.; Sun, J.; Han, Z.; Huang, H. Construction of Diverse Polycyclic N-Heterocycles via Cascade Allylic Amination/Diels-Alder Reaction. Chem. Commun. 2022, 58, 13258–13261.
- 15(a) Castro, A. M. M. Claisen Rearrangement over the Past Nine Decades. Chem. Rev. 2004, 104, 2939–3002; (b) Majumdar, K. C.; Nandi, R. K. The Claisen Rearrangement in the Syntheses of Bioactive Natural Products. Tetrahedron 2013, 69, 6921–6957; (c) Kotha, S.; Meshram, M. Application of Claisen Rearrangement and Olefin Metathesis in Organic Synthesis. Chem. Asian J. 2018, 13, 1758–1766.
- 16(a) Ameen, D.; Snape, T. J. Chiral 1,1-Diaryl Compounds as Important Pharmacophores. Med. Chem. Commun. 2013, 4, 893–907; (b) Gijsen, H. J. M.; Berthelot, D.; Zaja, M.; Brône, B.; Geuens, I.; Mercken, M. Analogues of Morphanthridine and the Tear Gas Dibenz[b,f][1,4]oxazepine (CR) as Extremely Potent Activators of the Human Transient Receptor Potential Ankyrin 1 (TRPA1) Channel. J. Med. Chem. 2010, 53, 7011–7020; (c) Kung, H. F.; Newman, S.; Choi, S.-R. Oya, S.; Hou, C.; Zhuang, Z.-P.; Acton, P. D.; Plössl, K.; Winkler, J.; Kung, M.-P. 2-(2-(Dimethylaminomethyl)phenoxy)-5-iodophenylamine: An Improved Serotonin Transporter Imaging Agent. J. Med. Chem. 2004, 47, 5258–5264.
- 17For selected reviews: (a) Nambo, M.; Crudden, C. M. Recent Advances in the Synthesis of Triarylmethanes by TransitionMetal Catalysis. ACS Catal. 2015, 5, 4734; (b) Mondal, S.; Roy, D.; Panda, G. Overview on the Recent Strategies for the Enantioselective Synthesis of 1,1-Diarylalkanes, Triarylmethanes and Related Molecules Containing the Diarylmethine Stereocenter. ChemCatChem 2018, 10, 1941. (c) Belal, M.; Li, Z.; Lu, X.; Yin, G. Recent Advances in the Synthesis of 1,1-Diarylalkanes by Transitionmetal Catalysis. Sci. China Chem. 2021, 64, 513–533.




