Palladium-Catalyzed Construction of Phthalides Bearing Two Adjacent Stereocenters through Retro-oxa-Michael Addition
Li-Xia Liu
State Key Laboratory of Catalysis, Dalian Institute of Chemical Physics, Chinese Academy of Sciences, Dalian, Liaoning, 116023 China
University of Chinese Academy of Sciences, Beijing, 100049 China
Search for more papers by this authorTong Niu
State Key Laboratory of Catalysis, Dalian Institute of Chemical Physics, Chinese Academy of Sciences, Dalian, Liaoning, 116023 China
Search for more papers by this authorYu-Qing Bai
State Key Laboratory of Catalysis, Dalian Institute of Chemical Physics, Chinese Academy of Sciences, Dalian, Liaoning, 116023 China
Search for more papers by this authorCorresponding Author
Yong-Gui Zhou
State Key Laboratory of Catalysis, Dalian Institute of Chemical Physics, Chinese Academy of Sciences, Dalian, Liaoning, 116023 China
State Key Laboratory of Organometallic Chemistry, Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences, Shanghai, 200032 China
E-mail: [email protected]Search for more papers by this authorLi-Xia Liu
State Key Laboratory of Catalysis, Dalian Institute of Chemical Physics, Chinese Academy of Sciences, Dalian, Liaoning, 116023 China
University of Chinese Academy of Sciences, Beijing, 100049 China
Search for more papers by this authorTong Niu
State Key Laboratory of Catalysis, Dalian Institute of Chemical Physics, Chinese Academy of Sciences, Dalian, Liaoning, 116023 China
Search for more papers by this authorYu-Qing Bai
State Key Laboratory of Catalysis, Dalian Institute of Chemical Physics, Chinese Academy of Sciences, Dalian, Liaoning, 116023 China
Search for more papers by this authorCorresponding Author
Yong-Gui Zhou
State Key Laboratory of Catalysis, Dalian Institute of Chemical Physics, Chinese Academy of Sciences, Dalian, Liaoning, 116023 China
State Key Laboratory of Organometallic Chemistry, Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences, Shanghai, 200032 China
E-mail: [email protected]Search for more papers by this authorComprehensive Summary
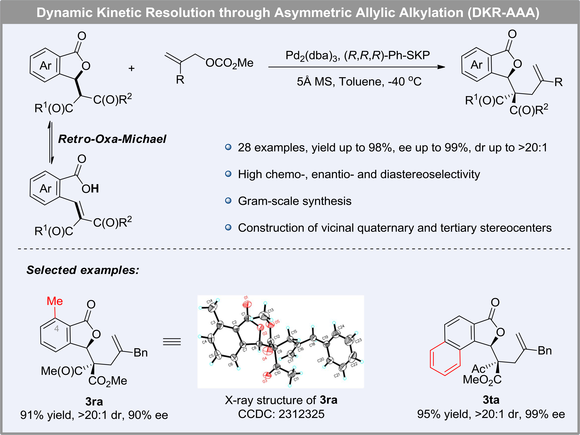
Optically active phthalides are prevalent in many natural and bioactive products. Herein, a novel dynamic kinetic resolution of isobenzofuranone derivatives through palladium-catalyzed asymmetric allylic alkylation has been developed to synthesize phthalide derivatives bearing vicinal quaternary and tertiary stereocenters with high yields, showing excellent chemo-, enantio- and diastereoselectivity. Furthermore, gram-scale experiment underwent smoothly and the transformation of product could build a bridged bicyclic skeleton.
Supporting Information
| Filename | Description |
|---|---|
| CJOC202400612-sup-0001-supinfo.pdfPDF document, 9.1 MB |
Appendix S1: Supporting Information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1(a) Lin, G.; Chan, S. S.-K.; Chung, H.-S.; Li, S.-L. Chemistry and Biological Activities of Naturally Occurring Phthalides. Stud. Nat. Prod. Chem. 2005, 32, 611–669;
(b) Beck, J. J.; Chou, S. C. The Structural Diversity of Phthalides from the Apiaceae. J. Nat. Prod. 2007, 70, 891–900;
(c) Karmakar, R.; Pahari, P.; Mal, D. Phthalides and Phthalans: Synthetic Methodologies and Their Applications in the Total Synthesis. Chem. Rev. 2014, 114, 6213–6284;
(d) Zhang, L.-B.; Lv, J.-L.; Liu, J.-W. Phthalide Derivatives with Anticoagulation Activities from Angelica Sinensis. J. Nat. Prod. 2016, 79, 1857–1861;
(e) León, A.; Del-Ángel, M.; Ávila, J. L.; Delgado, G. Phthalides: Distribution in Nature, Chemical Reactivity, Synthesis, and Biological Activity. In Progress in the Chemistry of Organic Natural Products. Progress in the Chemistry of Organic Natural Products, Vol. 104, Eds.: A. D. Kinghorn; H. Falk; S. Gibbons; J. Kobayashi, Springer, 2017, pp. 127–246.
10.1007/978-3-319-45618-8_2 Google Scholar
- 2(a) Snieckus, V. Directed ortho Metalation. Tertiary Amide and O-Carbamate Directors in Synthetic Strategies for Polysubstituted Aromatics. Chem. Rev. 1990, 90, 879–933; (b) Cox, C.; Danishefsky, S. J. Synthesis of the Functionalized Tricyclic Core of Lactonamycin by Oxidative Dearomatization. Org. Lett. 2000, 2, 3493–3496; (c) Napoletano, M.; Norcini, G.; Pellacini, F.; Marchini, F.; Morazzoni, G.; Ferlenga, P.; Pradella, L. Phthalazine PDE4 Inhibitors. Part 2: The Synthesis and Biological Evaluation of 6-Methoxy-1,4-disubstituted Derivatives. Bioorg. Med. Chem. Lett. 2001, 11, 33–37; (d) Li, Y.; Linden, A.; Hesse, M. Total Synthesis of the Spermidine Alkaloid 13-Hydroxyisocyclocelabenzine. Helv. Chim. Acta 2003, 86, 579–591; (e) Mal, D.; Pahari, P. Recent Advances in the Hauser Annulation. Chem. Rev. 2007, 107, 1892–1918; (f) Rathwell, K.; Brimble, M. A. Use of Stabilized Phthalide Anion Annulation Reactions in Synthesis: An Update. Synthesis 2007, 643–662; (g) Hobson, S. J.; Parkin, A.; Marquez, R. Oxidative Rearrangements of Isobenzofurans: Studies toward the Synthesis of the Ajudazols. Org. Lett. 2008, 10, 2813–2816.
- 3(a) Yu, S.-R.; You, S.-Q.; Chen, H.-Y. The Pharmacological Actions of 3-n-Butylphthalioe (AG-1). Acta Pharm. Sinica 1984, 19, 486–490; (b) Xiong, N.; Huang, J.; Chen, C.; Zhao, Y.; Zhang, Z.; Jia, M.; Zhang, Z.; Hou, L.; Yang, H.; Cao, X.; Liang, Z.; Zhang, Y.; Sun, S.; Lin, Z.; Wang, T. dl-3-n-Butylphthalide, a Natural Antioxidant, Protects Dopamine Neurons in Rotenone Models for Parkinson's Disease. Neurobiol. Aging 2012, 33, 1777–1791; (c) Diao, X.; Deng, P.; Xie, C.; Li, X.; Zhong, D.; Zhang, Y.; Chen, X. Metabolism and Pharmacokinetics of 3-n-Butylphthalide (NBP) in Humans: The Role of Cytochrome P450s and Alcohol Dehydrogenase in Biotransformation. Drug Metab. Dispos. 2013, 41, 430–444.
- 4 Jadulco, R.; Brauers, G.; Edrada, R. A.; Ebel, R.; Wray, V.; Proksch, S.; Proksch, P. New Metabolites from Sponge-Derived Fungi Curvularia Lunata and Cladosporium Herbarum. J. Nat. Prod. 2002, 65, 730–733.
- 5 Palermo, J. A.; Rodriguez Brasco, M. F.; Spagnuolo, C.; Seldes, A. M. Illudalane Sesquiterpenoids from the Soft Coral Alcyonium paessleri: The First Natural Nitrate Esters. J. Org. Chem. 2000, 65, 4482–4486.
- 6 Jeon, J.; Julianti, E.; Oha, H.; Park, W.; Oh, D.-C.; Oh, K.-B.; Shin, J. Stereochemistry of Hydroxy-Bearing Benzolactones: Isolation and Structural Determination of Chrysoarticulins A-C from a Marine-Derived Fungus Chrysosporium articulatum. Tetrahedron Lett. 2013, 54, 3111–3115.
- 7(a) Kitamura, M.; Ohkuma, T.; Inoue, S.; Sayo, N.; Kumobayashi, H.; Akutagawa, S.; Ohta, T.; Takaya, H.; Noyori, R. Homogeneous Asymmetric Hydrogenation of Functionalized Ketones. J. Am. Chem. Soc. 1988, 110, 629–631; (b) Tanaka, K.; Osaka, T.; Noguchi, K.; Hirano, M. Rhodium-Catalyzed Asymmetric One-Pot Transesterification and [2+2+2] Cycloaddition Leading to Enantioenriched 3,3-Disubstituted Phthalides. Org. Lett. 2007, 9, 1307–1310; (c) Zhang, B.; Xu, M.-H.; Lin, G.-Q. Catalytic Enantioselective Synthesis of Chiral Phthalides by Efficient Reductive Cyclization of 2-Acylarylcarboxylates under Aqueous Transfer Hydrogenation Conditions. Org. Lett. 2009, 11, 4712–4715; (d) Phan, D. H. T.; Kim, B.; Dong, V. M. Phthalides by Rhodium-Catalyzed Ketone Hydroacylation. J. Am. Chem. Soc. 2009, 131, 15608–15609; (e) Yang, J.; Yoshikai, N. Cobalt-Catalyzed Enantioselective Intramolecular Hydroacylation of Ketones and Olefins. J. Am. Chem. Soc. 2014, 136, 16748–16751; (f) Cheng, T.; Ye, Q.; Zhao, Q.; Liu, G. Dynamic Kinetic Resolution of Phthalides via Asymmetric Transfer Hydrogenation: A Strategy Constructs 1,3-Distereocentered 3-(2-Hydroxy-2-arylethyl)-isobenzofuran-1(3H)-one. Org. Lett. 2015, 17, 4972–4975; (g) Ge, Y.; Han, Z.; Wang, Z.; Feng, C.-G.; Zhao, Q.; Lin, G.-Q.; Ding, K. Ir-SpinPHOX Catalyzed Enantioselective Hydrogenation of 3-Ylidenephthalides. Angew. Chem. Int. Ed. 2018, 57, 13140–13144; (h) Chen, W.; Li, J.; Xie, H.; Wang, J. Rhodium(III)-Catalyzed Asymmetric Addition of Inert Arene C-H Bond to Aldehydes to Afford Enantioenriched Phthalides. Org. Lett. 2020, 22, 3586–3590.
- 8(a) Watanabe, M.; Hashimoto, N.; Araki, S.; Butsugan, Y. A Facile Synthesis of Optically Active 3-Ethyl- and 3-Butylphthalides via Catalytic Enantioselective Addition of Dialkylzinc Reagents to o-Phthalaldehyde. J. Org. Chem. 1992, 57, 742–744; (b) Zhong, F.; Luo, J.; Chen, G.-Y.; Dou, X.; Lu, Y. Highly Enantioselective Regiodivergent Allylic Alkylations of MBH Carbonates with Phthalides. J. Am. Chem. Soc. 2012, 134, 10222–10227; (c) Youn, S. W.; Song, H. S.; Park, J. H. Asymmetric Domino Multicatalysis for the Synthesis of 3-Substituted Phthalides: Cinchonine/NHC Cooperative System. Org. Lett. 2014, 16, 1028–1031; (d) Pan, Y.-L.; Zheng, H.-L.; Wang, J.; Yang, C.; Li, X.; Cheng. J.-P. Enantioselective Allylation of Oxocarbenium Ions Catalyzed by Bi(OAc)3/Chiral Phosphoric Acid. ACS Catal. 2020, 10, 8069–8076.
- 9 Mangas-Sánchez, J.; Busto, E.; Gotor-Fernández, V.; Gotor, V. Highly Stereoselective Chemoenzymatic Synthesis of the 3H-Isobenzofuran Skeleton. Access to Enantiopure 3-Methylphthalides. Org. Lett. 2012, 14, 1444–1447.
- 10For selected reviews on dynamic kinetic resolution, see: (a) Huerta, F. F.; Minidis, A. B. E.; Bäckvall, J.-E. Racemisation in Asymmetric Synthesis. Dynamic Kinetic Resolution and Related Processes in Enzyme and Metal Catalysis. Chem. Soc. Rev. 2001, 30, 321–331; (b) Pàmies, O.; Bäckvall, J.-E. Combination of Enzymes and Metal Catalysts. A Powerful Approach in Asymmetric Catalysis. Chem. Rev. 2003, 103, 3247–3262; (c) Pellissier, H. Recent Developments in Dynamic Kinetic Resolution. Tetrahedron 2008, 64, 1563–1601; (d) Pellissier, H. Organocatalyzed Dynamic Kinetic Resolution. Adv. Synth. Catal. 2011, 353, 659–676; (e) Pellissier, H. Recent Developments in Dynamic Kinetic Resolution. Tetrahedron 2011, 67, 3769–3802; (f) Bhat, V.; Welin, E. R.; Guo, X.; Stoltz, B. M. Advances in Stereoconvergent Catalysis from 2005 to 2015: Transition-Metal-Mediated Stereoablative Reactions, Dynamic Kinetic Resolutions, and Dynamic Kinetic Asymmetric Transformations. Chem. Rev. 2017, 117, 4528–4561.
- 11For selected reviews on asymmetric transfer hydrogenation, see: (a) Noyori, R.; Hashiguchi, S. Asymmetric Transfer Hydrogenation Catalyzed by Chiral Ruthenium Complexes. Acc. Chem. Res. 1997, 30, 97–102;
(b) Ikariya, T.; Blacker, A. J. Asymmetric Transfer Hydrogenation of Ketones with Bifunctional Transition Metal-Based Molecular Catalysts. Acc. Chem. Res. 2007, 40, 1300–1308;
(c) Wang, D.; Astruc, D. The Golden Age of Transfer Hydrogenation. Chem. Rev. 2015, 115, 6621–6686;
(d) Asymmetric Hydrogenation and Transfer Hydrogenation, Eds.: V. Ratovelomanana-Vidal; P. Phansavath, Wiley-VCH, 2021.
10.1002/9783527822294 Google Scholar
- 12(a) Liu, L.-X.; Huang, W.-J.; Xie, Q.-X.; Wu, B.; Yu, C.-B.; Zhou, Y.-G. Dynamic Kinetic Resolution of Flavonoids via Asymmetric Allylic Alkylation: Construction of Two Contiguous Stereogenic Centers on Nucleophiles. ACS Catal. 2021, 11, 12859–12863; (b) Xie, Q.-X.; Liu, L.-X.; Zhu, Z.-H.; Yu, C.-B.; Zhou, Y.-G. Asymmetric Transfer Hydrogenation of 2,3-Disubstituted Flavanones through Dynamic Kinetic Resolution Enabled by Retro-oxa-Michael Addition: Construction of Three Contiguous Stereogenic Centers. J. Org. Chem. 2022, 87, 7521–7530; (c) Liu, L.-X.; Huang, W.-J.; Yu, C.-B.; Zhou, Y.-G. Palladium-Catalyzed Stereoselective Construction of Chiral Allenes Bearing Nonadjacent Axial and Two Central Chirality. Org. Biomol. Chem. 2023, 21, 8516–8520.
- 13For selected articles on asymmetric allylic alkylation, see: (a) Trost, B. M.; Van Vranken, D. L. Asymmetric Transition Metal-Catalyzed Allylic Alkylations. Chem. Rev. 1996, 96, 395–422; (b) Trost, B. M.; Crawley, M. L. Asymmetric Transition-Metal-Catalyzed Allylic Alkylations: Applications in Total Synthesis. Chem. Rev. 2003, 103, 2921–2944; (c) Trost, B. M. Asymmetric Allylic Alkylation, an Enabling Methodology. J. Org. Chem. 2004, 69, 5813–5837; (d) Trost, B. M.; Machacek, M. R.; Aponick, A. Predicting the Stereochemistry of Diphenylphosphino Benzoic Acid (DPPBA)-Based Palladium-Catalyzed Asymmetric Allylic Alkylation Reactions: A Working Model. Acc. Chem. Res. 2006, 39, 747–760; (e) Lu, Z.; Ma, S. Metal-Catalyzed Enantioselective Allylation in Asymmetric Synthesis. Angew. Chem. Int. Ed. 2008, 47, 258–297; (f) Cheng, Q.; Tu, H.-F.; Zheng, C.; Qu, J.-P.; Helmchen, G.; You, S.-L. Iridium-Catalyzed Asymmetric Allylic Substitution Reactions. Chem. Rev. 2019, 119, 1855–1969; (g) Pàmies, O.; Margalef, J.; Cañellas, S.; James, J.; Judge, E.; Guiry, P. J.; Moberg, C.; Bäckvall, J.-E.; Pfaltz, A.; Pericàs, M. A.; Diéguez, M. Recent Advances in Enantioselective Pd-Catalyzed Allylic Substitution: From Design to Applications. Chem. Rev. 2021, 121, 4373–4505; (h) Jiang, R.; Ding, L.; Zheng, C.; You, S.-L. Iridium-Catalyzed Z-Retentive Asymmetric Allylic Substitution Reactions. Science 2021, 371, 380–386.
- 14(a) Wang, X.; Guo, P.; Han, Z.; Wang, X.; Wang, Z.; Ding, K. Spiroketal- Based Diphosphine Ligands in Pd-Catalyzed Asymmetric Allylic Amination of Morita-Baylis-Hillman Adducts: Exceptionally High Efficiency and New Mechanism. J. Am. Chem. Soc. 2014, 136, 405–411; (b) Liu, J.; Han, Z.; Wang, X.; Meng, F.; Wang, Z.; Ding, K. Palladium-Catalyzed Asymmetric Construction of Vicinal Tertiary and All-Carbon Quaternary Stereocenters by Allylation of β-Ketocarbonyls with Morita-Baylis-Hillman Adducts. Angew. Chem. Int. Ed. 2017, 56, 5050–5054; (c) Wang, X.; Han, Z.; Wang, Z.; Ding, K. A Type of Structurally Adaptable Aromatic Spiroketal Based Chiral Diphosphine Ligands in Asymmetric Catalysis. Acc. Chem. Res. 2021, 54, 668–684.
- 15(a) Tsuji, J.; Shimizu, I.; Minami, I.; Ohashi, Y.; Sugiura, T.; Takahashi, K. Allylic Carbonates. Efficient Allylating Agents of Carbonucleophiles in Palladium-Catalyzed Reactions under Neutral Conditions. J. Org. Chem. 1985, 50, 1523–1529; (b) Tsuji, J.; Minami, I. New Synthetic Reactions of Allyl Alkyl Carbonates, Allyl β-Keto Carboxylates, and Allyl Vinylic Carbonates Catalyzed by Palladium Complexes. Acc. Chem. Res. 1987, 20, 140–145; (c) Chen, G.; Mo, J.; Jiang, D.; Peng, Y. Synthesis of Chiral 1,1,1-Trifluoro-α,α- disubstituted 2,4-Diketones via Palladium-Catalyzed Asymmetric Allylation. Org. Lett. 2023, 25, 2388–2393.