Regulatory Mechanisms and Applications of Rare Earth Elements-Based Electrocatalysts†
Qinlong Gao
School of Materials and Energy, University of Electronic Science and Technology of China, Chengdu, Sichuan, 611731 China
These authors contributed equally to this work.
Search for more papers by this authorHaoyuan Wang
School of Materials and Energy, University of Electronic Science and Technology of China, Chengdu, Sichuan, 611731 China
Hefei National Research Center for Physical Sciences at the Microscale, University of Science and Technology of China, Hefei, Anhui, 230026 China
These authors contributed equally to this work.
Search for more papers by this authorChunxiao Liu
School of Materials and Energy, University of Electronic Science and Technology of China, Chengdu, Sichuan, 611731 China
Search for more papers by this authorLaihao Luo
School of Materials and Energy, University of Electronic Science and Technology of China, Chengdu, Sichuan, 611731 China
Hefei National Research Center for Physical Sciences at the Microscale, University of Science and Technology of China, Hefei, Anhui, 230026 China
Search for more papers by this authorXu Li
School of Materials and Energy, University of Electronic Science and Technology of China, Chengdu, Sichuan, 611731 China
Search for more papers by this authorQiu jiang
School of Materials and Energy, University of Electronic Science and Technology of China, Chengdu, Sichuan, 611731 China
Search for more papers by this authorYuan Ji
School of Materials and Energy, University of Electronic Science and Technology of China, Chengdu, Sichuan, 611731 China
Search for more papers by this authorCorresponding Author
Tingting Zheng
School of Materials and Energy, University of Electronic Science and Technology of China, Chengdu, Sichuan, 611731 China
E-mail: [email protected]; [email protected]Search for more papers by this authorCorresponding Author
Chuan Xia
School of Materials and Energy, University of Electronic Science and Technology of China, Chengdu, Sichuan, 611731 China
E-mail: [email protected]; [email protected]Search for more papers by this authorQinlong Gao
School of Materials and Energy, University of Electronic Science and Technology of China, Chengdu, Sichuan, 611731 China
These authors contributed equally to this work.
Search for more papers by this authorHaoyuan Wang
School of Materials and Energy, University of Electronic Science and Technology of China, Chengdu, Sichuan, 611731 China
Hefei National Research Center for Physical Sciences at the Microscale, University of Science and Technology of China, Hefei, Anhui, 230026 China
These authors contributed equally to this work.
Search for more papers by this authorChunxiao Liu
School of Materials and Energy, University of Electronic Science and Technology of China, Chengdu, Sichuan, 611731 China
Search for more papers by this authorLaihao Luo
School of Materials and Energy, University of Electronic Science and Technology of China, Chengdu, Sichuan, 611731 China
Hefei National Research Center for Physical Sciences at the Microscale, University of Science and Technology of China, Hefei, Anhui, 230026 China
Search for more papers by this authorXu Li
School of Materials and Energy, University of Electronic Science and Technology of China, Chengdu, Sichuan, 611731 China
Search for more papers by this authorQiu jiang
School of Materials and Energy, University of Electronic Science and Technology of China, Chengdu, Sichuan, 611731 China
Search for more papers by this authorYuan Ji
School of Materials and Energy, University of Electronic Science and Technology of China, Chengdu, Sichuan, 611731 China
Search for more papers by this authorCorresponding Author
Tingting Zheng
School of Materials and Energy, University of Electronic Science and Technology of China, Chengdu, Sichuan, 611731 China
E-mail: [email protected]; [email protected]Search for more papers by this authorCorresponding Author
Chuan Xia
School of Materials and Energy, University of Electronic Science and Technology of China, Chengdu, Sichuan, 611731 China
E-mail: [email protected]; [email protected]Search for more papers by this authorDedicated to the Special Issue of Emerging Investigators in 2024.
Comprehensive Summary
Amidst the pressing environmental challenges posed by the prevalent reliance on fossil fuels, it becomes imperative to seek sustainable alternatives and prioritize energy efficiency. Electrocatalysis, which is renowned for its high efficiency and environmental friendliness, has garnered significant attention. Rare earth elements (REEs), distinguished by their unique electronic and orbital structures, play a crucial role in electrocatalysis. The strategic integration of REEs into catalysts allows for the fine-tuning of atomic structures, which in turn, significantly boosts catalytic performance. Despite substantial advancements in rare earth-based materials for electrocatalysis, a comprehensive overview of the regulatory mechanisms involving REEs is lacking. In this mini-review, we systematically explore the regulatory mechanisms of REEs within electrocatalysts and their pivotal roles in essential electrocatalytic processes such as the CO2 reduction reaction, oxygen reduction reaction, and hydrogen evolution reaction. We commence with an elucidation of REEs, proceed to delineate their regulatory impacts on electrocatalysts and delve into their applications in key electroreduction reactions. We conclude with discussions on current limitations and prospects for further advancements in this burgeoning field of research.
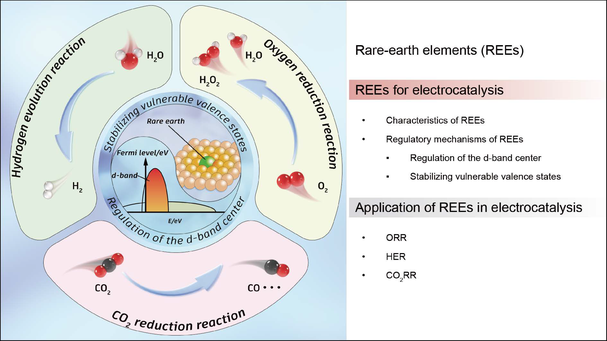
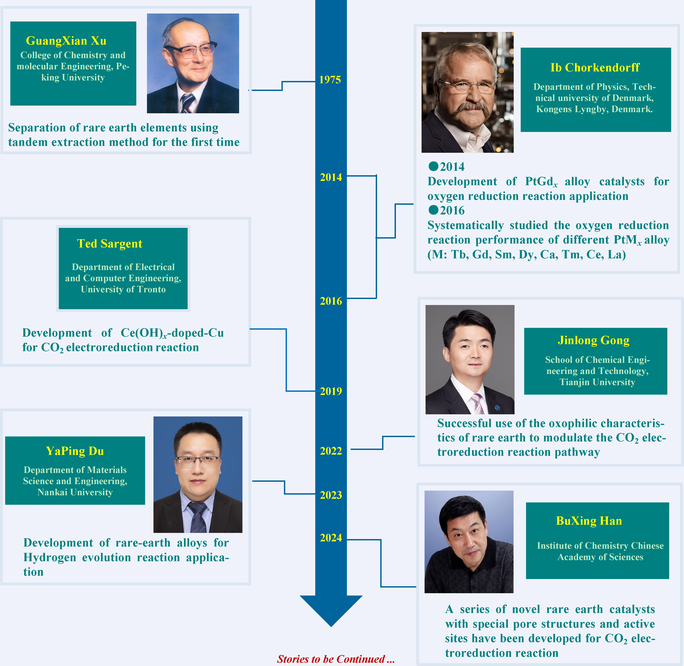
Key Scientists
References
- 1 Kuhl, K. P.; Cave, E. R.; Abram, D. N.; Jaramillo, T. F. New insights into the electrochemical reduction of carbon dioxide on metallic copper surfaces. Energy Environ. Sci. 2012, 5, 7050–7059.
- 2 Mok, D. H.; Li, H.; Zhang, G.; Lee, C.; Jiang, K.; Back, S. Data-driven discovery of electrocatalysts for CO2 reduction using active motifs-based machine learning. Nat. Commun. 2023, 14, 7303.
- 3 Birdja, Y. Y.; Perez-Gallent, E.; Figueiredo, M. C.; Gottle, A. J.; Calle-Vallejo, F.; Koper, M. T. M. Advances and challenges in understanding the electrocatalytic conversion of carbon dioxide to fuels. Nat. Energy 2019, 4, 732–745.
- 4 Duan, C. Selective CO2 electrohydrogenation. Nat. Catal. 2021, 4, 264–265.
- 5 Zhong, H.; Ghorbani-Asl, M.; Ly, K. H.; Zhang, J.; Ge, J.; Wang, M.; Liao, Z.; Makarov, D.; Zschech, E.; Brunner, E.; Weidinger, I. M.; Zhang, J.; Krasheninnikov, A. V.; Kaskel, S.; Dong, R.; Feng, X. Synergistic electroreduction of carbon dioxide to carbon monoxide on bimetallic layered conjugated metal-organic frameworks. Nat. Commun. 2020, 11, 1409.
- 6 Yang, L.; Shui, J.; Du, L.; Shao, Y.; Liu, J.; Dai, L.; Hu, Z. Carbon-Based Metal-Free ORR Electrocatalysts for Fuel Cells: Past, Present, and Future. Adv. Mater. 2019, 31, 1804799.
- 7 Cui, X.; Tang, C.; Zhang, Q. A Review of Electrocatalytic Reduction of Dinitrogen to Ammonia under Ambient Conditions. Adv. Energy Mater. 2018, 8, 1800369.
- 8 Jin, H.; Guo, C.; Liu, X.; Liu, J.; Vasileff, A.; Jiao, Y.; Zheng, Y.; Qiao, S.-Z. Emerging Two-Dimensional Nanomaterials for Electrocatalysis. Chem. Rev. 2018, 118, 6337–6408.
- 9 Wang, Y.; Chu, F.; Zeng, J.; Wang, Q.; Naren, T.; Li, Y.; Cheng, Y.; Lei, Y.; Wu, F. Single Atom Catalysts for Fuel Cells and Rechargeable Batteries: Principles, Advances, and Opportunities. ACS Nano 2021, 15, 210–239.
- 10 You, B.; Tang, M. T.; Tsai, C.; Abild-Pedersen, F.; Zheng, X.; Li, H. Enhancing Electrocatalytic Water Splitting by Strain Engineering. Adv. Mater. 2019, 31, 1807001.
- 11 Linnemann, J.; Kanokkanchana, K.; Tschulik, K. Design Strategies for Electrocatalysts from an Electrochemis's Perspective. ACS Catal. 2021, 11, 5318–5346.
- 12 Wan, Y.; Xu, J.; Lv, R. Heterogeneous electrocatalysts design for nitrogen reduction reaction under ambient conditions. Mater. Today 2019, 27, 69–90.
- 13 Gao, G.; Zhu, G.; Chen, X.; Sun, Z.; Cabot, A. Optimizing Pt-Based Alloy Electrocatalysts for Improved Hydrogen Evolution Performance in Alkaline Electrolytes: A Comprehensive Review. ACS Nano 2023, 17, 20804–20824.
- 14 Shi, K.; Si, D.; Teng, X.; Chen, L.; Shi, J. Pd/NiMoO4/NF electrocatalysts for the efficient and ultra-stable synthesis and electrolyte-assisted extraction of glycolate. Nat. Commun. 2024, 15, 2899.
- 15 Böhm, D.; Beetz, M.; Schuster, M.; Peters, K.; Hufnagel, A. G.; Döblinger, M.; Böller, B.; Bein, T.; Fattakhova-Rohlfing, D. Efficient OER Catalyst with Low Ir Volume Density Obtained by Homogeneous Deposition of Iridium Oxide Nanoparticles on Macroporous Antimony-Doped Tin Oxide Support. Adv. Funct. Mater. 2020, 30, 1906670.
- 16 Kalinin, S. V.; Dyck, O.; Balke, N.; Neumayer, S.; Tsai, W.-Y.; Vasudevan, R.; Lingerfelt, D.; Ahmadi, M.; Ziatdinov, M.; McDowell, M. T.; Strelcov, E. Toward Electrochemical Studies on the Nanometer and Atomic Scales: Progress, Challenges, and Opportunities. ACS Nano 2019, 13, 9735–9780.
- 17 Malek, A.; Eikerling, M. H. Chemisorbed Oxygen at Pt(111): a DFT Study of Structural and Electronic Surface Properties. Electrocatalysis 2018, 9, 370–379.
- 18 Zhang, M.; Zhang, K.; Ai, X.; Liang, X.; Zhang, Q.; Chen, H.; Zou, X. Theory-guided electrocatalyst engineering: From mechanism analysis to structural design. Chin. J. Catal. 2022, 43, 2987–3018.
- 19 Norskov, J. K.; Bligaard, T.; Rossmeisl, J.; Christensen, C. H. Towards the computational design of solid catalysts. Nat. Chem. 2009, 1, 37–46.
- 20 Jiang, Y.; Fu, H.; Liang, Z.; Zhang, Q.; Du, Y. Rare earth oxide based electrocatalysts: synthesis, properties and applications. Chem. Soc. Rev. 2024, 53, 714–763.
- 21 Meng, S.; Li, G.; Wang, P.; He, M.; Sun, X.; Li, Z. Rare earth-based MOFs for photo/electrocatalysis. Mater. Chem. Front. 2023, 7, 806–827.
- 22 de Damborenea, J.; Conde, A.; Arenas, M. A. 3 - Corrosion inhibition with rare earth metal compounds in aqueous solutions. In Woodhead Publishing Series in Metals and Surface Engineering, Rare Earth-Based Corrosion Inhibitors, Eds.: M. Forsyth; B. Hinton, Woodhead Publishing, 2014, pp. 84–116.
- 23 Ko, Y.-J.; Kim, J.-Y.; Lee, W. H.; Kim, M. G.; Seong, T.-Y.; Park, J.; Jeong, Y.; Min, B. K.; Lee, W.-S.; Lee, D. K.; Oh, H.-S. Exploring dopant effects in stannic oxide nanoparticles for CO2 electro-reduction to formate. Nat. Commun. 2022, 13, 2205.
- 24 Geng, S.; Tian, F.; Li, M.; Guo, X.; Yu, Y.; Yang, W.; Hou, Y. Hole-rich CoP nanosheets with an optimized d-band center for enhancing pH-universal hydrogen evolution electrocatalysis. J. Mater. Chem. A 2021, 9, 8561–8567.
- 25 Zhang, X.; Wang, Y.; Wu, X.; Wang, F.; Ou, Q.; Zhang, S. A Comprehensive Review on Mechanisms and Applications of Rare-Earth Based Perovskite Nanocrystals. Chin. J. Chem. 2024, 42, 1032–1056.
- 26 Lin, X.; Huang, Y.-C.; Hu, Z.; Li, L.; Zhou, J.; Zhao, Q.; Huang, H.; Sun, J.; Pao, C.-W.; Chang, Y.-C.; Lin, H.-J.; Chen, C.-T.; Dong, C.-L.; Wang, J.-Q.; Zhang, L. 5f Covalency Synergistically Boosting Oxygen Evolution of UCoO4 Catalyst. J. Am. Chem. Soc. 2022, 144, 416–423.
- 27 Sun, Y.; Xie, J.; Fu, Z.; Zhang, H.; Yao, Y.; Zhou, Y.; Wang, X.; Wang, S.; Gao, X.; Tang, Z.; Li, S.; Wang, X.; Nie, K.; Yang, Z.; Yan, Y. Boosting CO2 Electroreduction to C2H4 via Unconventional Hybridization: High-Order Ce4+ 4f and O 2p Interaction in Ce-Cu2O for Stabilizing Cu+. ACS Nano 2023, 17, 13974–13984.
- 28 Zeng, Z.; Xu, Y.; Zhang, Z.; Gao, Z.; Luo, M.; Yin, Z.; Zhang, C.; Xu, J.; Huang, B.; Luo, F.; Du, Y.; Yan, C. Rare-earth-containing perovskite nanomaterials: design, synthesis, properties and applications. Chem. Soc. Rev. 2020, 49, 1109–1143.
- 29 Li, M.; Zhao, Z.; Zhang, W.; Luo, M.; Tao, L.; Sun, Y.; Xia, Z.; Chao, Y.; Yin, K.; Zhang, Q.; Gu, L.; Yang, W.; Yu, Y.; Lu, G.; Guo, S. Sub-Monolayer YOx/MoOx on Ultrathin Pt Nanowires Boosts Alcohol Oxidation Electrocatalysis. Adv. Mater. 2021, 33, 2103762.
- 30 Hu, Y.; Jensen, J. O.; Cleemann, L. N.; Brandes, B. A.; Li, Q. Synthesis of Pt-Rare Earth Metal Nanoalloys. J. Am. Chem. Soc. 2020, 142, 953–961.
- 31 Zhang, S.; Saji, S. E.; Yin, Z.; Zhang, H.; Du, Y.; Yan, C.-H. Rare-Earth Incorporated Alloy Catalysts: Synthesis, Properties, and Applications. Adv. Mater. 2021, 33, 2005988.
- 32 Fu, H.; Jiang, Y.; Zhang, M.; Zhong, Z.; Liang, Z.; Wang, S.; Du, Y.; Yan, C. High-entropy rare earth materials: synthesis, application and outlook. Chem. Soc. Rev. 2024, 53, 2211–2247.
- 33 Guo, J.; Du, Y.; Zhang, H. A Brief Summary of Research Progress on the Application of Rare Earth Materials in Heterogeneous Catalysis. Acta Chim. Sinica 2020, 78, 625–633.
- 34 Feng, X.; Wang, Z.; Liu, X. Chiral Lewis Acid Rare-Earth Metal Complexes in Enantioselective Catalysis. In Chiral Lewis Acids. Topics in Organometallic Chemistry, Ed.: K. Mikami, Vol. 62, Springer, 2018, pp. 147–191.
- 35 Pearson, R. G. Hard and Soft Acids and Bases. J. Am. Chem. Soc. 1963, 85, 3533–3539.
- 36 Luo, M.; Guo, S. Strain-controlled electrocatalysis on multimetallic nanomaterials. Nat. Rev. Mater. 2017, 2, 17059.
- 37 Wang, X.; Tang, Y.; Lee, J.-M.; Fu, G. Recent advances in rare-earth-based materials for electrocatalysis. Chem. Catal. 2022, 2, 967–1008.
- 38 Kim, D.-H.; Lee, E.; Pak, C. Effect of rare-earth elements in Pd ternary alloy catalysts on activity toward oxygen reduction reaction. Catal. Today 2021, 359, 106–111.
- 39 Yoo, S. J.; Sung, Y.-E. Nanosized Pt-La alloy electrocatalysts with high activity and stability for the oxygen reduction reaction. Surf. Sci. 2015, 631, 272–277.
- 40 Li, Z.; Tian, Z.; Cheng, H.; Wang, T.; Zhang, W.; Lu, Y.; Lai, Y.; He, G. Engineering d-band center of FeN4 moieties for efficient oxygen reduction reaction electrocatalysts. Energy Storage Mater. 2023, 59, 102764.
- 41
Nørskov, J. K. Theory nof chemisorption and heterogeneous catalysis. Physica B+C 1984, 127, 193–202.
10.1016/S0378-4363(84)80030-3 Google Scholar
- 42 Xin, H.; Vojvodic, A.; Voss, J.; Norskov, J. K.; Abild-Pedersen, F. Effects of d-band shape on the surface reactivity of transition-metal alloys. Phys. Rev. B 2014, 89, 115114.
- 43 Nilsson, A.; Pettersson, L. G. M.; Hammer, B.; Bligaard, T.; Christensen, C. H.; Norskov, J. K. The electronic structure effect in heterogeneous catalysis. Catal. Lett. 2005, 100, 111–114.
- 44 Ruban, A.; Hammer, B.; Stoltze, P.; Skriver, H. L.; Norskov, J. K. Surface electronic structure and reactivity of transition and noble metals. J. Mol. Catal. A-Chem. 1997, 115, 421–429.
- 45 Garlyyev, B.; Pohl, M. D.; Colic, V.; Liang, Y.; Butt, F. K.; Holleitner, A.; Bandarenka, A. S. High oxygen reduction reaction activity of Pt5Pr electrodes in acidic media. Electrochem. Commun. 2018, 88, 10–14.
- 46 Li, M.; Pan, X.; Jiang, M.; Zhang, Y.; Tang, Y.; Fu, G. Interface engineering of oxygen-vacancy-rich CoP/CeO2 heterostructure boosts oxygen evolution reaction. Chem. Eng. J. 2020, 395, 125160.
- 47 Eriksson, B.; Montserrat-Siso, G.; Brown, R.; Skala, T.; Lindstrom, R. W.; Lindbergh, G.; Wickman, B.; Lagergren, C. Enhanced oxygen reduction activity with rare earth metal alloy catalysts in proton exchange membrane fuel cells. Electrochim. Acta 2021, 387, 138454.
- 48 Mavrikakis, M.; Hammer, B.; Norskov, J. K. Effect of strain on the reactivity of metal surfaces. Phys. Rev. Lett. 1998, 81, 2819–2822.
- 49 Greeley, J.; Stephens, I. E. L.; Bondarenko, A. S.; Johansson, T. P.; Hansen, H. A.; Jaramillo, T. F.; Rossmeisl, J.; Chorkendorff, I.; Norskov, J. K. Alloys of platinum and early transition metals as oxygen reduction electrocatalysts. Nat. Chem. 2009, 1, 552–556.
- 50 Stamenkovic, V. R.; Fowler, B.; Mun, B. S.; Wang, G.; Ross, P. N.; Lucas, C. A.; Markovic, N. M. Improved oxygen reduction activity on Pt3Ni(111) via increased surface site availability. Science 2007, 315, 493–497.
- 51 Stephens, I. E. L.; Bondarenko, A. S.; Perez-Alonso, F. J.; Calle-Vallejo, F.; Bech, L.; Johansson, T. P.; Jepsen, A. K.; Frydendal, R.; Knudsen, B. P.; Rossmeisl, J.; Chorkendorff, I. Tuning the Activity of Pt(111) for Oxygen Electroreduction by Subsurface Alloying. J. Am. Chem. Soc. 2011, 133, 5485–5491.
- 52 Liu, G.; Shih, A. J.; Deng, H.; Ojha, K.; Chen, X.; Luo, M.; McCrum, I. T.; Koper, M. T. M.; Greeley, J.; Zeng, Z. Site-specific reactivity of stepped Pt surfaces driven by stress release. Nature 2024, 626, 1005–1010.
- 53 Sheng, W.; Zhuang, Z.; Gao, M.; Zheng, J.; Chen, J. G.; Yan, Y. Correlating hydrogen oxidation and evolution activity on platinum at different pH with measured hydrogen binding energy. Nat. Commun. 2015, 6, 5848.
- 54 Li, Q.; Sun, C.; Fu, H.; Zhang, S.; Sun, X.; Liu, J.-C.; Du, Y.; Luo, F. Enhanced Alkaline Hydrogen Evolution Reaction through Lanthanide-Modified Rhodium Intermetallic Catalysts. Small 2024, 20, 2307052.
- 55 Li, M.; Wang, X.; Liu, K.; Zhu, Z.; Guo, H.; Li, M.; Du, H.; Sun, D.; Li, H.; Huang, K.; Tang, Y.; Fu, G. Ce-Induced Differentiated Regulation of Co Sites via Gradient Orbital Coupling for Bifunctional Water-Splitting Reactions. Adv. Energy Mater. 2023, 13, 2301162.
- 56 Wang, X.; Wang, J.; Wang, P.; Li, L.; Zhang, X.; Sun, D.; Li, Y.; Tang, Y.; Wang, Y.; Fu, G. Engineering 3d-2p-4f Gradient Orbital Coupling to Enhance Electrocatalytic Oxygen Reduction. Adv. Mater. 2022, 34, 2206540.
- 57 Feng, K.; Xu, J.; Chen, Y.; Li, S.; Kang, Z.; Zhong, J. Positively Charged Pt-Based Nanoreactor for Efficient and Stable Hydrogen Evolution. Adv. Sci. 2022, 9, 2203199.
- 58 Yang, H.; Ji, Y.; Shao, Q.; Zhu, W.; Fang, M.; Ma, M.; Liao, F.; Huang, H.; Zhang, Y.; Yang, J.; Fan, Z.; Li, Y.; Liu, Y.; Shao, M.; Kang, Z. Metastable-phase platinum oxide for clarifying the Pt-O active site for the hydrogen evolution reaction. Energy Environ. Sci. 2023, 16, 574–583.
- 59 Ni, W.; Li, C.; Zang, X.; Xu, M.; Huo, S.; Liu, M.; Yang, Z.; Yan, Y.-M. Efficient electrocatalytic reduction of CO2 on CuxO decorated graphene oxides: an insight into the role of multivalent Cu in selectivity and durability. Appl. Catal. B-Environ. 2019, 259, 118044.
- 60 De Luna, P.; Quintero-Bermudez, R.; Cao-Thang, D.; Ross, M. B.; Bushuyev, O. S.; Todorovic, P.; Regier, T.; Kelley, S. O.; Yang, P.; Sargent, E. H. Catalyst electro-redeposition controls morphology and oxidation state for selective carbon dioxide reduction. Nat. Catal. 2018, 1, 103–110.
- 61 Liang, Z.-Q.; Zhuang, T.-T.; Seifitokaldani, A.; Li, J.; Huang, C.-W.; Tan, C.-S.; Li, Y.; De Luna, P.; Dinh, C. T.; Hu, Y.; Xiao, Q.; Hsieh, P.-L.; Wang, Y.; Li, F.; Quintero-Bermudez, R.; Zhou, Y.; Chen, P.; Pang, Y.; Lo, S.-C.; Chen, L.-J.; Tan, H.; Xu, Z.; Zhao, S.; Sinton, D.; Sargent, E. H. Copper-on-nitride enhances the stable electrosynthesis of multi-carbon products from CO2. Nat. Commun. 2018, 9, 3828.
- 62 Yang, P.-P.; Zhang, X.-L.; Gao, F.-Y.; Zheng, Y.-R.; Niu, Z.-Z.; Yu, X.; Liu, R.; Wu, Z.-Z.; Qin, S.; Chi, L.-P.; Duan, Y.; Ma, T.; Zheng, X.-S.; Zhu, J.-F.; Wang, H.-J.; Gao, M.-R.; Yu, S.-H. Protecting Copper Oxidation State via Intermediate Confinement for Selective CO2 Electroreduction to C2+ Fuels. J. Am. Chem. Soc. 2020, 142, 6400–6408.
- 63 Cai, R.; Sun, M.; Yang, F.; Gu, D.; Ju, M.; Chen, Y.; Gu, M. D.; Huang, B.; Yang, S. Engineering Cu(I)/Cu(0) interfaces for efficient ethanol production from CO2 electroreduction. Chem 2024, 10, 211–233.
- 64 Chang, C.-J.; Lai, Y.-A.; Chu, Y.-C.; Peng, C.-K.; Tan, H.-Y.; Pao, C.-W.; Lin, Y.-G.; Hung, S.-F.; Chen, H.-C.; Chen, H. M. Lewis Acidic Support Boosts C-C Coupling in the Pulsed Electrochemical CO2 Reaction. J. Am. Chem. Soc. 2023, 145, 6953–6965.
- 65 Xie, M.; Shen, Y.; Ma, W.; Wei, D.; Zhang, B.; Wang, Z.; Wang, Y.; Zhang, Q.; Xie, S.; Wang, C.; Wang, Y. Fast Screening for Copper-Based Bimetallic Electrocatalysts: Efficient Electrocatalytic Reduction of CO2 to C2+ Products on Magnesium-Modified Copper. Angew. Chem. Int. Ed. 2022, 61, e202213423.
- 66 Zhou, Y.; Che, F.; Liu, M.; Zou, C.; Liang, Z.; De Luna, P.; Yuan, H.; Li, J.; Wang, Z.; Xie, H.; Li, H.; Chen, P.; Bladt, E.; Quintero-Bermudez, R.; Sham, T.-K.; Bals, S.; Hofkens, J.; Sinton, D.; Chen, G.; Sargent, E. H. Dopant-induced electron localization drives CO2 reduction to C2 hydrocarbons. Nat. Chem. 2018, 10, 974–980.
- 67 Xiao, H.; Goddard, W. A., III; Cheng, T.; Liu, Y. Cu metal embedded in oxidized matrix catalyst to promote CO2 activation and CO dimerization for electrochemical reduction of CO2. Proc. Natl. Acad. Sci. U. S. A. 2017, 114, 6685–6688.
- 68 Gao, D.; Scholten, F.; Cuenya, B. R. Improved CO2 Electroreduction Performance on Plasma-Activated Cu Catalysts via Electrolyte Design: Halide Effect. ACS Catal. 2017, 7, 5112–5120.
- 69 Chu, S.; Yan, X.; Choi, C.; Hong, S.; Robertson, A. W.; Masa, J.; Han, B.; Jung, Y.; Sun, Z. Stabilization of Cu+ by tuning a CuO–CeO2 interface for selective electrochemical CO2 reduction to ethylene. Green Chem. 2020, 22, 6540–6546.
- 70 Wakerley, D.; Lamaison, S.; Ozanam, F.; Menguy, N.; Mercier, D.; Marcus, P.; Fontecave, M.; Mougel, V. Bio-inspired hydrophobicity promotes CO2 reduction on a Cu surface. Nat. Mater. 2019, 18, 1222.
- 71 Wang, X.; Tong, Y.; Feng, W.; Liu, P.; Li, X.; Cui, Y.; Cai, T.; Zhao, L.; Xue, Q.; Yan, Z.; Yuan, X.; Xing, W. Embedding oxophilic rare-earth single atom in platinum nanoclusters for efficient hydrogen electro-oxidation. Nat. Commun. 2023, 14, 3767.
- 72 Zhou, X.; Shan, J.; Chen, L.; Xia, B. Y.; Ling, T.; Duan, J.; Jiao, Y.; Zheng, Y.; Qiao, S.-Z. Stabilizing Cu2+ Ions by Solid Solutions to Promote CO2 Electroreduction to Methane. J. Am. Chem. Soc. 2022, 144, 2079–2084.
- 73 Wang, X.; Wang, J.; Wang, P.; Li, L.; Zhang, X.; Sun, D.; Li, Y.; Tang, Y.; Wang, Y.; Fu, G. Engineering 3d-2p-4f Gradient Orbital Coupling to Enhance Electrocatalytic Oxygen Reduction. Adv. Mater. 2022, 34, 2206540.
- 74 Lin, X.; Huang, Y.-C.; Hu, Z.; Li, L.; Zhou, J.; Zhao, Q.; Huang, H.; Sun, J.; Pao, C.-W.; Chang, Y.-C.; Lin, H.-J.; Chen, C.-T.; Dong, C.-L.; Wang, J.-Q.; Zhang, L. 5f Covalency Synergistically Boosting Oxygen Evolution of UCoO4 Catalyst. J. Am. Chem. Soc. 2022, 144, 416–423.
- 75 Song, J.; Wei, C.; Huang, Z.-F.; Liu, C.; Zeng, L.; Wang, X.; Xu, Z. J. A review on fundamentals for designing oxygen evolution electrocatalysts. Chem. Soc. Rev. 2020, 49, 2196–2214.
- 76 Juhin, A.; de Groot, F.; Vanko, G.; Calandra, M.; Brouder, C. Angular dependence of core hole screening in LiCoO2: A DFT+U calculation of the oxygen and cobalt K-edge x-ray absorption spectra. Phys. Rev. B 2010, 81, 115115.
- 77 Feng, J.; Wu, L.; Liu, S.; Xu, L.; Song, X.; Zhang, L.; Zhu, Q.; Kang, X.; Sun, X.; Han, B. Improving CO2-to-C2+Product Electroreduction Efficiency via Atomic Lanthanide Dopant-Induced Tensile-Strained CuOx Catalysts. J. Am. Chem. Soc. 2023, 145, 9857–9866.
- 78 Ma, R.; Lin, G.; Zhou, Y.; Liu, Q.; Zhang, T.; Shan, G.; Yang, M.; Wang, J. A review of oxygen reduction mechanisms for metal-free carbon-based electrocatalysts. npj Comput. Mater. 2019, 5, 78.
- 79 Campos-Roldan, C. A.; Gonzalez-Huerta, R. G.; Alonso-Vante, N. Experimental Protocol for HOR and ORR in Alkaline Electrochemical Measurements. J. Electrochem. Soc. 2018, 165, J3001–J3007.
- 80 Liu, M.; Xiao, X.; Li, Q.; Luo, L.; Ding, M.; Zhang, B.; Li, Y.; Zou, J.; Jiang, B. Recent progress of electrocatalysts for oxygen reduction in fuel cells. J. Colloid Interface Sci. 2022, 607, 791–815.
- 81 Zaman, S.; Su, Y.-Q.; Dong, C.-L.; Qi, R.; Huang, L.; Qin, Y.; Huang, Y.-C.; Li, F.-M.; You, B.; Guo, W.; Li, Q.; Ding, S.; Xia, B. Y. Scalable Molten Salt Synthesis of Platinum Alloys Planted in Metal-Nitrogen-Graphene for Efficient Oxygen Reduction. Angew. Chem. Int. Ed. 2022, 61, e202115835.
- 82 Raj, I. A.; Vasu, K. I. Transition-Metal Oxides Based Oxygen Electrodes In Alkaline-Solution - Electrocatalysis by Some Perovskite Oxides Based on Manganese and Cobalt. Bull. Electrochem. 1993, 9, 560–563.
- 83 Escudero-Escribano, M.; Verdaguer-Casadevall, A.; Malacrida, P.; Gronbjerg, U.; Knudsen, B. P.; Jepsen, A. K.; Rossmeisl, J.; Stephens, I. E. L.; Chorkendorff, I. Pt5Gd as a Highly Active and Stable Catalyst for Oxygen Electroreduction. J. Am. Chem. Soc. 2012, 134, 16476–16479.
- 84 Zhang, S.; Li, Q.-q.; Luo, F.; Du, Y.-p. Research Progress of Rare Earth Alloy Electrocatalysts. Chin. Rare Earths 2022, 43, 1–16.
- 85 Hernandez-Fernandez, P.; Masini, F.; McCarthy, D. N.; Strebel, C. E.; Friebel, D.; Deiana, D.; Malacrida, P.; Nierhoff, A.; Bodin, A.; Wise, A. M.; Nielsen, J. H.; Hansen, T. W.; Nilsson, A.; Stephens, I. E. L.; Chorkendorff, I. Mass-selected nanoparticles of PtxY as model catalysts for oxygen electroreduction. Nat. Chem. 2014, 6, 732–738.
- 86 Escudero-Escribano, M.; Malacrida, P.; Hansen, M. H.; Vej-Hansen, U. G.; Velazquez-Palenzuela, A.; Tripkovic, V.; Schiotz, J.; Rossmeisl, J.; Stephens, I. E. L.; Chorkendorff, I. Tuning the activity of Pt alloy electrocatalysts by means of the lanthanide contraction. Science 2016, 352, 73–76.
- 87 Tripkovic, V.; Zheng, J.; Rizzi, G. A.; Marega, C.; Durante, C.; Rossmeisl, J.; Granozzi, G. Comparison between the Oxygen Reduction Reaction Activity of Pd5Ce and Pt5Ce: The Importance of Crystal Structure. ACS Catal. 2015, 5, 6032–6040.
- 88 Yoo, S. J.; Hwang, S. J.; Lee, J.-G.; Lee, S.-C.; Lim, T.-H.; Sung, Y.-E.; Wieckowski, A.; Kim, S.-K. Promoting effects of La for improved oxygen reduction activity and high stability of Pt on Pt-La alloy electrodes. Energy Environ. Sci. 2012, 5, 7521–7525.
- 89 Yoo, S. J.; Kim, S.-K.; Jeon, T.-Y.; Hwang, S. J.; Lee, J.-G.; Lee, S.-C.; Lee, K.-S.; Cho, Y.-H.; Sung, Y.-E.; Lim, T.-H. Enhanced stability and activity of Pt-Y alloy catalysts for electrocatalytic oxygen reduction. Chem. Commun. 2011, 47, 11414–11416.
- 90 Chen, Z.; Higgins, D.; Yu, A.; Zhang, L.; Zhang, J. A review on non-precious metal electrocatalysts for PEM fuel cells. Energy Environ. Sci. 2011, 4, 3167–3192.
- 91 Wang, X.; Zhang, J.; Wang, P.; Li, L.; Wang, H.; Sun, D.; Li, Y.; Tang, Y.; Lu, X. F.; Wang, Y.; Fu, G. Terbium-induced cobalt valence-band narrowing boosts electrocatalytic oxygen reduction. Energy Environ. Sci. 2023, 16, 5500–5512.
- 92 Fan, C.; Wang, X.; Wu, X.; Chen, Y.; Wang, Z.; Li, M.; Sun, D.; Tang, Y.; Fu, G. Neodymium-Evoked Valence Electronic Modulation to Balance Reversible Oxygen Electrocatalysis. Adv. Energy Mater. 2023, 13, 2203244.
- 93 Sun, Y.; Zhang, T.; Sun, P.; Wang, J.; Duan, W.; Zhuang, Y.; Wang, L.; Li, Z. Enhanced bifunctional oxygen electrochemical catalytic performance using La-doped CoFe2O4 spinel supported by 3D-G for Zn-air batteries. J. Energy Chem. 2024, 94, 778–788.
- 94 Nnabuife, S. G.; Ugbeh-Johnson, J.; Okeke, N. E.; Ogbonnaya, C. Present and Projected Developments in Hydrogen Production: A Technological Review. Carbon Capture Sci. Technol. 2022, 3, 100042.
- 95 Zhu, J.; Hu, L.; Zhao, P.; Lee, L. Y. S.; Wong, K.-Y. Recent Advances in Electrocatalytic Hydrogen Evolution Using Nanoparticles. Chem. Rev. 2020, 120, 851–918.
- 96 Peng, Z.; Zhang, Q.; Qi, G.; Zhang, H.; Liu, Q.; Hu, G.; Luo, J.; Liu, X. Nanostructured Pt@RuOx catalyst for boosting overall acidic seawater splitting. Chin. J. Struct. Chem. 2024, 43, 100191.
- 97
Liu, W.; Liu, W.; Hou, T.; Ding, J.; Wang, Z.; Yin, R.; San, X.; Feng, L.; Luo, J.; Liu, X. Coupling Co-Ni phosphides for energy-saving alkaline seawater splitting. Nano Res. 2024, 4797–4806.
10.1007/s12274-024-6433-8 Google Scholar
- 98
Qin, C.; Chen, S.; Gomaa, H.; Shenashen, M. A.; El-Safty, S. A.; Liu, Q.; An, C.; Liu, X.; Deng, Q.; Hu, N. Regulating HER and OER Performances of 2D Materials by the External Physical Fields. Acta Phys.-Chim. Sin. 2024, 40, 2307059.
10.3866/PKU.WHXB202307059 Google Scholar
- 99 Liu, W.; Niu, X.; Tang, J.; Liu, Q.; Luo, J.; Liu, X. Energy-efficient anodic reactions for sustainable hydrogen production via water electrolysis. Chem. Synth. 2023, 3, 44.
- 100 Campos-Roldan, C. A.; Chattot, R.; Pailloux, F.; Zitolo, A.; Roziere, J.; Jones, D. J.; Cavaliere, S. Lanthanide contraction effect on the alkaline hydrogen evolution and oxidation reactions activity in platinum-rare earth nanoalloys. J. Mater. Chem. A 2024, 12, 1253–1258.
- 101 Jiang, Y.; Liang, Z.; Fu, H.; Sun, M.; Wang, S.; Huang, B.; Du, Y. Pt-Modified High Entropy Rare Earth Oxide for Efficient Hydrogen Evolution in pH-Universal Environments. J. Am. Chem. Soc. 2024, 146, 9012–9025.
- 102 Zhang, D.; Ji, S.-J.; Suen, N.-T. Crystal and electronic structure manipulation of Laves intermetallics for boosting hydrogen evolution reaction. Chem. Commun. 2021, 57, 8504–8507.
- 103
Peng, W.; Li, X.; He, Z.; Li, Z.; Zhang, X.; Sun, X.; Li, Q.; Yang, H.; Han, J.; Huang, Y. Electron density modulation of MoP by rare earth metal as highly efficient electrocatalysts for pH-universal hydrogen evolution reaction. Appl. Catal. B-Environ. 2021, 299, 120657.
10.1016/j.apcatb.2021.120657 Google Scholar
- 104
Yoro, K.; Daramola, M. O. CO2 emission sources, greenhouse gases, and the global warming effect. In Advances in Carbon Capture, Eds: M. R. Rahimpour; M. Farsi; M. A. Makarem, Woodhead Publishing, 2020, pp. 1–28.
10.1016/B978-0-12-819657-1.00001-3 Google Scholar
- 105 Yu, J.; Wang, J.; Ma, Y.; Zhou, J.; Wang, Y.; Lu, P.; Yin, J.; Ye, R.; Zhu, Z.; Fan, Z. Recent Progresses in Electrochemical Carbon Dioxide Reduction on Copper-Based Catalysts toward Multicarbon Products. Adv. Funct. Mater. 2021, 31, 2102151.
- 106 Nitopi, S.; Bertheussen, E.; Scott, S. B.; Liu, X.; Engstfeld, A. K.; Horch, S.; Seger, B.; Stephens, I. E. L.; Chan, K.; Hahn, C.; Norskov, J. K.; Jaramillo, T. F.; Chorkendorff, I. Progress and Perspectives of Electrochemical CO2 Reduction on Copper in Aqueous Electrolyte. Chem. Rev. 2019, 119, 7610–7672.
- 107 Luo, M.; Wang, Z.; Li, Y. C.; Li, J.; Li, F.; Lum, Y.; Nam, D.-H.; Chen, B.; Wicks, J.; Xu, A.; Zhuang, T.; Leow, W. R.; Wang, X.; Dinh, C.-T.; Wang, Y.; Wang, Y.; Sinton, D.; Sargent, E. H. Hydroxide promotes carbon dioxide electroreduction to ethanol on copper via tuning of adsorbed hydrogen. Nat. Commun. 2019, 10, 5814.
- 108 Liu, J.; Li, P.; Bi, J.; Jia, S.; Wang, Y.; Kang, X.; Sun, X.; Zhu, Q.; Han, B. Switching between C2+ Products and CH4 in CO2 Electrolysis by Tuning the Composition and Structure of Rare-Earth/Copper Catalysts. J. Am. Chem. Soc. 2023, 145, 23037–23047.
- 109 Liu, W.; Bai, P.; Wei, S.; Yang, C.; Xu, L. Gadolinium Changes the Local Electron Densities of Nickel 3d Orbitals for Efficient Electrocatalytic CO2 Reduction. Angew. Chem. Int. Ed. 2022, 61, e202201166.
- 110 Zhao, J.; Zhang, P.; Yuan, T.; Cheng, D.; Zhen, S.; Gao, H.; Wang, T.; Zhao, Z.-J.; Gong, J. Modulation of *CHXO Adsorption to Facilitate Electrocatalytic Reduction of CO2 to CH4 over Cu-Based Catalysts. J. Am. Chem. Soc. 2023, 145, 6622–6627.
- 111 Wang, X.; Zhu, Y.; Li, H.; Lee, J.-M.; Tang, Y.; Fu, G. Rare-Earth Single-Atom Catalysts: A New Frontier in Photo/Electrocatalysis. Small Methods 2022, 6, 2200413.
- 112 Handoko, A. D.; Wei, F.; Jenndy; Yeo, B. S.; Seh, Z. W. Understanding heterogeneous electrocatalytic carbon dioxide reduction through operando techniques. Nat. Catal. 2018, 1, 922–934.
- 113 Mu, C.; Lv, C.; Meng, X.; Sun, J.; Tong, Z.; Huang, K. In Situ Characterization Techniques Applied in Photocatalysis: A Review. Adv. Mater. Interfaces 2023, 10, 2201842.




