Skeletal Editing of Cyclic Molecules Using Nitrenes
Yi-An Xu
Shenzhen Grubbs Institute, Department of Chemistry, Southern University of Science and Technology, Shenzhen, Guangdong, 518055 China
Search for more papers by this authorShao-Hua Xiang
Shenzhen Grubbs Institute, Department of Chemistry, Southern University of Science and Technology, Shenzhen, Guangdong, 518055 China
Search for more papers by this authorJin-Teng Che
Shenzhen Grubbs Institute, Department of Chemistry, Southern University of Science and Technology, Shenzhen, Guangdong, 518055 China
Search for more papers by this authorCorresponding Author
Yong-Bin Wang
Shenzhen Grubbs Institute, Department of Chemistry, Southern University of Science and Technology, Shenzhen, Guangdong, 518055 China
E-mail: [email protected]; [email protected]Search for more papers by this authorCorresponding Author
Bin Tan
Shenzhen Grubbs Institute, Department of Chemistry, Southern University of Science and Technology, Shenzhen, Guangdong, 518055 China
E-mail: [email protected]; [email protected]Search for more papers by this authorYi-An Xu
Shenzhen Grubbs Institute, Department of Chemistry, Southern University of Science and Technology, Shenzhen, Guangdong, 518055 China
Search for more papers by this authorShao-Hua Xiang
Shenzhen Grubbs Institute, Department of Chemistry, Southern University of Science and Technology, Shenzhen, Guangdong, 518055 China
Search for more papers by this authorJin-Teng Che
Shenzhen Grubbs Institute, Department of Chemistry, Southern University of Science and Technology, Shenzhen, Guangdong, 518055 China
Search for more papers by this authorCorresponding Author
Yong-Bin Wang
Shenzhen Grubbs Institute, Department of Chemistry, Southern University of Science and Technology, Shenzhen, Guangdong, 518055 China
E-mail: [email protected]; [email protected]Search for more papers by this authorCorresponding Author
Bin Tan
Shenzhen Grubbs Institute, Department of Chemistry, Southern University of Science and Technology, Shenzhen, Guangdong, 518055 China
E-mail: [email protected]; [email protected]Search for more papers by this authorAbstract
Comprehensive Summary
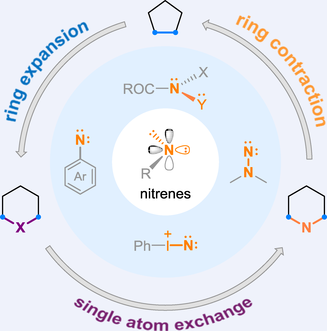
Nitrenes, as neutral monovalent nitrogen-centered molecular species, can insert into various bond or remove nitrogen atoms from amines. Nitrene assisted single-atom skeletal editing, discovered decades ago, provides an efficient approach for the precise alteration of cyclic skeletons. In this review, we briefly summarize early studies on skeletal editing of cyclic frameworks involving nitrene species, and introduce several recent important advances systematically.

References
- 1
Tiemann, F. Ueber die Einwirkung von Benzolsulfonsäurechlorid auf Amidoxime. Ber. Dtsch. Chem. Ges. 1891, 24, 4162–4167.
10.1002/cber.189102402316 Google Scholar
- 2 Shainyan, B. A.; Kuzmin, A. V.; Moskalik, M. Y. Carbenes and Nitrenes. An Overview. Comput. Theor. Chem. 2013, 1006, 52–61.
- 3 Gritsan, N. P.; Platz, M. S. Kinetics, Spectroscopy, and Computational Chemistry of Arylnitrenes. Chem. Rev. 2006, 106, 3844–3867.
- 4 Wentrup, C. Carbenes and Nitrenes: Recent Developments in Fundamental Chemistry. Angew. Chem. Int. Ed. 2018, 57, 11508–11521.
- 5 Borden, W. T.; Gritsan, N. P.; Hadad, C. M.; Karney, W. L.; Kemnitz, C. R.; Platz, M. S. The Interplay of Theory and Experiment in the Study of Phenylnitrene. Acc. Chem. Res. 2000, 33, 765–771.
- 6 Gritan, N. P.; Gudmundsdóttir, A. D.; Tigelaar, D.; Zhu, Z.; Karney, W. L.; Hadad, C. M.; Platz, M. S. A Laser Flash Photolysis and Quantum Chemical Study of the Fluorinated Derivatives of Singlet Phenylnitrene. J. Am. Chem. Soc. 2001, 123, 1951–1962.
- 7 McConaghy, J. S.; Lwowski, W. Singlet and Triplet Nitrenes. I. Carbethoxynitrene Generated by α Elimination. J. Am. Chem. Soc. 1967, 89, 2357–2364.
- 8 Scholz, S. O.; Farney, E. P.; Kim, S.; Bates, D. M.; Yoon, T. P. Spin-Selective Generation of Triplet Nitrenes: Olefin Aziridination through Visible-Light Photosensitization of Azidoformates. Angew. Chem. Int. Ed. 2016, 55, 2239–2242.
- 9 Zhang, Y.; Dong, X.; Wu, Y.; Li, G.; Lu, H. Visible-Light-Induced Intramolecular C(sp2)−H Amination and Aziridination of Azidoformates via a Triplet Nitrene Pathway. Org. Lett. 2018, 20, 4838–4842.
- 10 Wang, Y.-C.; Lai, X.-J.; Huang, K.; Yadav, S.; Qiu, G.; Zhang, L.; Zhou, H. Unravelling Nitrene Chemistry from Acyclic Precursors: Recent Advances and Challenges. Org. Chem. Front. 2021, 8, 1677–1693.
- 11 Uchida, T.; Katsuki, T. Asymmetric Nitrene Transfer Reactions: Sulfimidation, Aziridination and C-H Amination Using Azide Compounds as Nitrene Precursors. Chem. Rec. 2014, 14, 117–129.
- 12 Wang, H.; Jung, H.; Song, F.; Zhu, S.; Bai, Z.; Chen, D.; He, G.; Chang, S.; Chen, G. Nitrene-Mediated Intermolecular N-N Coupling for Efficient Synthesis of Hydrazides. Nat. Chem. 2021, 13, 378–385.
- 13 Dequirez, G.; Pons, V.; Dauban, P. Nitrene Chemistry in Organic Synthesis: Still in Its Infancy? Angew. Chem. Int. Ed. 2012, 51, 7384–7395.
- 14 Kwart, H.; Khan, A. A. Copper-Catalyzed Decomposition of Benzenesulfonyl Azide in Cyclohexene Solution. J. Am. Chem. Soc. 1967, 89, 1951–1953.
- 15 Macara, J.; Caldeira, C.; Poeira, D. L.; Marques, M. M. B. Reactivity of Hypervalent Iodine(III) Reagents Bearing Transferable N-Based Groups. Eur. J. Org. Chem. 2023, 26, e202300109.
- 16 Campos, K. R.; Coleman, P. J.; Alvarez, J. C.; Dreher, S. D.; Garbaccio, R. M.; Terrett, N. K.; Tillyer, R. D.; Truppo, M. D.; Parmee, E. R. The Importance of Synthetic Chemistry in the Pharmaceutical Industry. Science 2019, 363, eaat0805.
- 17 Taylor, R. D.; MacCoss, M.; Lawson, A. D. G. Rings in Drugs. J. Med. Chem. 2014, 57, 5845–5859.
- 18 Hong, B.; Luo, T.; Lei, X. Late-Stage Diversification of Natural Products. ACS Cent. Sci. 2020, 6, 622–635.
- 19 Cernak, T.; Dykstra, K. D.; Tyagarajan, S.; Vachal, P.; Krska, S. W. The Medicinal Chemist's Toolbox for Late-Stage Functionalization of Drug-like Molecules. Chem. Soc. Rev. 2016, 45, 546–576.
- 20 Huigens, R. W. III; Morrison, K. C.; Hicklin, R. W.; Flood, T. A. Jr; Richter, M. F.; Hergenrother, P. J. A Ring-Distortion Strategy to Construct Stereochemically Complex and Structurally Diverse Compounds from Natural Products. Nat. Chem. 2013, 5, 195–202.
- 21 Blakemore, D. C.; Castro, L.; Churcher, I.; Rees, D. C.; Thomas, A. W.; Wilson, D. M.; Wood, A. Organic Synthesis Provides Opportunities to Transform Drug Discovery. Nat. Chem. 2018, 10, 383–394.
- 22 Jurczyk, J.; Woo, J.; Kim, S. F.; Dherange, B. D.; Sarpong, R.; Levin, M. D. Single-atom Logic for Heterocycle Editing. Nat. Synth. 2022, 1, 352–364.
- 23 Joynson, B. W.; Ball, L. T. Skeletal Editing: Interconversion of Arenes and Heteroarenes. Helv. Chim. Acta 2023, 106, e202200182.
- 24 Liu, Z.; Sivaguru, P.; Ning, Y.; Wu, Y.; Bi, X. Skeletal Editing of (Hetero)Arenes Using Carbenes. Chem. Eur. J. 2023, 29, e202301227.
- 25 Huisgen, R.; Appl, M. Der Chemismus der Ringerweiterung beim Zerfall des Phenylazids in Anilin. Chem. Bur. 1958, 91, 12–21.
- 26 Doering, W. von E.; Odum, R. A. Ring Enlargement in the Photolysis of Phenyl Azide. Tetrahedron 1966, 22, 81–93.
- 27 Sundberg, R. J.; Das, B. P.; Smith, R. H. Photochemical Deoxygenation of Aromatic Nitro Compounds in Triethyl Phosphite. Substituent Effects and Evidence for the Involvement Aryl Nitrenes. J. Am. Chem. Soc. 1969, 91, 658–668.
- 28 Atherton, F. R.; Lambert, R. W. Nitrenes Generated from Nitro-compounds by Various Phosphorus Reagents in Heterocyclic Synthesis. A Convenient Route to Substituted 3H-Azepines. J. Chem. Soc., Perkin Trans. 1 1973, 1079–1084.
- 29 Mykura, R.; Sánchez-Bento, R.; Matador, E.; Duong, V. K.; Varela, A.; Angelini, L.; Carbajo, R. J.; Llaveria, J.; Ruffoni, A.; Leonori, D. Synthesis of Polysubstituted Azepanes by Dearomative Ring Expansion of Nitroarenes. Nat. Chem. 2024, 16, 771–779.
- 30 Marsh, F. D.; Simmons, H. E. N-Cyanoazepines from Cyanonitrene and Aromatic Compounds. J. Am. Chem. Soc. 1965, 87, 3529–3530.
- 31 Abramovitch, R. A.; Bailey, T. D.; Takaya, T.; Uma, V. The Reaction of Methanesulfonyl Nitrene with Benzene. Attempts to Generate Sulfonyl Nitrenes from Sources Other than the Azides. J. Org. Chem. 1974, 39, 340–345.
- 32
Ayyangar, N. R.; Bambal, R. B.; Lugade, A. G. Pressure-induced Synthesis of an N-Sulphonyl-1H-azepine by Sulphonyl-nitrene Insertion into Benzene. J. Chem. Soc., Chem. Commun. 1981, 790–791.
10.1039/c39810000790 Google Scholar
- 33 Saikia, I.; Kashiyap, B.; Phukan, P. A Facile Noncatalytic Pathway for the Nitrene Transfer Process: Expeditous Access to Aziridines. Chem. Commun. 2011, 47, 2967–2969.
- 34 Zenzola, M.; Doran, R.; Degennaro, L.; Luisi, R.; Bull, J. Transfer of Electrophilic NH Using Convenient Sources of Ammonia: Direct Synthesis of NH Sulfoximines from Sulfoxides. Angew. Chem. Int. Ed. 2016, 55, 7203–7207.
- 35 Desai, A. V.; Sharma, S.; Let, S.; Ghosh S. K. N-Donor Linker Based Metal-organic Frameworks (MOFs): Advancement and Prospects as Functional Materials. Coord. Chem. Rev. 2019, 395, 146–192.
- 36 Fernández, E.; Guiry, P. J.; Connole, K. P. T.; Brown, J. M. Quinap and Congeners: Atropos PN ligands for Asymmetric Catalysis. J. Org. Chem. 2014, 79, 5391–5400.
- 37 Baumann, M,; Baxendake, I. R. An Overview of the Synthetic Routes to the Best Selling Drugs Containing 6-Membered Heterocycles. Beilstein J. Org. Chem. 2013, 9, 2265–2319.
- 38 Reisenbauer, J. C.; Ori, G.; Franchino, A.; Finkelstein, P.; Morandi, B. Late-stage Diversification of Indole Skeletons Through Nitrogen Atom Insertion. Science 2022, 377, 1104–1109.
- 39 Reisenbauer, J. C.; Paschke, A. K.; Krizic, J.; Botlik, B. B.; Finkelstein, P.; Morandi, B. Direct Access to Quinazolines and Pyrimidines from Unprotected Indoles and Pyrroles through Nitrogen Atom Insertion. Org. Lett. 2023, 25, 8419–8423.
- 40 Wang, J.; Lu, H.; He, Y.; Jing, C.; Wei, H. Cobalt-Catalyzed Nitrogen Atom Insertion in Arylcycloalkenes. J. Am. Chem. Soc. 2022, 144, 22433–22439.
- 41 Finkelstein, P.; Reisenbauer, J. C.; Botlik, B. B.; Green, O.; Florin, A.; Morandi, B. Nitrogen Atom Insertion into Indenes to Access Isoquinolines. Chem. Sci. 2023, 14, 2954–2959.
- 42 Fujita, T.; Maeda, T.; Kim, B. J.; Tatami, A.; Miyamoto, D.; Kawaguchi, H.; Tsuchiya, N.; Yoshida, M.; Kawashima, W.; Morita, H. Photolytic Aziridination by Thianthrene Sulfilimines Derivatives. J. Sulfur Chem. 2008, 29, 459–465.
- 43 Morita, H.; Tatami, A.; Maeda, T.; Kim, B. J.; Kawashima, W.; Yoshimura, T.; Abe, H.; Akasaka, T. Generation of Nitrene by the Photolysis of N-Substituted Iminodibenzothiophene. J. Org. Chem. 2008, 73, 7159–7163.
- 44 Heilmann, T.; Lopez-Soria, J. M.; Ulbrich, J.; Kircher, J.; Li, Z.; Worbs, B.; Golz, C.; Mata, R. A.; Alcarazo, M. N-(Sulfonio)Sulfilimine Reagents: Non-Oxidizing Sources of Electrophilic Nitrogen Atom for Skeletal Editing. Angew. Chem. Int. Ed. 2024, e202403826.
- 45 Intrieri, D.; Zardi, P.; Caselli, A.; Gallo, E. Organic azides: “energetic reagents” for the intermolecular amination of C-H bonds. Chem. Commun. 2014, 50, 11440–11453.
- 46 Shin, K.; Kim, H.; Chang, S. Transition-Metal-Catalyzed C-N Bond Forming Reactions Using Organic Azides as the Nitrogen Source: A Journey for the Mild and Versatile C-H Amination. Acc. Chem. Res. 2015, 48, 1040–1052.
- 47 Antoni, P. W.; Mackenroth, A. V.; Mulks, F. F.; Rudolph, M.; Helmchen, G.; Hashmi, A. S. K. Dibenzothiophenesulfilimines: A Convenient Approach to Intermolecular Rhodium-Catalysed C−H Amidation. Chem. Eur. J. 2020, 26, 8235–8238.
- 48 Li, H.; Li, N.; Wu, J.; Yu, T.; Zhang, R.; Xu, L.; Wei, H. Rhodium-Catalyzed Intramolecular Nitrogen Atom Insertion into Arene Rings. J. Am. Chem. Soc. 2023, 145, 17570–17576.
- 49 Overberger, C. G.; Lombardino, J. G.; Hiskey, R. G. Azo Compounds. Oxidation of 1,1-Disubstituted Hydrazines. The Synthesis and Oxidation of cis- and trans-1-Amino-2,6-diphenylpiperidine. A New Stereospecific Ring Closure. J. Am. Chem. Soc. 1957, 79, 6430–6435.
- 50 Carpino, L. A. Oxidation of N-Aminodihydroisoindoles. Synthesis of cis- and trans-1,2-Diphenylbenzocyclobutanes. J. Am. Chem. Soc. 1962, 84, 2196–2201.
- 51 Overberger, C. G.; Valentine, M.; Anselme, J.-P. The Synthesis of cis- and trans-N-Amino- and N-Nitroso-2,5-diphenylpyrrolidines. Their Abnormal Oxidation and Reduction with Mercuric Oxide and Sodium Hydrosulfite. J. Am. Chem. Soc. 1969, 91, 687–694.
- 52 Dervan, P. B.; Uyehara, T. Stereochemical Test of Cyclic 1,1-Dialkyldiazene Fragmentation Reactions. Thermal Decomposition of N-(cis-(and trans-)2,3-(and 2,5-)Dimethylpyrrolidine)-nitrenes. J. Am. Chem. Soc. 1979, 101, 2076–2082.
- 53 Schultz, P. G.; Dervan, P. B. Synthesis and Direct Spectroscopic Observation of N-(2,2,5,5-Tetramethylpyrrolidyl)nitrene. Comparison of Five- and Six-Membered Cyclic 1,1-Dialkyldiazenes. J. Am. Chem. Soc. 1980, 102, 878–880.
- 54 Schultz, P. G.; Dervan, P. B. Photochemistry of 1,1-Diazenes. Direct and Sensitized Photolyses of N-(2,2,5,5-Tetramethylpyrrolidyl)nitrene, dl-N-(2,5-Diethyl-2,5-dimethylpyrrolidyl)nitrene, and N-(2,2,6,6-Tetramethylpiperidyl)nitrene. J. Am. Chem. Soc. 1982, 104, 6660–6668.
- 55
Hinsberg, W. D.; Schultz, P. G.; Dervan, P. B. Direct Studies of 1,1-Diazenes. Syntheses, Infrared and Electronic Spectra, and Kinetics of the Thermal Decomposition of N-(2,2,6,6-Tetramethylpiperidyl)nitrene and N-(2,2,5,5-Tetramethylpyrrolidyl)nitrene. J. Am. Chem. Soc. 1982, 104, 755–773.
10.1021/ja00367a020 Google Scholar
- 56 Miller, R. D.; Gölitz, P.; Janssen, J.; Lemmens, J. Alternative Precursors to 1,4-Acyl Alkyl Biradicals: Cyclic N-Acyl-1,1-diazenes. J. Am. Chem. Soc. 1984, 106, 7277–7279.
- 57 Horner, M. G.; Rudolph, M. J.; Wolff, S.; Agosta, W. C. Thermal and Photochemical Decomposition of N-[2-(3,3-Dimethyl-1-butynyl)- 2,5,5-trimethyl-1-pyrrolidinyl]nitrene. J. Am. Chem. Soc. 1992, 114, 6034–6037.
- 58 Kennedy, S. H.; Dherange, B. D.; Berger, K. J.; Levin, M. D. Skeletal Editing Through Direct Nitrogen Deletion of Secondary Amines. Nature 2021, 593, 223–227.
- 59 Auberson, Y. P.; Brocklehurst, C.; Furegati, M.; Fessard, T. C.; Koch, G.; Decker, A.; La Vecchia, L.; Briard, E. Improving Nonspecific Binding and Solubility: Bicycloalkyl Groups and Cubanes as para-Phenyl Bioisosteres. ChemMedChem 2017, 12, 590–598.
- 60 Monteleone, S.; Fuchs, J. E.; Liedl, K. R. Molecular Connectivity Predefines Polypharmacology: Aliphatic Rings, Chirality, and sp3 Centers Enhance Target Selectivity. Front. Pharmacol. 2017, 8, 552–560.
- 61 Wright, B. A.; Matviitsuk, A.; Black, M. J.; García-Reynaga, P.; Hanna, L. E.; Herrmann, A. T.; Ameriks, M. K.; Sarpong, R.; Lebold, T. P. Skeletal Editing Approach to Bridge-Functionalized Bicyclo[1.1.1]pentanes from Azabicyclo[2.1.1]hexanes. J. Am. Chem. Soc. 2023, 145, 10960–10966.
- 62 Lwowski, W. Nitrenes and the Decomposition of Carbonylazides. Angew. Chem. Int. Ed. 1967, 6, 897–906.
- 63 Zou, X.; Zou, J.; Yang, L.; Li, G.; Lu, H. Thermal Rearrangement of Sulfamoyl Azide: Reactivity and Mechanistic Study. J. Org. Chem. 2017, 82, 4677–4688.
- 64 Qin, H.; Cai, W.; Wang, S.; Guo, T.; Li, G.; Lu, H. N-Atom Deletion in Nitrogen Heterocycles. Angew. Chem. Int. Ed. 2021, 60, 20678–20683.
- 65 Chen, W.; Ma, L.; Paul, A.; Seidel, D. Direct α-C-H Bond Functionalization of Unprotected Cyclic Amines. Nat. Chem. 2018, 10, 165–169.
- 66 Hui, C.; Brieger, L.; Strohmann, C.; Antonchick, A. P. Stereoselective Synthesis of Cyclobutanes by Contraction of Pyrrolidines. J. Am. Chem. Soc. 2021, 143, 18864–18870.
- 67 Monreal-Corona, R.; Solà, M.; Pla-Quintana, A.; Poater, A. Stereoretentive Formation of Cyclobutanes from Pyrrolidines: Lessons Learned from DFT Studies of the Reaction Mechanism. J. Org. Chem. 2023, 88, 4619–4626.
- 68 Sundberg, R. J.; Suter, S. R.; Brenner, M. Photolysis of Ortho-Substituted Aryl Azides in Diethylamine. Formation and Autoxidation of 2-Diethylamino-1H-Azepine Intermediates. J. Am. Chem. Soc. 1972, 94, 513–520.
- 69 Patel, S. C.; Burns, N. Z. Conversion of Aryl Azides to Aminopyridines. J. Am. Chem. Soc. 2022, 144, 17797–17802.
- 70 Davydov, D. A.; Giricheva, M. A.; Malysheva, Y. B.; Fukin, G. K.; Budruev, A. V. Photoinitiated Rearrangement of Aromatic Azides to 2-Aminonicotinates. J. Org. Chem. 2023, 88, 14998–15006.
- 71 Perera, T. A.; Reinheimer, E. W.; Hudnall, T. W. Photochemically Switching Diamidocarbene Spin States Leads to Reversible Büchner Ring Expansions. J. Am. Chem. Soc. 2017, 139, 14807–14814.
- 72 Pearson, T. J.; Shimazumi, R.; Driscoll, J. L.; Dherange, B. D.; Park, D.; Levin, M. D. Aromatic Nitrogen Scanning by ipso-Selective Nitrene Internalization. Science 2023, 381, 1474–1479.




