Visible-Light-Mediated Photocatalyst-Free Hydroacylation of Azodicarboxylic Acid Derivatives with 4-Acyl-1,4-dihydropyridines
Li Liu
Department of Organic Chemistry, College of Chemistry, Beijing University of Chemical Technology, Beijing, 100029 China
Sinopec Beijing Research Institute of Chemical Industry, Beijing, 100013 China
The authors contributed equally.
Search for more papers by this authorJing Wang
Department of Organic Chemistry, College of Chemistry, Beijing University of Chemical Technology, Beijing, 100029 China
The authors contributed equally.
Search for more papers by this authorXiaoying Feng
Department of Organic Chemistry, College of Chemistry, Beijing University of Chemical Technology, Beijing, 100029 China
Search for more papers by this authorKun Xu
Faculty of Environment and Life, Beijing University of Technology, Beijing, 100124 China
Search for more papers by this authorWei Liu
Department of Organic Chemistry, College of Chemistry, Beijing University of Chemical Technology, Beijing, 100029 China
Search for more papers by this authorXia Peng
Department of Organic Chemistry, College of Chemistry, Beijing University of Chemical Technology, Beijing, 100029 China
Search for more papers by this authorCorresponding Author
Hongguang Du
Department of Organic Chemistry, College of Chemistry, Beijing University of Chemical Technology, Beijing, 100029 China
E-mail: [email protected]; [email protected]Search for more papers by this authorCorresponding Author
Jiajing Tan
Department of Organic Chemistry, College of Chemistry, Beijing University of Chemical Technology, Beijing, 100029 China
E-mail: [email protected]; [email protected]Search for more papers by this authorLi Liu
Department of Organic Chemistry, College of Chemistry, Beijing University of Chemical Technology, Beijing, 100029 China
Sinopec Beijing Research Institute of Chemical Industry, Beijing, 100013 China
The authors contributed equally.
Search for more papers by this authorJing Wang
Department of Organic Chemistry, College of Chemistry, Beijing University of Chemical Technology, Beijing, 100029 China
The authors contributed equally.
Search for more papers by this authorXiaoying Feng
Department of Organic Chemistry, College of Chemistry, Beijing University of Chemical Technology, Beijing, 100029 China
Search for more papers by this authorKun Xu
Faculty of Environment and Life, Beijing University of Technology, Beijing, 100124 China
Search for more papers by this authorWei Liu
Department of Organic Chemistry, College of Chemistry, Beijing University of Chemical Technology, Beijing, 100029 China
Search for more papers by this authorXia Peng
Department of Organic Chemistry, College of Chemistry, Beijing University of Chemical Technology, Beijing, 100029 China
Search for more papers by this authorCorresponding Author
Hongguang Du
Department of Organic Chemistry, College of Chemistry, Beijing University of Chemical Technology, Beijing, 100029 China
E-mail: [email protected]; [email protected]Search for more papers by this authorCorresponding Author
Jiajing Tan
Department of Organic Chemistry, College of Chemistry, Beijing University of Chemical Technology, Beijing, 100029 China
E-mail: [email protected]; [email protected]Search for more papers by this authorComprehensive Summary
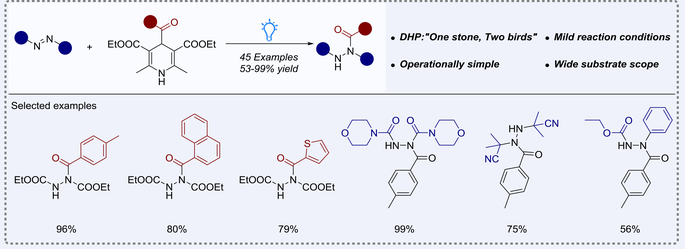
A visible-light-enabled, photocatalyst-free hydroacylation reaction of azodicarboxylic acid derivatives was described. This radical conjugate addition (RCA) protocol relied on the dual role of 4-acyl-1,4-dihydropyridine (acyl-DHP) reagents that besides being as radical reservoirs, they also enabled the conversion of radical adducts to anion intermediates via reduction. Under “catalyst-oxidant-additive free” conditions, a wide range of structurally different acyl hydrazide products were readily obtained in 56%—99% yields. The utility of this transformation was further demonstrated by the scale-up synthesis and downstream derivatization.
Supporting Information
| Filename | Description |
|---|---|
| cjoc202300726-sup-0001-Supinfo.pdfPDF document, 9.1 MB |
Appendix S1: Supporting Information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1(a) Beeler, A. B. Introduction: Photochemistry in Organic Synthesis. Chem. Rev. 2016, 116, 9629–9630; (b) Holmberg-Douglas, N.; Nicewicz, D. A. Photoredox-Catalyzed C—H Functionalization Reactions. Chem. Rev. 2022, 122, 1925–2016; (c) Juliá, F.; Constantin, T.; Leonori, D. Applications of Halogen-Atom Transfer (XAT) for the Generation of Carbon Radicals in Synthetic Photochemistry and Photocatalysis. Chem. Rev. 2022, 122, 2292–2352; (d) Wang, X. Y.; Luo, N. C.; Wang, F. Advances and challenges of photocatalytic methane C—C coupling. Chin. J. Chem. 2022, 40, 1492–1505; (e) Zhong, Y. W. Photofunction-directed coordination molecular engineering. Chin. J. Chem. 2021, 39, 543–549; (f) Marek, I. Introduction: Carbon-Carbon Bond Cleavage in Stereoselective Synthesis. Chem. Rev. 2021, 121, 1–2.
- 2(a) Lechner, V. M.; Nappi, M.; Deneny, P. J.; Folliet, S.; Chu, J. C. K.; Gaunt, M. J. Visible-Light-Mediated Modification and Manipulation of Biomacromolecules. Chem. Rev. 2022, 122, 1752–1829; (b) Peng, X.; Xu, K.; Zhang, Q.; Liu, L.; Tan, J. J. Dehydroalanine modification sees the light: a photochemical conjugate addition strategy. Trends Chem. 2022, 4, 643–657; (c) Zhang, Y. D.; Zhang, Y. X.; Zhang, Y. X.; Han, L. L.; Che, Q. L.; Tan, J. W.; Zou, P.; Chen, Y. Y. Photo-Modulation of Gene-Editing Enzymes CRISPR/Cas9 with Bifunctional Small-Molecule Ligands. Chin. J. Chem. 2023, 41, 3639–3644, and references therein.
- 3(a) Crisenza, G. E. M.; Mazzarella, D.; Melchiorre, P. Synthetic Methods Driven by the Photoactivity of Electron Donor-Acceptor Complexes. J. Am. Chem. Soc. 2020, 142, 5461–5476; (b) Wei, Y.; Zhou, Q. Q.; Tan, F.; Lu, L. Q.; Xiao, W. J. Visible-Light-Driven Organic Photochemical Reactions in the Absence of External Photocatalysts. Synthesis 2019, 51, 3021–3054; (c) Lima, C. G. S.; Lima, T. M.; Duarte, M.; urberg, I. D. J; Paixão, M. W. Organic Synthesis Enabled by Light-Irradiation of EDA Complexes: Theoretical Background and Synthetic Applications. ACS Catal. 2016, 6, 1389–1407.
- 4(a) Huang, W. H.; Cheng, X. Hantzsch Esters as Multifunctional Reagents in Visible-Light Photoredox Catalysis. Synlett 2017, 28, 148–158; (b) Wang, P. Z.; Chen, J. R.; Xiao, W. J. Hantzsch esters: an emerging versatile class of reagents in photoredox catalyzed organic synthesis. Org. Biomol. Chem. 2019, 17, 6936–6951; (c) Bhunia, A.; Studer, A. Recent advances in radical chemistry proceeding through pro-aromatic radicals. Chem. 2021, 7, 2060–2100; (d) Corcé, V.; Ollivier, C.; Fensterbank, L. Boron, silicon, nitrogen and sulfur-based contemporary precursors for the generation of alkyl radicals by single electron transfer and their synthetic utilization. Chem. Soc. Rev. 2022, 51, 1470–1510; (e) Gu, F. J.; Huang, W. H.; Liu, X.; Chen, W. X.; Cheng, X. Substituted Hantzsch Esters as Versatile Radical Reservoirs in Photoredox Reactions. Adv. Synth. Catal. 2018, 360, 925–931.
- 5For selected reports, see: (a) Zeng, F. L.; Xie, K. C.; Liu, Y. T.; Wang, H.; Yin, P. C.; Qu, L. B.; Chen, X. C.; Yu, B. Visible-light-promoted catalyst-/additive-free synthesis of aroylated heterocycles in a sustainable solvent. Green Chem. 2022, 24, 1732–1737; (b) Matsuo, B. T.; Oliveira, P. H. R.; Correia J. T. M.; Paixão, M. W. Carbamoylation of Azomethine Imines via Visible-Light Photoredox Catalysis. Org. Lett. 2021, 23, 6775–6779; (c) Yu, W. Q.; Fan, J. H.; Chen, P.; Xiong, B. Y.; Xie, J.; Tang, K. W.; Liu, Y. Transition-metal-free alkylation strategy: facile access to alkylated oxindoles via alkyl transfer. Org. Biomol. Chem. 2022, 20, 1958–1968; (d) Gandolfo, E.; Tang, X.; Roy, S. R.; Melchiorre, P. Photochemical Asymmetric Nickel-Catalyzed Acyl Cross-Coupling. Angew. Chem. Int. Ed. 2019, 58, 16854–16858; (e) Yao, Z.; Yang, J.; Luo, Z. L.; Wang, H. Y.; Zhang, X.; Ye, J. H.; Xu, L.; Shi, Q. Photo-driven metal-free multicomponent reaction between aldehydes, anilines and 4-substituted-DHPs for the synthesis of secondary amines. Green Chem. 2022, 24, 7968–7973; (f) Shan, X. W.; Wang, X. X.; Chen, E. X.; Liu, J. Y.; Lu, K.; Zhao, X. Visible-Light-Promoted Trifluoromethylthiolation and Trifluoromethylselenolation of 1,4-Dihydropyridines. J. Org. Chem. 2023, 88, 319–328; (g) Zhao, X. X.; Li, B.; Xia, W. J. Visible-Light-Promoted Photocatalyst-Free Hydroacylation and Diacylation of Alkenes Tuned by NiCl2·DME. Org. Lett. 2020, 22, 1056–1061; (h) Wang, S. Y.; Zhou, Q. Q.; Zhang, X. H.; Wang, P. Site-Selective Itaconation of Complex Peptides by Photoredox Catalysis. Angew. Chem. Int. Ed. 2022, 61, e202111388; (i) Goti, G.; Bieszczad, B.; Vega-Peñaloza, A.; Melchiorre, P. Stereocontrolled Synthesis of 1,4-Dicarbonyl Compounds by Photochemical Organocatalytic Acyl Radical Addition to Enals. Angew. Chem. Int. Ed. 2019, 58, 1213–1217; (j) Jiang, P. X.; Liu, L.; Tan, J. J.; Du, H. G. Visible-light-promoted photocatalyst-free alkylation and acylation of benzothiazoles. Org. Biomol. Chem. 2021, 19, 4487–4491; (k) Zhang, F. H.; Guo, X. C.; Zeng, X. R.; Wang, Z. B. Asymmetric 1,4-functionalization of 1,3-enynes via dual photoredox and chromium catalysis. Nat. Commun. 2022, 13, 5036–5046.
- 6 Bieszczad, B.; Perego, L. A.; Melchiorre, P. Photochemical C—H Hydroxyalkylation of Quinolines and Isoquinolines. Angew. Chem. Int. Ed. 2019, 58, 16878–16883.
- 7 Liu, L.; Deng, Z. K.; Xu, K.; Jiang, P. X.; Du, H. G.; Tan, J. J. Access to Deuterated Unnatural α-Amino Acids and Peptides by Photochemical Acyl Radical Addition. Org. Lett. 2021, 23, 5299–5304.
- 8(a) Liu, L.; Jiang, P. X.; Liu, Y. G.; Du, H. G.; Tan, J. J. Direct radical alkylation and acylation of 2H-indazoles using substituted Hantzsch esters as radical reservoirs. Org. Chem. Front. 2020, 7, 2278–2283; (b) Guo, Y. F.; Zhuang, Z.; Liu, Y. G.; Yang, X.; Tan, C.; Zhao, X. W.; Tan, J. J. Advances in C1-deuterated aldehyde synthesis. Coordin. Chem. Rev. 2022, 463, 214525–214542; (c) Yi, S. Y.; Wang, L. B.; Cheng, X. X.; Fujiki, M.; Zhang, W. Chiroptical Generation, Switching, and Long-Term Memory in Supramolecular Azobenzene-Pendant Polymer: Regulation by Cellulose Peralkyl Esters, D-/L-Glucose Permethyl Esters, Solvents, UV Light Irradiation, and Thermal Annealing Process. Chin. J. Chem. 2023, 41, 3625–3632.
- 9(a) Kanzian, T.; Mayr, H. Electrophilic Reactivities of Azodicarboxylates. Chem. Eur. J. 2010, 16, 11670–11677; (b) Ryu, I.; Tani, A.; Fukuyama, T.; Ravelli, D.; Montanaro, S.; Fagnoni, M. Efficient C—H/ C—N and C—H/C—CO—N Conversion via Decatungstate-Photoinduced Alkylation of Diisopropyl Azodicarboxylate. Org. Lett. 2013, 15, 2554–2557; (c) Buzzetti, L.; Crisenza, G. E. M.; Melchiorre, P. Mechanistic Studies in Photocatalysis. Angew. Chem. Int. Ed. 2019, 58, 3730–3747; (d) Zheng, H. F.; Fan, Y. J.; Blenko. A. L.; Lin, W. B. Sequential Modifications of Metal-Organic Layer Nodes for Highly Efficient Photocatalyzed Hydrogen Atom Transfer. J. Am. Chem. Soc. 2023, 145, 9994–10000; (e) Hu, A. H,; Guo, J.-J.; Pan, H.; Zuo, Z. W. Selective functionalization of methane, ethane, and higher alkanes by cerium photocatalysis. Science 2018, 361, 668–672.
- 10For selected reports, see: (a) Shamsabadi, A.; Chudasama, V. An overview of the synthesis of acyl hydrazides from aldehydes and reactions of the products thereof. Org. Biomol. Chem. 2017, 15, 17–33; (b) Lee, D.; Otte, R. D. Transition-Metal-Catalyzed Aldehydic C−H Activation by Azodicarboxylates. J. Org. Chem. 2004, 69, 3569–3571; (c) Inamdar, S. M.; More, V. K.; Mandal, S. K. CuO Nanoparticles Supported on Silica: A Simple, Efficient, and Recyclable Catalyst for Hydroacylation Reactions of Aldehydes with Azodicarboxylate. Chem. Lett. 2012, 41, 1484–1486; (d) Zhang, H. B.; Wang, Y.; Gu, Y.; Xu, P. F. Lewis- and Brønsted-acid cooperative catalytic radical coupling of aldehydes and azodicarboxylate. RSC Adv. 2014, 4, 27796–27799; (e) Pérez, J. M.; Ramón, D. J. Cobalt-Impregnated Magnetite as General Heterogeneous Catalyst for the Hydroacylation Reaction of Azodicarboxylates. Adv. Synth. Catal. 2014, 356, 3039–3047; (f) Ni, B. K.; Zhang, Q. Y.; Garre, S.; Headley, A. D. Ionic Liquid (IL) as an Effective Medium for the Highly Efficient Hydroacylation Reaction of Aldehydes with Azodicarboxylates. Adv. Synth. Catal. 2009, 351, 875–880; (g) Chudasama, V.; Akhbar, A. R.; Bahou, K. A.; Fitzmaurice, R. J.; Caddick, S. Metal-free, hydroacylation of C=C and N=N bonds via aerobic C–H activation of aldehydes, and reaction of the products thereof. Org. Biomol. Chem. 2013, 11, 7301–7317; (h) Spiliopoulou, N.; Constantinou, C. T.; Triandafillidi, I.; Kokotos, C. G. Synthetic Approaches to Acyl Hydrazides and Their Use as Synthons in Organic Synthesis. Synthesis 2020, 52, 3219–3230.
- 11(a) Wang, P.; Zhu, S. S.; Lu, D. F.; Gong, Y. F. Intermolecular Trifluoromethyl-Hydrazination of Alkenes Enabled by Organic Photoredox Catalysis. Org. Lett. 2020, 22, 1924–1928; (b) Sugimoto, O.; Arakaki, T.; Kamio, H.; Tanji, K. The use of a Mitsunobu reagent for the formation of heterocycles: a simple method for the preparation of 3-alkyl-5-aryl-1,3,4-oxadiazol-2(3H)-ones from carboxylic acids. Chem. Commun. 2014, 50, 7314–7317; (c) Yang, J. Y.; Song, M. H.; Zhou, H. Y.; Wang, G. G.; Ma, B.; Qi, Y. Y.; Huo, C. D. Visible-Light-Mediated Hydroacylation of Azobenzenes with α-Keto Acids. Org. Lett. 2020, 22, 8407–8412; (d) Zhou, Q.; Gong, X.; Yan, G. Recent Advances in Radical Reactions of Azo Compounds. Adv. Synth. Catal. 2023, 365, 1565–1579; (e) Singh, P.; Mritunjay. Progress of Dialkyl Azodicarboxylates in Organic Transformations. Asian J. Org. Chem. 2021, 10, 964–979.
- 12(a) Papadopoulos, G. N.; Limnios, D.; Kokotos, C. G. Photoorganocatalytic hydroacylation of dialkyl azodicarboxylates by utilising activated ketones as photocatalysts. Chem. Eur. J. 2014, 20, 13811–13814; (b) Koutoulogenis, G. S.; Kokotou, M. G.; Voutyritsa, E.; Limnios, D.; Kokotos, C. G. Visible-Light-Mediated Catalytic Hydroacylation of Dialkyl Azodicarboxylates by Graphite Flakes. Org. Lett. 2017, 19, 1760–1763; (c) Lei, J.; Sha, W.; Xie, X.; Weng, W. T. Copper-Catalyzed C(sp)–H Bond Hydrazidation. Org. Lett. 2023, 25, 320–324; (d) Spiliopoulou, N.; Gkizis, P. L.; Triandafillidi, I.; Nikitas, N. F.; Batsika, C. S.; Bisticha, A.; Kokotos, C. G. A Unified Mechanism for the PhCOCOOH- mediated Photochemical Reactions: Revisiting its Action and Comparison to Known Photoinitiators. Chem. Eur. J. 2022, 28, e202200023; (e) Xie, P.; Shi, S.; Hu, X.; Xue, C.; Du, D. Sunlight Photocatalytic Synthesis of Aryl Hydrazides by Decatungstate-Promoted Acylation under Room Temperature. ChemistrySelect 2021, 6, 3922–3925; (f) Stini, N. A.; Poursaitidis, E. T.; Nikitas, N. F.; Kartsinis, M.; Spiliopoulou, N.; Ananida-Dasenaki, P.; Kokotos, C. G. Light-accelerated “on-water” hydroacylation of dialkyl azodicarboxylates. Org. Biomol. Chem. 2023, 21, 1284–1293.
- 13(a) Yamashina, M.; Sei, Y.; Akita, M.; Yoshizawa, M. Safe storage of radical initiators within a polyaromatic nanocapsule. Nat. Commun. 2014, 5, 4662–4668; (b) Jia, M.; Guo, S.; Gao, S.; Wang, Q. S.; Sun, J. H. Thermal decomposition mechanism of diisopropyl azodicarboxylate and its thermal hazard assessment. Thermochim. Acta 2020, 688, 178601–178607; (c) Fantazie, R. M.; Herweh, J. E. 1,1'-Azobisformamide. I. Photochemical decomposition in solution. J. Am. Chem. Soc. 1974, 96, 1187–1192; (d) Fahr, E.; Lind, H. The Chemistry of α-Carbonyl Azo Compounds. Angew. Chem. Int. Ed. Engl. 1966, 5, 372–384; (e) Petrella, A.; Boghetich, G.; Petrella, M.; Mastrorilli, P.; Petruzzelli, V.; Petruzzelli, D. Photocatalytic Degradation of Azo Dyes. Pilot Plant Investigation. Ind. Eng. Chem. Res. 2014, 53, 2566–2571, and references therein.
- 14For details, please see the Electronic Supporting Information.
- 15(a) Palani, V.; Perea, M. A.; Sarpong, R. Site-Selective Cross-Coupling of Polyhalogenated Arenes and Heteroarenes with Identical Halogen Groups. Chem. Rev. 2022, 122, 10126–10169;
(b) Jin, C. C.; Xu, K.; Fan, X.; Liu C. Y.; Tan, J. J. Direct benzylic functionalization of pyridines: Palladium-catalyzed mono-α-arylation of α-(2-pyridinyl)acetates with heteroaryl halides. Chin. Chem. Lett. 2020, 31, 91–94;
(c) Patonay, T.; Konya, K. Synthesis and Modification of Heterocycles by Metal-Catalyzed Cross-coupling Reactions in Topics in Heterocyclic Chemistry, Springer, Switzerland, 2016.
10.1007/978-3-319-32610-8 Google Scholar
- 16(a) Elliott, Q.; Alabugin, I. V. AIBN as an Electrophilic Reagent for Cyano Group Transfer. J. Org. Chem. 2023, 88, 2648–2654; (b) Zhou, H.; Liu, Y. L.; Tang, S. Recent Advances on Radical-Mediated Cyanoalkylation/Cyanation Using AIBN and Analogues as the Radical Sources. Synlett 2023, 34, 106–123.
- 17 Ahmed, N.; Shamsabadi, A.; Chudasama, V. Formation of Synthetically Versatile 2-Aminobenzophenones from Readily Accessed Acyl Hydrazides. ACS Omega 2019, 4, 22601.
- 18(a) Pálvölgyi, Á. M.; Ehrschwendtner, F.; Schnürch, M.; Bica-Schröder, K. Photocatalyst-free hydroacylations of electron-poor alkenes and enones under visible-light irradiation. Org. Biomol. Chem. 2022, 20, 7245–7249; (b) Nakajima, K.; Zhang, Y.; Nishibayashi, Y. Alkylation Reactions of Azodicarboxylate Esters with 4-Alkyl-1,4-Dihydropyridines under Catalyst-Free Conditions. Org. Lett. 2019, 21, 4642–4645; (c) Wang, Q. L.; Huang, H. W.; Mao, G. J.; Deng, G. J. Bromine radical-enhanced HAT activity leading to stoichiometric couplings of methylarenes with acid chlorides. Green Chem. 2022, 24, 8324–8329; (d) Sun, Z. Z.; Huang, H. W.; Wang, Q. L.; Huang, C. Y.; Mao, G. J.; Deng, G. J. Visible light-mediated radical-cascade addition/cyclization of arylacrylamides with aldehydes to form quaternary oxindoles at room temperature. Org. Chem. Front. 2022, 9, 3506–3514.




