Cobalt-Catalyzed Switchable [4 + 1] and [4 + 1 + 1] Spirocyclization of Aromatic Amides with 2-Diazo-1H-indene-1,3(2H)-dione: Access to Spiro Indene-2,1'-isoindolinones and Spiro Isochroman-3,1'-isoindolinones
Corresponding Author
Bin Li
State Key Laboratory of Antiviral Drugs, Pingyuan Laboratory, Key Laboratory of Green Chemical Media and Reactions, Key Laboratory for Yellow River and Huai River Water Environmental Pollution Control, Ministry of Education, School of Environment, School of Chemistry and Chemical Engineering, Henan Normal University, Xinxiang, Henan, 453007 China
E-mail: [email protected]; [email protected]Search for more papers by this authorMengmeng Xie
State Key Laboratory of Antiviral Drugs, Pingyuan Laboratory, Key Laboratory of Green Chemical Media and Reactions, Key Laboratory for Yellow River and Huai River Water Environmental Pollution Control, Ministry of Education, School of Environment, School of Chemistry and Chemical Engineering, Henan Normal University, Xinxiang, Henan, 453007 China
Search for more papers by this authorJingyu Li
State Key Laboratory of Antiviral Drugs, Pingyuan Laboratory, Key Laboratory of Green Chemical Media and Reactions, Key Laboratory for Yellow River and Huai River Water Environmental Pollution Control, Ministry of Education, School of Environment, School of Chemistry and Chemical Engineering, Henan Normal University, Xinxiang, Henan, 453007 China
Search for more papers by this authorNana Shen
State Key Laboratory of Antiviral Drugs, Pingyuan Laboratory, Key Laboratory of Green Chemical Media and Reactions, Key Laboratory for Yellow River and Huai River Water Environmental Pollution Control, Ministry of Education, School of Environment, School of Chemistry and Chemical Engineering, Henan Normal University, Xinxiang, Henan, 453007 China
Search for more papers by this authorXinying Zhang
State Key Laboratory of Antiviral Drugs, Pingyuan Laboratory, Key Laboratory of Green Chemical Media and Reactions, Key Laboratory for Yellow River and Huai River Water Environmental Pollution Control, Ministry of Education, School of Environment, School of Chemistry and Chemical Engineering, Henan Normal University, Xinxiang, Henan, 453007 China
Search for more papers by this authorCorresponding Author
Xuesen Fan
State Key Laboratory of Antiviral Drugs, Pingyuan Laboratory, Key Laboratory of Green Chemical Media and Reactions, Key Laboratory for Yellow River and Huai River Water Environmental Pollution Control, Ministry of Education, School of Environment, School of Chemistry and Chemical Engineering, Henan Normal University, Xinxiang, Henan, 453007 China
E-mail: [email protected]; [email protected]Search for more papers by this authorCorresponding Author
Bin Li
State Key Laboratory of Antiviral Drugs, Pingyuan Laboratory, Key Laboratory of Green Chemical Media and Reactions, Key Laboratory for Yellow River and Huai River Water Environmental Pollution Control, Ministry of Education, School of Environment, School of Chemistry and Chemical Engineering, Henan Normal University, Xinxiang, Henan, 453007 China
E-mail: [email protected]; [email protected]Search for more papers by this authorMengmeng Xie
State Key Laboratory of Antiviral Drugs, Pingyuan Laboratory, Key Laboratory of Green Chemical Media and Reactions, Key Laboratory for Yellow River and Huai River Water Environmental Pollution Control, Ministry of Education, School of Environment, School of Chemistry and Chemical Engineering, Henan Normal University, Xinxiang, Henan, 453007 China
Search for more papers by this authorJingyu Li
State Key Laboratory of Antiviral Drugs, Pingyuan Laboratory, Key Laboratory of Green Chemical Media and Reactions, Key Laboratory for Yellow River and Huai River Water Environmental Pollution Control, Ministry of Education, School of Environment, School of Chemistry and Chemical Engineering, Henan Normal University, Xinxiang, Henan, 453007 China
Search for more papers by this authorNana Shen
State Key Laboratory of Antiviral Drugs, Pingyuan Laboratory, Key Laboratory of Green Chemical Media and Reactions, Key Laboratory for Yellow River and Huai River Water Environmental Pollution Control, Ministry of Education, School of Environment, School of Chemistry and Chemical Engineering, Henan Normal University, Xinxiang, Henan, 453007 China
Search for more papers by this authorXinying Zhang
State Key Laboratory of Antiviral Drugs, Pingyuan Laboratory, Key Laboratory of Green Chemical Media and Reactions, Key Laboratory for Yellow River and Huai River Water Environmental Pollution Control, Ministry of Education, School of Environment, School of Chemistry and Chemical Engineering, Henan Normal University, Xinxiang, Henan, 453007 China
Search for more papers by this authorCorresponding Author
Xuesen Fan
State Key Laboratory of Antiviral Drugs, Pingyuan Laboratory, Key Laboratory of Green Chemical Media and Reactions, Key Laboratory for Yellow River and Huai River Water Environmental Pollution Control, Ministry of Education, School of Environment, School of Chemistry and Chemical Engineering, Henan Normal University, Xinxiang, Henan, 453007 China
E-mail: [email protected]; [email protected]Search for more papers by this authorComprehensive Summary
Herein, we report a condition-controlled divergent synthesis of spiro indene-2,1'-isoindolinones and spiro isochroman-3,1'-isoindolinones through cobalt-catalyzed formal [4 + 1] and [4 + 1 + 1] spirocyclization of aromatic amides with 2-diazo-1H-indene-1,3(2H)- dione. When the reaction is carried out under air in ethyl acetate, spiro indene-2,1'-isoindolinones are formed through Co(II)-catalyzed C—H/N—H [4 + 1] spirocyclization. When the reaction is run under O2 in CH3CN, on the other hand, spiro isochroman-3,1'-isoindolinones are generated through Baeyer-Villiger oxidation of the in situ formed spiro indene-2,1'-isoindolinones with O2 as a cheaper and environmental-friendly oxygen source. In general, these protocols have advantages such as using non-precious and earth-abundant metal catalyst, no extra additive, high efficiency and regioselectivity. A gram-scale synthesis and the removal of the directing group further highlight its utility.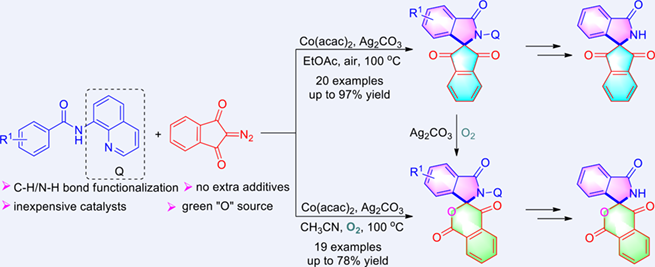
Supporting Information
| Filename | Description |
|---|---|
| cjoc202300509-sup-0001-Supinfo.pdfPDF document, 3.3 MB |
Appendix S1: Supporting Information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1(a) Zheng, Y.; Tice, C. M.; Singh, S. B. The Use of Spirocyclic Scaffolds in Drug Discovery, Bioorg. Med. Chem. Lett. 2014, 24, 3673–3682;
(b) Smith, L. K.; Baxendale, I. R. Total Syntheses of Natural Products Containing Spirocarbocycles, Org. Biomol. Chem. 2015, 13, 9907–9933;
(c) Zheng, Y. J.; Tice, C. M. The Utilization of Spirocyclic Scaffolds in Novel Drug Discovery. Expert. Opin. Drug Dis. 2016, 11, 831–834;
(d) Hiesinger, K.; Dar’in, D.; Proschak, E.; Krasavin, M. Spirocyclic Scaffolds in Medicinal Chemistry. J. Med. Chem. 2021, 64, 150–183;
(e) Yan, C. G. 1,3-Indanedione: An Versatile Building Block. Green Synth. Catal. 2023, 4, 78–88.
10.1016/j.gresc.2022.12.004 Google Scholar
- 2(a) Sharma, S.; Oh, Y.; Mishra, N. K.; De, U.; Jo, H.; Sachan, R.; Kim, H. S.; Jung, Y. H.; Kim, I. S. Rhodium-Catalyzed [3 + 2] Annulation of Cyclic N-Acyl Ketimines with Activated Olefins: Anticancer Activity of Spiroisoindolinones. J. Org. Chem. 2017, 82, 3359–3367; (b) Wrobel, J.; Dietrich, A.; Woolson, S. A.; Millen, J.; McCaleb, M.; Harrison, M. C.; Hohman, T. C.; Sredy, J.; Sullivan, D. Novel SpiroSuccinimides with Incorporated Isoindolone and Benzisothiazole 1,1-Dioxide Moieties as Aldose Reductase Inhibitors and Antihyperglycemic Agents. J. Med. Chem. 1992, 35, 4613–4627; (c) Chia, Y. C.; Chang, F. R.; Wu, C. C.; Teng, C. M.; Chen, K. S.; Wu, Y. C. Effect of Isoquinoline Alkaloids of Different Structural Types on Antiplatelet Aggregation in vitro. Planta Med. 2006, 72, 1238–1241; (d) Wang, J.; Chen, F.; Liu, Y.; Liu, Y.; Li, K.; Yang, X.; Liu, S.; Zhou, X.; Yang, J. Spirostaphylotrichin X From a Marine-Derived Fungus as an Anti-influenza Agent Targeting RNA Polymerase PB2. J. Nat. Prod. 2018, 81, 2722–2730.
- 3(a) Ding, A.; Meazza, M.; Guo, H.; Yang, J. W.; Rios, R. New Development in the Enantioselective Synthesis of Spiro Compounds. Chem. Soc. Rev. 2018, 47, 5946–5996; (b) Alves, A. J. S.; Alves, N. G.; Soares, M. I. L.; Melo, T. M. V. D. P. Strategies and Methodologies for the Construction of Spiro-γ-lactams: An Update. Org. Chem. Front. 2021, 8, 3543–3593.
- 4(a) He, J.; Wasa, M.; Chan, K. S. L.; Shao, Q.; Yu, J. Q. Palladium-Catalyzed Transformations of Alkyl C−H Bonds. Chem. Rev. 2017, 117, 8754–8786; (b) Rej, S.; Chatani, N. Rhodium-Catalyzed C(sp2)− or C(sp3)−H Bond Functionalization Assisted by Removable Directing Groups. Angew. Chem. Int. Ed. 2019, 58, 8304–8329; (c) Wu, Y.; Pi, C.; Wu, Y.; Cui, X. Directing Group Migration Strategy in Transition-Metal-Catalysed Direct C−H Functionalization. Chem. Soc. Rev. 2021, 50, 3677–3689; (d) Li, C. J. On Inventing Cross-Dehydrogenative Coupling (CDC): Forming C−C Bond from Two Different C−H Bonds. Chin. J. Chem. 2022, 40, 838–845; (e) Lu, L. J.; Li, H.; Lei, A. W. Oxidative Cross-Coupling Reactions between Two Nucleophiles. Chin. J. Chem. 2022, 40, 256–266.
- 5(a) Su, B.; Cao, Z. C.; Shi, Z. J. Exploration of Earth-Abundant Transition Metals (Fe, Co, and Ni) as Catalysts in Unreactive Chemical Bond Activations. Acc. Chem. Res. 2015, 48, 886–896; (b) Zweig, J. E.; Kim, D. E.; Newhouse, T. R. Methods Utilizing First-Row Transition Metals in Natural Product Total Synthesis. Chem. Rev. 2017, 117, 11680–11752; (c) Li, Y.; Hu, Y.; Wu, X. F. Non-Noble Metal-Catalysed Carbonylative Transformations. Chem. Soc. Rev. 2018, 47, 172–194; (d) Gandeepan, P.; Müller, T.; Zell, D.; Cera, G.; Warratz, S.; Ackermann, L. 3d Transition Metals for C−H Activation. Chem. Rev. 2019, 119, 2192–2452.
- 6(a) Moselage, M.; Li, J.; Ackermann, L. Cobalt-Catalyzed C−H Activation. ACS Catal. 2016, 6, 498–525; (b) Kommagalla, Y.; Chatani, N. Cobalt(II)-Catalyzed CAH Functionalization Using an N,N’-Bidentate Directing Group. Coord. Chem. Rev. 2017, 350, 117–135; (c) Cizikovs, A.; Lukasevics, L.; Grigorjeva, L. Cobalt-Catalyzed C−H Bond Functionalization Using Traceless Directing Group. Tetrahedron 2021, 93, 132307; (d) Banjare, S. K.; Nanda, T.; Pati, B. V.; Biswal, P.; Ravikumar, P. C. O-Directed C−H Functionalization via Cobaltacycles: A Sustainable Approach for C−C and C-Heteroatom Bond Formations. Chem. Commun. 2021, 57, 3630–3647; (e) Wang, C. Y.; Dong, S. D.; Zhu, T. Y.; Liu, Y. Q.; Wu, Z. H.; Feng, R. K. Cobalt-Catalyzed Decarbonylative C(8)-Acyloxylation of 1-Naphthalamine Derivatives with α-Oxocarboxylic Acids. Chin. J. Org. Chem. 2022, 42, 1799–1810; (f) Yan, R.; Yu, H.; Wang, Z. X. Cobalt-Catalyzed C−H Allylation of Arenes with Allylic Amines. Chin. J. Chem. 2021, 39, 1205–1210; (g) Zhang, J. Y.; Liu, X. Y. Single-Step Synthesis of Atropisomers with Vicinal C−C and C−N Diaxes via Cobalt-Catalyzed C−H Activation. Chin. J. Org. Chem. 2022, 42, 3899–3890; (h) Yang, D. D.; Niu, J. L. Electrooxidative Cobalt-Catalyzed Regio- and Enantioselective Annulation of Benzamides with Alkenes. Chin. J. Org. Chem. 2023, 43, 1887–1889.
- 7(a) Xiang, Y.; Wang, C.; Ding, Q.; Peng, Y. Diazo Compounds: Versatile Synthons for the Synthesis of Nitrogen Heterocycles via Transition Metal-Catalyzed Cascade C−H Activation/Carbene Insertion/Annulation Reactions. Adv. Synth. Catal. 2019, 361, 919–944; (b) Nunewar, S.; Kumar, S.; Talakola, S.; Nanduri, S.; Kanchupalli, V. Co(III), Rh(III) & Ir(III)-Catalyzed Direct C−H Alkylation/Alkenylation/Arylation with Carbene Precursors. Chem. Asian J. 2021, 16, 443–459; (c) Solovyev, I.; Eremeyeva, M.; Zhukovsky, D.; Dar’in, D.; Krasavin, M. Cyclic Diazo Compounds in the Construction of Spirocyclic Scaffolds. Tetrahedron Lett. 2021, 62, 152671; (d) Kumar, S.; Nunewar, S.; Oluguttula, S.; Nanduri, S.; Kanchupalli, V. Recent Advances in Rh(III)/Ir(III)-Catalyzed C−H Functionalization/Annulation via Carbene Migratory Insertion. Org. Biomol. Chem. 2021, 19, 1438–1458; (e) Tian, M. M.; Yang, L. Y.; Liu, B. X.; Chang, J. B. Synthesis of 9,10-Phenanthrenes via Rh(III)-Catalyzed [4 + 2] Annulation of 2-Biphenylboronic Acids with Diazo Compounds. Chin. J. Chem. 2023, 41, 1327–1332.
- 8(a) Berger, M.; Chauhan, R.; Rodrigues, C. A. B.; Maulide, N. Bridging C−H Activation: Mild and Versatile Cleavage of the 8-Aminoquinoline Directing Group. Chem. - Eur. J. 2016, 22, 16805–16808; (b) Deguchi, T.; Xin, H. L.; Morimoto, H.; Ohshima, T. Direct Catalytic Alcoholysis of Unactivated 8-Aminoquinoline Amides. ACS Catal. 2017, 7, 3157–3161; (c) Fitzgerald, L. S.; O’Duill, M. L. A Guide to Directing Group Removal: 8-Aminoquinoline. Chem. - Eur. J. 2021, 27, 8411–8436; (d) Liu, B.; Romine, A. M.; Rubel, C. Z.; Engle, K. M.; Shi, B. F. Transition- Metal-Catalyzed, Coordination-Assisted Functionalization of Nonactivated C(sp3)−H Bonds. Chem. Rev. 2021, 121, 14957–15074.
- 9 Xu, M. R.; Yuan, Y.; Wang, Y.; Tao, Q. H.; Wang, C. Y.; Li, Y. Z. Controllable α- or β-Functionalization of α-Diazoketones with Aromatic Amides via Cobalt-Catalyzed C−H Activation: A Regioselective Approach to Isoindolinones. Org. Lett. 2019, 21, 6264–6269.
- 10 Li, M. H.; Si, X. J.; Zhang, H.; Yang, D. D.; Niu, J. L.; Song, M. P. Directed Cobalt-Catalyzed C−H Activation to Form C−C and C−O Bonds in One Pot via Three-Component Coupling. Org. Lett. 2021, 23, 914–919.
- 11(a) Guo, C.; Li, B.; Liu, H.; Zhang, X.; Fan, X. Synthesis of Fused or Spiro Polyheterocyclic Compounds via the Dehydrogenative Annulation Reactions of 2-Arylindazoles with Maleimides. Org. Lett. 2019, 21, 7189–7193; (b) Li, B.; Guo, C. H.; Shen, N. N.; Zhang, X. Y.; Fan, X. S. Synthesis of Maleimide Fused Benzocarbazoles and Imidazo[1,2- a]pyridines via Rhodium(III)-Catalyzed [4 + 2] Oxidative Cycloaddition. Org. Chem. Front. 2020, 7, 3698–3704; (c) Li, H.; Shen, M. Y.; Li, B.; Zhang, X. Y.; Fan, X. S. Solvent-Dependent Selective Synthesis of CF3-Tethered Indazole Derivatives Based on Multiple Bond Activations. Org. Lett. 2023, 25, 720–725; (d) Song, X.; Wang, K. L.; Zhang, X. Y.; Fan, X. S. Unsymmetrical Relay C−H Alkenylation and [2 + 2] Cycloaddition of N-Arylsydnones with Allenyl Acetates Leading to Quinoline-Fused Cyclobutanes. Org. Chem. Front. 2023, 10, 1191–1197.
- 12(a) Li, B.; Zhang, B. B.; Zhang, X. Y.; Fan, X. S. Regio-selective Synthesis of Diversely Substituted Benzo[a]carbazoles through Rh(III)-Catalyzed Annulation of 2-Arylindoles with α-Diazo Carbonyl Compounds. Chem. Commun. 2017, 53, 1297–1300; (b) Li, B.; Shen, N. N.; Zhang, X. Y.; Fan, X. S. Synthesis of Fused Imidazo[1,2-a]pyridines Derivatives through Cascade C(sp2)−H Functionalizations. Org. Biomol. Chem. 2019, 17, 9140–9150; (c) Li, B.; Shen, N. N.; Yang, Y. J.; Zhang, X. Y.; Fan, X. S. Synthesis of Naphtho[1’,2’:4,5]imidazo-[1,2-a]pyridines via Rh(III)-Catalyzed C−H Functionalization of 2-Arylimidazo[1,2-a]pyridines with Cyclic 2-Diazo-1,3-diketones Featuring with a Ring Opening and Reannulation. Org. Chem. Front. 2020, 7, 919–925; (d) Li, B.; Shen, N. N.; Yang, Y. J.; Zhang, X. Y.; Fan, X. S. Tunable Synthesis of Indeno[1,2-c]furans and 3-Benzoylindenones via FeCl3-Catalyzed Carbene/Alkyne Metathesis Reaction of o-Alkynylbenzoyl Diazoacetates. Org. Lett. 2021, 23, 388–393; (e) Li, B.; Shen, N. N.; Wang, K. L.; Fan, X. S.; Zhang, X. Y. Rh(III)-Catalyzed Reaction of 2-Aryl-3-acyl-1H-indoles with α-Diazo Carbonyl Compounds: Synthesis of 5-Carbonyl Substituted Benzo[a]carbazoles via [5 + 1] Annulation. Asian J. Org. Chem. 2022, 11, e202100710.
- 13(a) Lian, X. L.; Lei, H.; Quan, X. J.; Ren, Z. H.; Wang, Y. Y.; Guan, Z. H. Oxidation of 2-Arylindoles for Synthesis of 2-Arylbenzoxazinones with Oxone as the Sole Oxidant. Chem. Commun. 2013, 49, 8196–8198; (b) Yamashita, M.; Iida, A. Copper-Mediated Oxidative Tandem Reactions with Molecular Oxygen: Synthesis of 2-Arylbenzoxazinone Derivatives from Indoles. Tetrahedron Lett. 2014, 55, 2991–2993; (c) Feng, Y. D.; Li, Y. D.; Cheng, G. L.; Wang, L. H.; Cui, X. L. Copper-Catalyzed Synthesis of 2-Arylquinazolinones from 2-Arylindoles with Amines or Ammoniums. J. Org. Chem. 2015, 80, 7099–7107.
- 14 Yuan, W. K.; Shi, B. F. Synthesis of Chiral Spirolactams via Sequential C−H Olefination/Asymmetric [4 + 1] Spirocyclization under a Simple CoII/Chiral Spiro Phosphoric Acid Binary System. Angew. Chem. Int. Ed. 2021, 60, 23187–23192.




