Recent Advances in the Synthesis and Polymerization of New CO2-Based Cyclic†
Matteo Lanzi
Institute of Chemical Research of Catalonia (ICIQ), The Barcelona Institute of Science and Technology (BIST), Av. Països Catalans 16, 43007 Tarragona, Spain
Search for more papers by this authorCorresponding Author
Arjan W. Kleij
Institute of Chemical Research of Catalonia (ICIQ), The Barcelona Institute of Science and Technology (BIST), Av. Països Catalans 16, 43007 Tarragona, Spain
Catalan Institute of Research and Advanced Studies (ICREA), Pg. Lluís Companys 23, 08010 Barcelona, Spain
*E-mail: [email protected]Search for more papers by this authorMatteo Lanzi
Institute of Chemical Research of Catalonia (ICIQ), The Barcelona Institute of Science and Technology (BIST), Av. Països Catalans 16, 43007 Tarragona, Spain
Search for more papers by this authorCorresponding Author
Arjan W. Kleij
Institute of Chemical Research of Catalonia (ICIQ), The Barcelona Institute of Science and Technology (BIST), Av. Països Catalans 16, 43007 Tarragona, Spain
Catalan Institute of Research and Advanced Studies (ICREA), Pg. Lluís Companys 23, 08010 Barcelona, Spain
*E-mail: [email protected]Search for more papers by this authorDedicated to the Special Issue of C1 Chemistry.
Comprehensive Summary
Carbon dioxide can be converted into functional heterocycles known as cyclic carbonates, whose recent reactivity has been expanded towards the formation of tailor-made engineering polymers. This minireview gives an overview of the most topical developments in this area with a special focus on the synthetic methods employed to prepare these CO2 based synthons. In addition, their application potential in the area of polymer science using a variety of polymerization techniques is discussed that have in common the ring-opening of the carbonate monomers. Future perspectives are provided that provide impetus for the scientific communities aligning research to the use of sustainable processes for polymers from recyclable carbon sources such as CO2.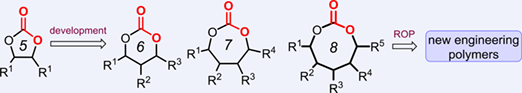
References
- 1 Abdel Baki, Z.; Dib, H.; Sahin, T. Overview: Polycarbonates via Ring-Opening Polymerization, Differences between Six- and Five-Membered Cyclic Carbonates: Inspiration for Green Alternatives. Polymers 2022, 14, 2031.
- 2 Yu, W.; Maynard, E.; Chiaradia, V.; Arno, M. C.; Dove, A. P. Aliphatic Polycarbonates from Cyclic Carbonate Monomers and Their Application as Biomaterials. Chem. Rev. 2021, 121, 10865–10907.
- 3 Pascual, A.; Tan, J. P. K.; Yuen, A.; Chan, J. M. W.; Coady, D. J.; Mecerreyes, D.; Hedrick, J. L.; Yang, Y. Y.; Sardon, H. Broad-Spectrum Antimicrobial Polycarbonate Hydrogels with Fast Degradability. Biomacromolecules 2015, 16, 1169–1178.
- 4 Arno, M. C.; Brannigan, R. P.; Policastro, G. M.; Becker, M. L.; Dove, A. P. pH-Responsive, Functionalizable Spyrocyclic Polycarbonate: A Versatile Platform for Biocompatible Nanoparticles. Biomacromolecules 2018, 19, 3427–3434.
- 5 Czysch, C.; Medina-Montano, C.; Zhong, Z.; Fuchs, A.; Stickdorn, J.; Winterwerber, P.; Schmitt, S.; Deswarte, K.; Raabe, M.; Scherger, M.; Combes, F.; de Vrieze, J.; Kasmi, S.; Sandners, N. N.; Lienenklaus, S.; Koynov, K.; Räder, H.; Lambrecht, B. N.; David, S. A.; Bros, M.; Schild, H.; Grabbe, S.; de Geest, B. G.; Nuhn, L. Transient Lymph Node Immune Activation by Hydrolysable Polycarbonate Nanogels. Adv. Funct. Mater. 2022, 32, e2203490.
- 6 Xu, J.; Feng, E.; Song, J. Renaissance of aliphatic polycarbonates: New techniques and biomedical applications. J. Appl. Polym. Sci. 2014, 131, 39822.
- 7 Liu, Q.; Wu, L.; Jackstell, R.; Beller, M. Using carbon dioxide as a building block in organic synthesis. Nat. Commun. 2015, 6, 5933.
- 8 Dabral, S.; Schaub, T. The Use of Carbon Dioxide (CO2) as a Building Block in Organic Synthesis from an Industrial Perspective. Adv. Synth. Catal. 2019, 361, 223–246.
- 9 Paul, S.; Zhu, Y.; Romain, C.; Brooks, R.; Saini, P. K.; Williams, C. K. Ring-opening copolymerization (ROCOP): synthesis and properties of polyesters and polycarbonates, Chem. Commun. 2015, 51, 6459–6479.
- 10 Bhat, G.; Darensbourg, D. J. Progress in the catalytic reactions of CO2 and epoxides to selectively provide cyclic or polymeric carbonates. Green Chem. 2022, 24, 5007–5034.
- 11 Mikami, K.; Lonnecker, A. T.; Gustafson, T. P.; Zinnel, N. F.; Pai, P.-J.; Russell, D. H.; Wooley, K. L. Polycarbonates Derived from Glucose via an Organocatalytic Approach. J. Am. Chem. Soc. 2013, 135, 6826–6829.
- 12 Nobuoka, H.; Ajiro, H. Novel synthesis method of ester free trimethylene carbonate derivatives. Tetrahedron Lett. 2019, 60, 164–170.
- 13 Tan, E. W. P.; Hedrick, J. L.; Arrechea, P. L.; Erdmann, T.; Kiyek, V.; Lottier, S.; Yang, Y. Y.; Park, N. H. Overcoming Barriers in Polycarbonate Synthesis: A Streamlined Approach for the Synthesis of Cyclic Carbonate Monomers. Macromolecules 2021, 54, 1767–1774.
- 14 Kindermann, N.; Jose, T.; Kleij, A. W. Synthesis of Carbonates from Alcohols and CO2. Top. Curr. Chem. 2017, 375, 1–15.
- 15 Reithofer, M. R.; Sum, Y. N.; Zhang, Y. Synthesis of cyclic carbonates with carbon dioxide and cesium carbonate. Green Chem. 2013, 15, 2086–2090.
- 16 Tamura, M.; Honda, M.; Nakagawa, Y.; Tomishige, K. Direct conversion of CO2 with diols, aminoalcohols and diamines to cyclic carbonates, cyclic carbamates and cyclic ureas using heterogeneous catalysts. J. Chem. Technol. Biotechnol. 2014, 89, 19–33.
- 17 Gregory, G. L.; Ulmann, M.; Buchard, A. Synthesis of 6-membered cyclic carbonates from 1,3-diols and low CO2 pressure: a novel mild strategy to replace phosgene reagents. RSC Adv. 2015, 5, 39404–39408.
- 18 Huang, J.; Jehanno, C.; Worch, J. C.; Ruipérez, F.; Sardon, H.; Dove, A. P.; Coulembier, O. Selective Organocatalytic Preparation of Trimethylene Carbonate from Oxetane and Carbon Dioxide. ACS Catal. 2020, 10, 5399–5404.
- 19 Alves, M.; Grignard, B.; Boyaval, A.; Méreau, R.; De Winter, J.; Gerbaux, P.; Detrembleur, C.; Tassaing, T.; Jérôme, C. Organocatalytic Coupling of CO2 with Oxetane. ChemSusChem 2017, 10, 1128–1138.
- 20 Grignard, B.; Gennen, S.; Jérôme, C.; Kleij, A: W.; Detrembleur, C. Advances in the use of CO2 as a renewable feedstock for the synthesis of polymers. Chem. Soc. Rev. 2019, 48, 4466–4514.
- 21 Whiteoak, C. J.; Martin, E.; Belmonte, M. M.; Benet-Buchholz, J.; Kleij, A. W. An Efficient Iron Catalyst for the Synthesis of Five- and Six-Membered Organic Carbonates under Mild Conditions. Adv. Synth. Catal. 2012, 354, 469–476.
- 22 McGuire, T. M.; López-Vidal, E. M.; Gregory, G. L.; Buchard, A. Synthesis of 5- to 8-membered cyclic carbonates from diols and CO2: A one-step, atmospheric pressure and ambient temperature procedure. J. CO2 Utiliz. 2018, 27, 283–288.
- 23 Rintjema, J.; Guo, W.; Martin, E.; Escudero-Adán, E. C.; Kleij, A. W. Highly Chemoselective Catalytic Coupling of Substituted Oxetanes and Carbon Dioxide. Chem. Eur. J. 2015, 21, 10754–10762.
- 24 Qiao, C.; Villar-Yanez, A.; Sprachmann, J.; Limburg, B.; Bo, C.; Kleij, A. W. Organocatalytic Trapping of Elusive Carbon Dioxide based Heterocycles through a Kinetically Controlled Cascade Process. Angew. Chem. Int. Ed. 2020, 59, 18446–18451.
- 25 Guerin, W.; Khadri Diallo, A.; Kirilov, E.; Helou, M.; Slawinski, M.; Brusson, J.-M.; Carpentier, J.-F.; Guillaume, S. F. Enantiopure Isotactic PCHC Synthesized by Ring-Opening Polymerization of Cyclohexene Carbonate. Macromolecules 2014, 47, 13, 4230–4235.
- 26 Kawamoto, M.; Mori, Y.; Tsuge, A.; Endo, T. Anionic ring-opening polymerization behavior of trans-cyclohexene carbonate using metal tert-butoxides: Construction of living anionic ring-opening polymerization by lithium tert-butoxide. J. Polym. Sci. 2022, 60, 1416–1421.
- 27 Ariga, T.; Takata, T.; Endo, T. Cationic Ring-Opening Polymerization of Cyclic Carbonates with Alkyl Halides To Yield Polycarbonate without the Ether Unit by Suppression of Elimination of Carbon Dioxide. Macromolecules 1997, 30, 737–744.
- 28 Endo, T.; Shibasaki, Y.; Sanda, F. Controlled ring-opening polymerization of cyclic carbonates and lactones by an activated monomer mechanism. J. Polym. Sci. A, Polym. Chem. 2002, 40, 2190–2198.
- 29 Tezuka, K.; Komatsu, K.; Haba, O. The anionic ring-opening polymerization of five-membered cyclic carbonates fused to the cyclohexane ring. Polym. J. 2013, 45, 1183–1187.
- 30 Lonnecker, A. T.; Lim, Y. H.; Wooley, K. L. Functional Polycarbonate of a D-Glucal-Derived Bicyclic Carbonate via Organocatalytic Ring- Opening Polymerization. ACS Macro Lett. 2017, 6, 748–753.
- 31 Ramesh, M. S.; Rajaram, S. Organocatalyzed regio-regular polymerization of α-aryl trimethylene carbonate. Polymer 2021, 227, 123803.
- 32 Schandl, S.; Koch, T.; Stampfl, J.; Ehrmann, K.; Liska, R. Pure aliphatic polycarbonate networks via photoinduced anionic ring-opening polymerization at elevated temperature. Reactive Funct. Polym. 2023, 182, 105460.
- 33 Song, Y.; Yang, X.; Shen, Y.; Dong, M.; Lin, Y.-N.; Hall, M. B.; Wooley, K. L. Invoking Side-Chain Functionality for the Mediation of Regioselectivity during Ring-Opening Polymerization of Glucose Carbonates. J. Am. Chem. Soc. 2020, 142, 16974–16981.
- 34 Shen, Y.; Yang, X.; Song, Y.; Tran, D. K.; Wang, H.; Wilson, J.; Dong, M.; Vazquez, M.; Sun, G.; Wooley, K. L. Complexities of Regioselective Ring-Opening vs Transcarbonylation-Driven Structural Metamorphosis during Organocatalytic Polymerizations of Five-Membered Cyclic Carbonate Glucose Monomers. JACS Au 2022, 2, 515–521.
- 35 Nederberg, F.; Lohmeijer, B. G. G.; Leibfarth, F.; Pratt, R. C.; Choi, J.; Dove, A. P.; Waymouth, R. M.; Hedrick, J. L. Organocatalytic Ring Opening Polymerization of Trimethylene Carbonate. Biomacromolecules 2007, 8, 153–160.
- 36 Kamber, N. E.; Jeong, W.; Waymouth, R. M.; Pratt, R. C.; Lohmeijer, B. G. G.; Hedrick, J. L. Organocatalytic Ring-Opening Polymerization. Chem. Rev. 2007, 107, 5813–5840.
- 37 Brege, A.; Méreau, R.; McGehee, K.; Grignard, B.; Detrembleur, C.; Jerome, C.; Tassaing, T. The Coupling of CO2 with Diols Promoted by Organic Dual Systems: Towards Products Divergence via Benchmarking of the Performance Metrics. J. CO2 Utiliz. 2020, 38, 88–98.
- 38 Hedrick, J. L.; Piunova, V.; Park, N. H.; Erdmann, T.; Arrechea, P. L. Simple and Efficient Synthesis of Functionalized Cyclic Carbonate Monomers Using Carbon Dioxide. ACS Macro Lett. 2022, 11, 368–375.
- 39 Palenzuela, M.; Sarisuta, K.; Navarro, M.; Kumamoto, N.; Chanthaset, N.; Monot, J.; Ajiro, H.; Martín-Vaca, B.; Bourissou, D. 5-Methylene-1,3-dioxane-2-one: A First-Choice Comonomer for Trimethylene Carbonate. Macromolecules 2023, 56, 678–689.
- 40 Whiteoak, C. J.; Kielland, N.; Laserna, V.; Escudero-Adán, E. C.; Martin, E.; Kleij, A. W. A Powerful Aluminum Catalyst for the Synthesis of Highly Functional Organic Carbonates. J. Am. Chem. Soc. 2013, 135, 1228–1231.
- 41 Laserna, V.; Fiorani, G.; Whiteoak, C. J.; Martin, E.; Escudero-Adán, E. C.; Kleij, A. W. Carbon Dioxide as a Protecting Group: Highly Efficient and Selective Catalytic Access to Cyclic cis-Diol Scaffolds. Angew. Chem. Int. Ed. 2014, 53, 10416–10419.
- 42 Qiao, C.; Shi, W.; Brandolese, A.; Benet-Buchholz, J.; Escudero-Adán, E. C.; Kleij, A. W. A Novel Catalytic Route to Polymerizable Bicyclic Cyclic Carbonate Monomers from Carbon Dioxide. Angew. Chem. Int. Ed. 2022, 61, e202205053.
- 43 Zhu, K. J.; Hendren, R. W.; Jensen, K.; Pitt, C. G. Synthesis, properties, and biodegradation of poly(1,3-trimethylene carbonate). Macromolecules 1991, 24, 1736–1740.
- 44 Ni, J.; Lanzi, M.; Lamparelli, D. H.; Kleij, A. W. Ring-Opening Polymerization of Functionalized Aliphatic Bicyclic Carbonates. Polym. Chem. 2023, DOI: https://doi.org/10.1039/d3py00860f.
- 45 Maisonneuve, L.; Lamarzelle, O.; Rix, E.; Grau, E.; Cramail, H. Isocyanate-Free Routes to Polyurethanes and Poly(hydroxy Urethane)s. Chem. Rev. 2015, 115, 12407–12439.
- 46 Gomez-Lopez, A.; Panchireddy, S.; Grignard, B.; Calvo, I.; Jérôme, C.; Detrembleur, C.; Sardon, H. Poly(hydroxyurethane) Adhesives and Coatings: State-of-the-Art and Future Directions. ACS Sustainable Chem. Eng. 2021, 9, 9541–9562.
- 47 Miao, P.; Jiao, Z.; Liu, J.; He, M.; Song, G.; Wei, Z.; Leng, X.; Li, Y. Mechanically Robust and Chemically Recyclable Polyhydroxyurethanes from CO2-Derived Six-Membered Cyclic Carbonates. ACS Appl. Mater. Interfaces 2023, 15, 2246–2255.
- 48 Gennen, S.; Grignard, B.; Tassaing, T.; Jérôme, C.; Detrembleur, C. CO2-Sourced a-Alkylidene Cyclic Carbonates: A Step Forward in the Quest for Functional Regioregular Poly(urethane)s and Poly(carbonate)s. Angew. Chem. Int. Ed. 2017, 56, 10394–10398.
- 49
Ngassam Tounzoua, C.; Grignard, B.; Detrembleur, C. Exovinylene Cyclic Carbonates: Multifaceted CO2-Based Building Blocks for Modern Chemistry and Polymer Science. Angew. Chem. Int. Ed. 2022, 134, e202116066.
10.1002/ange.202116066 Google Scholar
- 50 Habets, T.; Siragusa, F.; Grignard, B.; Detrembleur, C. Advancing the Synthesis of Isocyanate-Free Poly(Oxazolidones)s: Scope and Limitations. Macromolecules 2020, 53, 6396–6408.
- 51 Dabral, S.; Licht, U.; Rudolf, P.; Bollmann, G.; Hashmi, A. S. K.; Schaub, T. Synthesis and Polymerisation of α-Alkylidene Cyclic Carbonates from Carbon Dioxide, Epoxides and the Primary Propargylic Alcohol 1,4-Butynediol. Green Chem. 2020, 22, 1553–1558.
- 52 Wong, A. R.; Barrera, M.; Pal, A.; Lamb, J. R. Improved Characterization of Polyoxazolidinones by Incorporating Solubilizing Side Chains. Macromolecules 2022, 55, 11006–11012.
- 53 Ngassam Tounzoua, C.; Grignard, B.; Brege, A.; Jérôme, C.; Tassaing, T.; Mereau, R.; Detrembleur, C. A Catalytic Domino Approach toward Oxo-Alkyl Carbonates and Polycarbonates from CO2, Propargylic Alcohols, and (Mono- and Di-)Alcohols. ACS Sustainable Chem. Eng. 2020, 8, 9698−9710.
- 54 Siragusa, F.; van Den Broeck, E.; Ocando, C.; Müller, A. J.; de Smet, G.; Maes, B. U. W.; de Winter, J.; van Speybroeck, V.; Grignard, B.; Detrembleur, C. Access to Biorenewable and CO2-Based Polycarbonates from Exovinylene Cyclic Carbonates. ACS Sustainable Chem. Eng. 2021, 9, 1714−1728.
- 55 Siragusa, F.; Habets, T.; Méreau, R.; Evano, G.; Grignard, B.; Detrembleur, C. Catalyst-Free Approach for the Degradation of Bio- and CO2-Sourced Polycarbonates: A Step toward a Circular Plastic Economy. ACS Sustainable Chem. Eng. 2022, 10, 8863–8875.
- 56 Ouhib, F.; Grignard, B.; Van Den Broeck, E.; Luxen, A.; Robeyns, K.; Van Speybroeck, V.; Jerome, C.; Detrembleur, C. A Switchable Domino Process for the Construction of Novel CO2-Sourced Sulfur-Containing Building Blocks and Polymers. Angew. Chem. Int. Ed. 2019, 58, 11768–11773.
- 57 Habets, T.; Olmedo-Martínez, J. L.; Del Olmo, R.; Grignard, B.; Mecerreyes, D.; Detrembleur, C. Facile Access to CO2-Sourced Polythiocarbonate Dynamic Networks and Their Potential as Solid-State Electrolytes for Lithium Metal Batteries. ChemSusChem 2023, 16, e202300225.
- 58 Ouhib, F.; Meabe, L.; Mahmoud, A.; Eshraghi, N.; Grignard, B.; Thomassin, J.-M.; Aqil, A.; Boschini, F.; Jérôme, C.; Mecerreyes, D.; Detrembleur, C. CO2-Sourced Polycarbonates as Solid Electrolytes for Room Temperature Operating Lithium Batteries. J. Mater. Chem. A 2019, 7, 9844–9853.
- 59 Ouhib, F.; Meabe, L.; Mahmoud, A.; Grignard, B.; Thomassin, J.-M.; Boschini, F.; Zhu, H.; Forsyth, M.; Mecerreyes, D.; Detrembleur, C. Influence of the Cyclic versus Linear Carbonate Segments in the Properties and Performance of CO2-Sourced Polymer Electrolytes for Lithium Batteries. ACS Appl. Polym. Mater. 2020, 2, 922–931.
- 60 Siragusa, F.; Demarteau, J.; Habets, T.; Olazabal, I.; Robeyns, K.; Evano, G.; Mereau, R.; Tassaing, T.; Grignard, B.; Sardon, H.; Detrembleur, C. Unifying Step-Growth Polymerization and On-Demand Cascade Ring-Closure Depolymerization via Polymer Skeletal Editing. Macromolecules 2022, 55, 4637–4646.
- 61 Habets, T.; Siragusa, F.; Müller, A. J.; Grossman, Q.; Ruffoni, D.; Grignard, B.; Detrembleur, C. Facile construction of functional poly(mono-thiocarbonate) copolymers under mild operating conditions. Polym. Chem. 2022, 13, 3076–3090.
- 62 Maquilón, C.; Della Monica, F.; Limburg, B.; Kleij, A. W. Photocatalytic Synthesis of Substituted Cyclic Carbonate Monomers for Ring- Opening Polymerization. Adv. Synth. Catal. 2021, 363, 4033–4040.
- 63 McGuire, T. M.; Pérale, C.; Castaing, R.; Kociok-Köhn, G.; Buchard, A. Divergent Catalytic Strategies for the Cis/Trans Stereoselective Ring-Opening Polymerization of a Dual Cyclic Carbonate/Olefin Monomer. J. Am. Chem. Soc. 2019, 141, 13301–13305.
- 64 Guo, W.; Martínez-Rodríguez, L.; Martín, E.; Escudero-Adán, E. C.; Kleij, A. W. Highly Efficient Catalytic Formation of (Z)-1,4-But-2-ene Diols Using Water as a Nucleophile. Angew. Chem. Int. Ed. 2016, 55, 11037–11040.
- 65 Wu, Y.-C.; Fan, H.-Z.; Zhang, W.; Wang, M.-Y.; Cai, Z.; Zhu, J.-B. Biobased Bifunctional Monomers toward Functionalizable Polycarbonates and Poly(cyclic olefin)s with Tunable Properties. Macromolecules 2022, 55, 9232–9241.
- 66 Grace Hester, H.; Abel, B. A.; Coates, G. W. Ultra-High-Molecular- Weight Poly(Dioxolane): Enhancing the Mechanical Performance of a Chemically Recyclable Polymer. J. Am. Chem. Soc. 2023, 145, 8800–8804.
- 67 Li, X.-L.; Clarke, R. W.; An, H.-Y.; Gowda, R. R.; Jiang, J.-Y.; Xu, T.-Q.; Chen, E. Y.-X. Dual Recycling of Depolymerization Catalyst and Biodegradable Polyester that Markedly Outperforms Polyolefins. Angew. Chem. Int. Ed. 2023, 62, e202303791.
- 68 Zhang, W.; Dai, J.; Wu, Y.-C.; Chen, J.-X.; Shan, S.-Y.; Cai, Z.; Zhu, J.-B. Highly Reactive Cyclic Carbonates with a Fused Ring toward Functionalizable and Recyclable Polycarbonates. ACS Macro Lett. 2022, 11, 173–178.
- 69 Hardy, C.; Kociok-Köhn, G.; Buchard, A. Variations around the presence and position of sulfur in sugar-derived cyclic monomers: influence on polymerisation thermodynamics, polymer sequence and thermal properties. Polym. Chem. 2023, 14, 623–632.
- 70 McGuire, T. M.; Bowles, J.; Deane, E.; Farrar, E. H. E.; Grayson, M. N.; Buchard, A. Control of Crystallinity and Stereocomplexation of Synthetic Carbohydrate Polymers from D- and L-Xylose. Angew. Chem. Int. Ed. 2021, 60, 4524–4528.
- 71 Della Monica, F.; Kleij, A. W. From terpenes to sustainable and functional polymers. Polym. Chem. 2020, 11, 5109–5127.
- 72 Brandolese, A.; Della Monica, F.; Pericàs, M. A.; Kleij, A. W. Catalytic Ring-Opening Copolymerization of Fatty Acid Epoxides: Access to Functional Biopolyesters. Macromolecules 2022, 55, 2566–2573.




