CO2-Free Calcium Carbide Manufacturing: Demanded Strategy in the Carbon-Neutral Chemical Industry
Dmitriy E. Samoylenko
Institute of Chemistry, Saint Petersburg State University, 7/9 Universitetskaya nab, St. Petersburg, 199034 Russia
Search for more papers by this authorKonstantin S. Rodygin
Institute of Chemistry, Saint Petersburg State University, 7/9 Universitetskaya nab, St. Petersburg, 199034 Russia
Search for more papers by this authorCorresponding Author
Valentine P. Ananikov
Institute of Chemistry, Saint Petersburg State University, 7/9 Universitetskaya nab, St. Petersburg, 199034 Russia
Zelinsky Institute of Organic Chemistry, Russian Academy of Sciences, Leninsky pr. 47, Moscow, 119991 Russia
E-mail: [email protected]Search for more papers by this authorDmitriy E. Samoylenko
Institute of Chemistry, Saint Petersburg State University, 7/9 Universitetskaya nab, St. Petersburg, 199034 Russia
Search for more papers by this authorKonstantin S. Rodygin
Institute of Chemistry, Saint Petersburg State University, 7/9 Universitetskaya nab, St. Petersburg, 199034 Russia
Search for more papers by this authorCorresponding Author
Valentine P. Ananikov
Institute of Chemistry, Saint Petersburg State University, 7/9 Universitetskaya nab, St. Petersburg, 199034 Russia
Zelinsky Institute of Organic Chemistry, Russian Academy of Sciences, Leninsky pr. 47, Moscow, 119991 Russia
E-mail: [email protected]Search for more papers by this authorComprehensive Summary
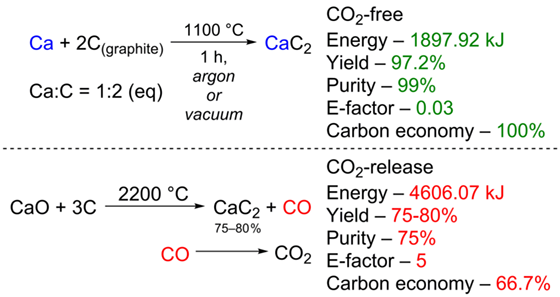
Calcium carbide is considered a possible key component in the sustainable carbon cycle, including convenient recycling of carbon wastes to industrial uptake. However, currently employed CaC2 manufacturing process produces significant amounts of CO2. One of the main factors of its appearance is the formation of carbon oxide during the reaction. The reaction of lime ore with coal inevitably results in the formation of CO and the loss of one carbon atom. CO is usually burnt, forming CO2 to maintain the required high temperature during synthesis – 2200 °C. In the present study, we discuss that the use of calcium metal instead of lime represents a good opportunity to prevent CO2 emission since the reaction of Ca with carbon occurs in an atom-efficient manner and results in only CaC2 at a much lower temperature of 1100 °C. Here, the reaction of Ca with carbon was successfully tested to synthesize CaC2. The desired product was isolated in gram-scale amounts in 97.2% yield and 99% purity. The environmental friendliness of the proposed method originates from the calculations of the E-factor. Rationalization is provided concerning the cost factor of Ca within the considered process.
Supporting Information
| Filename | Description |
|---|---|
| cjoc202300358-sup-0001-Supinfo.pdfPDF document, 1 MB |
Appendix S1: Supporting Information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1 Kuhns, R. J.; Shaw, G. H. The Carbon Dioxide Problem and Solution. In Navigating the Energy Maze: The Transition to a Sustainable Future, Eds.: Kuhns, R. J.; Shaw, G. H., Springer International Publishing, Cham, 2018, pp. 99–115.
- 2 Ritchie, H.; Roser, M.; Rosado, P. CO₂ and Greenhouse Gas Emissions. https://ourworldindata.org/co2-and-other-greenhouse-gas-emissions (accessed 04.22).
- 3 Jackson, R. B.; Le Quéré, C.; Andrew, R. M.; Canadell, J. G.; Peters, G. P.; Roy, J.; Wu, L. Warning signs for stabilizing global CO2 emissions. Environ. Res. Lett. 2017, 12, 110202.
- 4 Terlouw, T.; Bauer, C.; Rosa, L.; Mazzotti, M. Life cycle assessment of carbon dioxide removal technologies: a critical review. Energy Environ. Sci. 2021, 14, 1701–1721.
- 5(a) Gao, S.; Lin, Y.; Jiao, X.; Sun, Y.; Luo, Q.; Zhang, W.; Li, D.; Yang, J.; Xie, Y. Partially oxidized atomic cobalt layers for car-bon dioxide electroreduction to liquid fuel. Nature 2016, 529, 68–71; (b) Xie, J.; Huang, Y.; Wu, M.; Wang, Y. Electrochemical Carbon Dioxide Splitting. ChemElectroChem 2019, 6, 1587–1604; (c) Küngas, R. Review—Electrochemical CO2 Reduction for CO Production: Comparison of Low- and High-Temperature Electrolysis Technologies. J. Electrochem. Soc. 2020, 167, 044508; (d) Yu, K. M. K.; Curcic, I.; Gabriel, J.; Tsang, S. C. E. Recent Advances in CO2 Capture and Utilization. ChemSusChem 2008, 1, 893–899.
- 6 Simoes, A. J. G.; Hidalgo, C. A. The Economic Complexity Observatory: An Analytical Tool for Understanding the Dynamics of Economic Development. https://oec.world/en/profile/hs/calcium-carbide?yearSelector4
- 7=2020 (accessed 05.23).
- 8(a) Lu, H.; Li, Z. Synthesis of 1,2,3-Triazolyl-Based Ketoximes Using Calcium Carbide as an Acetylene Source. Eur. J. Org. Chem. 2020, 845–851; (b) Gao, L.; Li, Z. Synthesis of Aromatic Terminal Allenes and Aliphatic Terminal Alkynes from Hydra-zones Using Calcium Carbide as an Acetylene Source. Org. Chem. Front. 2020, 7, 702–708; (c) Wang, D.; Liu, Z.; Liu, Q. One-Pot Synthesis of Methyl-Substituted Benzenes and Methyl-Substituted Naphthalenes from Acetone and Calcium Carbide. Ind. Eng. Chem. Res. 2019, 58, 6226–6234; (d) Hosseini, A.; Schreiner, P. R. Synthesis of Exclusively 4-Substituted β-Lactams through the Kinugasa Reaction Utilizing Calcium Carbide. Org. Lett. 2019, 21, 3746–3749; (e) Gao, L.; Li, Z. Direct Synthesis of 1-Arylprop-1-ynes with Calcium Carbide as an Acetylene Source. Synlett 2019, 30, 1580–1584; (f) Turberg, M.; Ardila-Fierro, K. J.; Bolm, C.; Hernández, J. G. Altering Copper-Catalyzed A3 Couplings by Mechanochemistry: One-Pot Synthesis of 1,4-Diamino-2-butynes from Aldehydes, Amines, and Calcium Carbide. Angew. Chem. Int. Ed. 2018, 57, 10718–10722; (g) Fu, R.; Li, Z. Direct Synthesis of 2-Methylbenzofurans from Calcium Carbide and Salicylalde-hyde p-Tosylhydrazones. Org. Lett. 2018, 20, 2342–2345; (h) Metlyaeva, S. A.; Rodygin, K. S.; Lotsman, K. A.; Samoylenko, D. E.; Ananikov, V. P. Biomass- and calcium carbide-based recyclable polymers. Green Chem. 2021, 23, 2487–2495; (i) Voronin, V. V.; Ledovskaya, M. S.; Rodygin, K. S.; Ananikov, V. P. Examining the vinyl moiety as a protecting group for hydroxyl (-OH) functionality under basic conditions. Org. Chem. Front. 2020, 7, 1334–1342; (j) Ma, X.; Li, Z. Synthesis of Diarylethynes from Aryldiazonium Salts by Using Calcium Carbide as an Alkyne Source in a Deep Eutectic Solvent. Synlett 2021, 32, 631–635; (k) Chen, W.; Li, Z. One-Pot Synthesis of 3-Methyl-2-arylimidazo[1,2-a]pyridines Using Calcium Carbide as an Alkyne Source. J. Org. Chem. 2022, 87, 76–84; (l) Liu, Z.; Li, Z. Synthesis of 1,3-Diynes Using Calcium Carbide as an Alkyne Source. Eur. J. Org. Chem. 2021, 2021, 302–308; (m) Yang, P.-W.; Liu, X.-X.; Li, X.-Q.; Wei, M.-X. Transition metal-free and solvent-free calcium carbide promotes the formation of β-keto sulfoxide from acyl chloride and DMSO. Org. Chem. Front. 2021, 8, 2914–2918; (n) Xu, Z.; Zhou, S.; Zhu, M. Ni catalyst supported on nitrogen- doped activated carbon for selective hydrogenation of acetylene with high concentration. Catal. Commun. 2021, 149, 106241; (o) Zhang, Q.; Yuan, H.-Y.; Lin, X.-T.; Fukaya, N.; Fujitani, T.; Sato, K.; Choi, J.-C. Calcium carbide as a dehydrating agent for the synthesis of carbamates, glycerol carbonate, and cyclic carbonates from carbon dioxide. Green Chem. 2020, 22, 4231–4239.
- 9(a) Wu, H.; Wang, X.; Bai, Y.; Jiang, L.; Wu, C.; Hu, B. a.; Wei, Q.; Liu, X.; Li, N. The effects of preparation temperature on microstructure and electrochemical performance of calcium carbide-derived carbon. J. Solid State Electrochem. 2013, 17, 2453–2460; (b) Wu, H.; Wang, X.; Jiang, L.; Wu, C.; Zhao, Q.; Liu, X.; Hu, B. a.; Yi, L. The effects of electrolyte on the super-capacitive performance of activated calcium carbide-derived carbon. J. Power Sources 2013, 226, 202–209; (c) Wu, H.; Wang, X.; Wang, X.; Zhang, X.; Jiang, L.; Hu, B.; Wang, Y. The effect of activation technology on the electrochemical per-formance of calcium carbide skeleton carbon. J. Solid State Electrochem. 2012, 16, 2941–2947; (d) Du, Y. J.; Zhang, Y. Y.; Liu, S. Y. Investigation of Strength and California Bearing Ratio Properties of Natural Soils Treated by Calcium Carbide Residue. In Geo-Frontiers. Advances in Geotechnical Engineering, 2011, pp. 1237–1244; (e) Jiang, N.-J.; Du, Y.-J.; Liu, S.-Y.; Wei, M.-L.; Horpibulsuk, S.; Arulrajah, A. Multi-scale laboratory evaluation of the physical, mechanical, and microstructural properties of soft highway subgrade soil stabilized with calcium carbide residue. Can. Geotech. J. 2015, 53, 373–383; (f) Lebedev, A. N.; Rodygin, K. S.; Mironenko, R. M.; Saybulina, E. R.; Ananikov, V. P. Metal-catalyzed chemical activation of calcium carbide: New way to hierarchical met-al/alloy-on-carbon catalysts. J. Catal. 2022, 407, 281–289; (g) Lotsman, K. A.; Rodygin, K. S. Calcium carbide residue – a promising hidden source of hydrogen. Green Chem. 2023, 25, 3524–3532.
- 10(a) Ma, X.; Li, Z. Synthesis of Diarylethynes from Aryldiazonium Salts by Using Calcium Carbide as an Alkyne Source in a Deep Eutectic Solvent. Synlett 2021, 32, 631–635; (b) Ma, X.; Wang, Z.; Liu, Z.; Li, Z. One-Pot Three-Component Synthesis of 2-Methyl-3-aminobenzofurans Using Calcium Carbide as a Concise Solid Alkyne Source. Chin. J. Chem. 2021, 39, 2990–2994.
- 11 Rodygin, K. S.; Samoylenko, D. E.; Seitkalieva, M. M.; Lotsman, K. A.; Metlyaeva, S. A.; Ananikov, V. P. Generation, regeneration, and recovery of Cu catalytic system by changing the polarity of electrodes. Green Chem. 2022, 24, 1132–1140.
- 12 Fu, R.; Lu, Y.; Yue, G.; Wu, D.; Xu, L.; Song, H.; Cao, C.; Yu, X.; Zong, Y. Direct Synthesis of 3-Coumaranones with Calcium Carbide as an Acetylene Source. Org. Lett. 2021, 23, 3141–3145.
- 13 Hosseini, A.; Seidel, D.; Miska, A.; Schreiner, P. R. Fluoride-Assisted Activation of Calcium Carbide: A Simple Method for the Ethynylation of Aldehydes and Ketones. Org. Lett. 2015, 17, 2808–2811.
- 14 Lin, Z.; Yu, D.; Sum, Y. N.; Zhang, Y. Synthesis of Functional Acetylene Derivatives from Calcium Carbide. ChemSusChem 2012, 5, 625–628.
- 15 Thavornsin, N.; Sukwattanasinitt, M.; Wacharasindhu, S. Direct synthesis of poly(p-phenyleneethynylene)s from calcium carbide. Polym. Chem. 2014, 5, 48–52.
- 16Calcium Carbide Market - Growth, Trends, Covid-19 Impact, and Forecast (2022 - 2027). https://www.mordorintelligence.com/industry-reports/calcium-carbide-market# (accessed 04.22).
- 17 Cui, D.; Deng, Z.; Liu, Z. China's non-fossil fuel CO2 emissions from industrial processes. Appl. Energy 2019, 254, 113537.
- 18 Jiang, M.; Wang, Z.-H.; Ning, P.; Tian, S.-L.; Huang, X.-F.; Bai, Y.-W.; Shi, Y.; Ren, X.-G.; Chen, W.; Qin, Y.-S.; Zhou, J.; Miao, R.-R. Dust removal and purification of calcium carbide furnace off-gas. J. Taiwan Inst. Chem. Eng. 2014, 45, 901–907.
- 19 Chen, X.; Zheng, D. Thermodynamic characteristics of reac-tants and energy conversion in steam reforming of calcium carbide furnace off-gas to produce hydrogen. Int. J. Hydrog. Energy 2014, 39, 10996–11005.
- 20 Beloe, E. G. Improvements in the Production of Metallic Carbides and of Illuminating Gas derived therefrom. GB189416342A, 1895-11-27, 1895.
- 21 Danneel, H. Sammelreferate.: Calciumcarbid. Zeitschrift für Elektrochemie und angewandte physikalische. Chemie 1930, 36, 474–482.
- 22(a) Ruff, O.; Josephy, B. Arbeiten aus dem Gebiet hoher Temperaturen. XVIII. Reines Calciumcarbid und dessen Bild-ungswärme. Z. Anorg. Allg. Chem. 1926, 153, 17–32; (b) Thompson, M. D.; Gonzalez, L. R.; Blacke, K. B. Note on the Preparation of Pure Calcium Carbide. Metall. Res. Technol. 1914, 12, 779–780.
- 23(a) Zhang, X.-K.; Tong, Z.-X.; Li, D.; Hu, X.; He, Y.-L. Analysis and optimization about electromagnet-ics-temperature-component distribution in calcium carbide electric furnace. Appl. Therm. Eng. 2021, 185, 115980; (b) Zhang, X.-K.; Tong, Z.-X.; He, Y.-L.; Hu, X. Influence of feed architecture on heat and mass transfer in calcium carbide electric furnace. Int. J. Heat Mass Transf. 2021, 164, 120593; (c) Zhang, X.-K.; He, Y.-L.; Tang, S.-Z.; Wang, F.-L.; Xie, T. An electromagnetics- temperature-component multiphysical coupled model for electric furnace in calcium carbide smelting process. Appl. Therm. Eng. 2020, 165, 114552. Sigma-Aldrich Product Spesification. https://www.sigmaaldrich.com/specification-sheets/142/546/282863-BULK_______ALDRICH__.pdf.
- 24 Evers, J.; Weiss, A.; Kaldis, E.; Muheim, J. Purification of calcium and barium by reactive distillation. J. Less-common Met. 1973, 30, 83–95.
- 25 Rodygin, K. S.; Lotsman, K. A.; Samoylenko, D. E.; Kuznetsov, V. M.; Ananikov, V. P. Towards Sustainable Carbon Return from Waste to Industry via C2-Type Molecular Unit. Int. J. Mol. Sci. 2022, 23, 11828.
- 26(a) Li, G.; Liu, Q.; Liu, Z.; Zhang, Z. C.; Li, C.; Wu, W. Production of Calcium Carbide from Fine Biochars. Angew. Chem. Int. Ed. 2010, 49, 8480–8483; (b) El-Naas, M. H.; Munz, R. J.; Ajersch, F. Modelling of a Plasma Reactor for the Synthesis of Calcium Carbide. Can. Metall. Quart. 1998, 37, 67–74; (c) Pillai, R. C.; Sabolsky, E. M.; Rowan, S. L.; Celik, I. B.; Morrow, S. Solid-State Synthesis of Calcium Carbide by Using 2.45 GHz Microwave Reactor. Ind. Eng. Chem. Res. 2015, 54, 11001–11010.
- 27 Gyrdymova, Y. V.; Samoylenko, D. E.; Rodygin, K. S. [13C+D] Double Labeling with Calcium Carbide: Incorporation of Two Labels in One Step. Chem. Asian J. 2023, 18, e202201063.
- 28 Degtyareva, E. S.; Borkovskaya, E. V.; Ananikov, V. P. Applying Green Metrics to Eco-Friendly Synthesis of Sulfur-Substituted Conjugated Dienes Based on Atom-Economic Hydrothiolation. ACS Sustainable Chem. Eng. 2019, 7, 9680–9689.
- 29 Roschangar, F.; Sheldon, R. A.; Senanayake, C. H. Overcoming barriers to green chemistry in the pharmaceutical industry – the Green Aspiration Level™ concept. Green Chem. 2015, 17, 752–768.
- 30 Mi, Y.; Zheng, D.; Guo, J.; Chen, X.; Jin, P. Assessment of energy use and carbon footprint for low-rank coal-based oxygen-thermal and electro-thermal calcium carbide manufacturing processes. Fuel Process. Technol. 2014, 119, 305–315.
- 31(a) Ji, L.; Liu, Z.; Wang, R.; Wu, J.; Lin, X.; Liu, Q. Transformation of silicon-bearing minerals during CaC2 production and its effect on CaC2 formation. J. Taiwan Inst. Chem. Eng. 2016, 66, 80–87; (b) Li, G.; Liu, Q.; Liu, Z. CaC2 Production from Pulverized Coke and CaO at Low Temperatures—Influence of Minerals in Coal-Derived Coke. Ind. Eng. Chem. Res. 2012, 51, 10748–10754.
- 32
Gmehling, J. Thermochemical Data of Pure Substances, Part I/II. Von I. Barin. VCH, Weinheim – New York – Basel – Cam-bridge 1989. X, 1 829 S., 10 Abb., 2406 Tab., geb., DM 680,–. Chem. Ing. Tech. 1990, 62, 644.
10.1002/cite.330620809 Google Scholar
- 33(a) Constable, D. J. C.; Curzons, A. D.; Cunningham, V. L. Met-rics to ‘green’ chemistry—which are the best? Green Chem. 2002, 4, 521–527; (b) Trost, B. M. The atom economy–a search for synthetic efficiency. Science 1991, 254, 1471.
- 34(a) Zaikov, Y. P.; Batukhtin, V. P.; Shurov, N. I.; Ivanovskii, L. E.; Suzdaltsev, A. V. Calcium Production by the Electrolysis of Molten CaCl2—Part I. Interaction of Calcium and Cop-per-Calcium Alloy with Electrolyte. Metall. Mater. Trans. B 2014, 45, 961–967;
(b) Vrana, L. M. Calcium and Calcium Alloys. In Kirk-Othmer Encyclopedia of Chemical Technology, 2001;
(c) Hutton, R. S. Calcium Metal. Nature 1904, 71, 180–181;
10.1038/071180b0 Google Scholar(d) Lukasko, J. J. Electrolytic Production of Calcium Metal. ECS Proc. Vol. 1990, 1990-17, 588–599; (e) Neelameggham, N. R.; Brown, R. E.; Davis, B. R. Calcium Reductants – A Historical Review. In Rare Metal Technology, 2014, pp. 65–76; (f) Wu, L.; Luo, H. Thermodynamic calculation of calcium metal prepared by vacuum aluminothermic reduction method. IOP Conf. Ser.: Mater. Sci. Eng. 2019, 490, 022034; (g) Loomis, C. C. The Production of Metallic Calcium by Thermal Reduction. Trans. Electrochem. Soc. 1946, 89, 207; (h) Tasyurek, K. C.; Bugdayci, M.; Yucel, O. Parameters of the Metallic Calcium Reduction from Magnesium Production Residues. In 10th International Symposium on High-Temperature Metallurgical Processing, Eds: Jiang, T.; Hwang, J.-Y.; Gregurek, D.; Peng, Z.; Downey, J. P.; Zhao, B.; Yücel, O.; Keskinkilic, E.; Padilla, R., Springer International Publishing, Cham, 2019, pp. 479–487; (i) Taşyürek, K. C.; Buğdaycı, M.; Yücel, O. Reduction Conditions of Metallic Calcium from Magnesium Production Residues. Metals 2018, 8, 383; (j) El-Sadek, M. H.; El-Barawy, K.; Morsi, I. M. Production of calcium metal by aluminothermic reduction of Egyptian limestone ore. Can. Metall. Quart. 2019, 58, 213–222; (k) Mc Creary, W. J. High-purity calcium. J. Met. 1958, 10, 615–617.
- 35Kotsar’, M. L.; Talanov, A. A. Thermodynamics of high-purity calcium production. Russ. J. Inorg. Chem. 2016, 61, 344–350.
10.1134/S003602361603013X Google Scholar
- 36(a) Sholl, D. S.; Lively, R. P. Seven chemical separations to change the world. Nature. 2016, 532, 435-437; (b) Halvorsen, I. J.; Skogestad, S. Energy efficient distillation. J. Nat. Gas Eng. 2011, 3, 571–580; (c) Gadalla, M. A.; Olujic, Z.; Jansens, P. J.; Jobson, M.; Smith, R. Reducing CO2 Emissions and Energy Consumption of Heat-Integrated Distillation Systems. Environ. Sci. Technol. 2005, 39, 6860–6870.
- 37(a) Bogdanov, D.; Farfan, J.; Sadovskaia, K.; Aghahosseini, A.; Child, M.; Gulagi, A.; Oyewo, A. S.; de Souza Noel Simas Bar-bosa, L.; Breyer, C. Radical transformation pathway towards sustainable electricity via evolutionary steps. Nat. Commun. 2019, 10, 1077; (b) Khan, J.; Arsalan, M. H. Solar power tech-nologies for sustainable electricity generation – A review. Re-new. Sustain. Energy Rev. 2016, 55, 414–425.




