Catalyst-Free Regio- and Diastereoselective Synthesis of Heterocyclic Nucleosides in the Eco-friendly Solvent 2-Methyltetrahydrofuran†
Xiaodong Gu
Department of Chemistry, Hong Kong Baptist University, Kowloon, Hong Kong, China
‡These authors contributed equally to this work.
Search for more papers by this authorQingwei Du
Department of Chemistry, Southern University of Science and Technology, Shenzhen, Guangdong, 518055 China
‡These authors contributed equally to this work.
Search for more papers by this authorWeijian Song
Department of Chemistry, Southern University of Science and Technology, Shenzhen, Guangdong, 518055 China
Search for more papers by this authorCorresponding Author
Jun Joelle Wang
Department of Chemistry, Hong Kong Baptist University, Kowloon, Hong Kong, China
E-mail: [email protected]Search for more papers by this authorXiaodong Gu
Department of Chemistry, Hong Kong Baptist University, Kowloon, Hong Kong, China
‡These authors contributed equally to this work.
Search for more papers by this authorQingwei Du
Department of Chemistry, Southern University of Science and Technology, Shenzhen, Guangdong, 518055 China
‡These authors contributed equally to this work.
Search for more papers by this authorWeijian Song
Department of Chemistry, Southern University of Science and Technology, Shenzhen, Guangdong, 518055 China
Search for more papers by this authorCorresponding Author
Jun Joelle Wang
Department of Chemistry, Hong Kong Baptist University, Kowloon, Hong Kong, China
E-mail: [email protected]Search for more papers by this author†Dedicated to the Special Issue of Emerging Investigators in 2022.
Comprehensive Summary
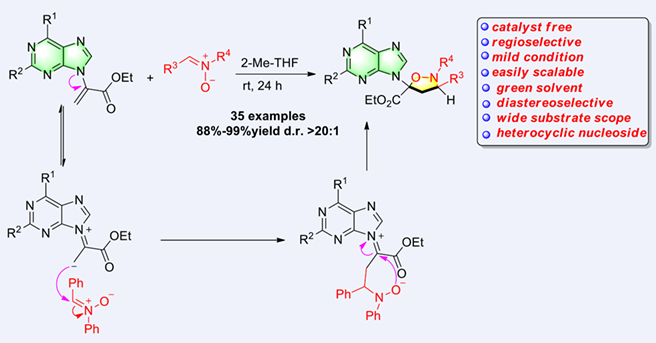
An efficient and practical synthesis of heterocyclic nucleosides is developed by a catalyst-free highly regioselective and diastereoselective [3+2] annulation of α-purine-substituted acrylates with nitrones. The reaction operates with excellent functional group tolerance, very mild reaction conditions, and with the green, sustainable, and eco-friendly 2-methyltetrahydrofuran (2-MeTHF) as solvent. Compared with other reactions of electron-deficient olefin dipolarophiles with nitrones, different regioselective cycloaddition products were observed in this work. This 1,3-dipolar cycloaddition reaction gives a series of isoxazolidinyl nucleosides in good to excellent yields with promising applications in biochemistry and medicinal chemistry.
Supporting Information
| Filename | Description |
|---|---|
| cjoc202200550-sup-0001-Supinfo.pdfPDF document, 9.1 MB |
Appendix S1: Supporting Information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1(a) Huryn, D. M.; Okabe M. AIDS-driven nucleoside chemistry. Chem. Rev. 1992, 92, 1745–1768; (b) Romeo, G.; Chiacchio, U.; Corsaro, A.; Merino, P. Chemical Synthesis of Heterocyclic−Sugar Nucleoside Analogues. Chem. Rev. 2010, 110, 3337–3370; (c) Merino, P. Heterocyclic Nucleosides: Chemical Synthesis and Biological Properties. Curr. Med. Chem. 2006, 13, 539–545; (d) Parker, W. B. Enzymology of Purine and Pyrimidine Antimetabolites Used in the Treatment of Cancer. Chem. Rev. 2009, 109, 2880–2893; (e) Shelton, J.; Lu, X.; Hollenbaugh, J. A.; Cho, J. H.; Amblard, F.; Schinazi, R. F. Metabolism, Biochemical Actions, and Chemical Synthesis of Anticancer Nucleosides, Nucleotides, and Base Analogs. Chem. Rev. 2016, 116, 14379–14455.
- 2 Searle, P. A.; Molinski, T. F. Trachycladines A and B: 2'-C-methyl-5'- deoxyribofuranosyl nucleosides from the marine sponge Trachycladus laevispirulifer. J. Org. Chem. 1995, 60, 4296–4298.
- 3 Peifer, M.; Berger, R.; Shurtleff, V. W.; Conrad, J. C.; MacMillan, D. W. A General and Enantioselective Approach to Pentoses: A Rapid Synthesis of PSI-6130, the Nucleoside Core of Sofosbuvir. J. Am. Chem. Soc. 2014, 136, 5900–5903.
- 4 Lee, Y.-S.; Hyean Kim, B. Heterocyclic nucleoside analogues: design and synthesis of antiviral, modified nucleosides containing isoxazole heterocycles. Bioorg. Med. Chem. Lett. 2002, 12, 1395–1397.
- 5 Lazrek, H. B.; Taourirte, M.; Oulih, T.; Barascut, J. L.; Imbach, J. L.; Pannecouque, C.; Witrouw, M.; De Clercq, E. Synthesis and anti-HIV activity of new modified 1,2,3-triazole acyclonucleosides. Nucleosides Nucleotides Nucleic Acids 2001, 20, 1949–1960.
- 6(a) Memeo, M. G.; Valletta, E.; Macchi, B.; Porta, A.; Bovio, B.; Moiola, M.; Quadrelli, P. Ene Reaction of Nitrosocarbonyl Mesitylene with the Cinnamyl Alcohol: Metabolic Activity and Apoptosis of the Synthetized 6-Chloropurine N,O-Nucleoside Analogues. ACS Omega 2018, 3, 7621–7629; (b) Fialho, D. M.; Roche, T. P.; Hud, N. V. Prebiotic Syntheses of Noncanonical Nucleosides and Nucleotides. Chem. Rev. 2020, 120, 4806–4830; (c) Piotrowska, D. G.; Balzarini, J.; Andrei, G.; Schols, D.; Snoeck, R.; Wroblewski, A. E.; Gotkowska, J. Novel isoxazolidine analogues of homonucleosides and homonucleotides. Tetrahedron 2016, 72, 8294–8308; (d) Hao, E. J.; Li, G. X.; Liang, Y. R.; Xie, D. M. S.; Wang, D. C.; Jiang, X. H.; Cheng, J. Y.; Shi, Z. X.; Wang, Y.; Guo, H. M. Design, Synthesis, and Activity Evaluation of Novel Acyclic Nucleosides as Potential Anticancer Agents In Vitro and In Vivo. J. Med. Chem. 2021, 64, 2077−2109; (e) Guo, M.; Kang, J.; Hou, J.; Zhang, Q.; Yu, W.; Chang, J. Synthesis and Anti-HBV Evaluation of 5-Halogenated 2'-Deoxy-2'-β-fluoro-4'-azido Pyrimidine Nucleosides. Chin. J. Chem. 2020, 40, 221–225.
- 7(a) Padwa, A.; Pearson, W. H. Synthetic Applications of 1,3-Dipolar Cycloaddition Chemistry Toward Heterocycles and Natural Products, John Wiley & Sons, Inc., 2002;
10.1002/0471221902 Google Scholar(b) Zou, X.; Yang, W.; Zhu, J.; Deng, W. Catalytic Enantioselective Formal Synthesis of MDM2 Antagonist RG7388 and Its Analogues. Chin. J. Chem. 2020, 38, 435–438; (c) Zhang, J.; Sheong, F. K.; Lu, Z.; Zhang, H.; Lin, Z. Unexpected Electronic Behavior of Organic Azide and Metal-Carbyne in Their 1,3-Dipolar Cycloaddition Reaction. Chin. J. Chem. 2020, 38, 1565–1570; (d) Zhang, G. Q.; Alshreimi, A. S.; Alonso, L.; Antar, A.; Yu, H. C.; Islam, S. M.; Anderson, L. L. Nitrone and Alkyne Cascade Reactions for Regio- and Diastereoselective 1-Pyrroline Synthesis. Angew. Chem. Int. Ed. 2021, 60, 13089–13097; (e) Reddy, S.; Reddy, G. S.; Beatriz, A.; Corey, E. J. Contrasting Diastereoselectivity between Cyclic Nitrones and Azomethine Ylides. Stereocontrolled Pathways to cis-anti-anti-cis-Oxazatetraquinanes from a Bicyclic Nitrone. Org. Lett. 2021, 23, 5445–5447; (f) Tang, Q.; Zhang, K. Polymer Synthesis Based on Self-Accelerating 1,3-Dipolar Cycloaddition Click Reactions. Chin. J. Chem. 2021, 39, 3093–3100.
- 8(a) Adams, D. R.; Boyd, A. S.; Ferguson, R.; Grierson, D. S.; Monneret, C. Heterocyclic Nucleosede Analogues by Cycloaddition Reactions of 1-Vinylthymine with 1,3-Depoles. Nucleosides Nucleotides 1998, 17, 1053–1075; (b) Merino, P.; Franco, S.; Garces, N.; Merchan, F. L.; Tejero, T. Modified nucleosides from nitrones: a new and efficient stereoselective approach to isoxazolidinyl thymidine derivatives. Chem. Commun. 1998, 493–494; (c) Chiacchio, U.; Corsaro, A.; Gumina, G.; Rescifina, A.; Iannazzo, D.; Piperno, A.; Romeo, G.; Romeo, R. Homochiral α-d- and β-d-Isoxazolidinylthymidines via 1,3-Dipolar Cycloaddition. J. Org. Chem. 1999, 64, 9321–9327; (d) Xiang, Y.; Gi, H.-J.; Niu, D.; Schinazi, R. F.; Zhao, K. An Efficient Route to β-d-Isoxazolidinyl Nucleosides via Diastereoselective Michael Addition of Hydroxylamine to Unsaturated Esters. J. Org. Chem. 1997, 62, 7430–7434.
- 9(a) Robak, T.; Robak, P. Purine nucleoside analogs in the treatment of rarer chronic lymphoid leukemias. Curr. Pharm. Des. 2012, 18, 3373–3388; (b) Robak, P.; Robak, T. Older and new purine nucleoside analogs for patients with acute leukemias. Cancer Treat. Rev. 2013, 39, 851–861; (c) Bonate, P. L.; Arthaud, L.; Cantrell, W. R. Jr. Discovery and development of clofarabine: a nucleoside analogue for treating cancer. Nat. Rev. Drug Discov. 2006, 5, 855–863.
- 10(a) Gerster, J. F.; Jones, J. W.; Robins, R. K. Purine Nucleosides. IV. The Synthesis of 6-Halogenated 9-β-D-Ribofuranosylpurines from Inosine and Guanosine. J. Org. Chem. 1963, 28, 945–948; (b) Liu, J.; Robins, M. J. SNAr Displacements with 6-(Fluoro, Chloro, Bromo, Iodo, and Alkylsulfonyl)purine Nucleosides: Synthesis, Kinetics, and Mechanism. J. Am. Chem. Soc. 2007, 129, 5962–5968.
- 11 Li, J. P.; Zhao, G. F.; Wang, H. X.; Xie, M. S.; Qu, G. R.; Guo, H. M. Highly Enantioselective Synthesis of Chiral Cyclopropyl Nucleosides via Catalytic Asymmetric Intermolecular Cyclopropanation. Org. Lett. 2017, 19, 6494–6497.
- 12(a) Gao, Y.-W.; Niu, H.-Y.; Zhang, Q.-Y.; Xie, M.-S.; Qu, G.-R.; Guo, H.-M. Regio- and Enantioselective [3+2] Cycloaddition of α-Purine Substituted Acrylates with Allenes: An Approach to Chiral Carbocyclic Nucleosides. Adv. Synth. Catal. 2018, 360, 2813–2819; (b) Huang, K. X.; Xie, M. S.; Zhang, Q. Y.; Qu, G. R.; Guo, H. M. Enantioselective Synthesis of Carbocyclic Nucleosides via Asymmetric [3 + 2] Annulation of α-Purine-Substituted Acrylates with MBH Carbonates. Org. Lett. 2018, 20, 389–392; (c) Huang, K. X.; Xie, M. S.; Zhang, Q. Y.; Niu, H. Y.; Qu, G. R.; Guo, H. M. Synthesis of Chiral Six-Membered Carbocyclic Purine Nucleosides via Organocatalytic Enantioselective [3 + 3] Annulation. Org. Lett. 2018, 20, 5398–5401.
- 13(a) Yang, Q. L.; Xie, M. S.; Xia, C.; Sun, H. L.; Zhang, D. J.; Huang, K. X.; Guo, Z.; Qu, G. R.; Guo, H. M. A rapid and divergent access to chiral azacyclic nucleoside analogues via highly enantioselective 1,3-dipolar cycloaddition of β-nucleobase substituted acrylates. Chem. Commun. 2014, 50, 14809–14812; (b) Zhang, D. J.; Xie, M. S.; Qu, G. R.; Gao, Y. W.; Guo, H. M. Synthesis of Azacyclic Nucleoside Analogues via Asymmetric [3 + 2] Cycloaddition of 9-(2-Tosylvinyl)-9H-purines. Org. Lett. 2016, 18, 820–823; (c) Xie, M. S.; Wang, Y.; Li, J. P.; Du, C.; Zhang, Y. Y.; Hao, E. J.; Zhang, Y. M.; Qu, G. R.; Guo, H. M. A straightforward entry to chiral carbocyclic nucleoside analogues via the enantioselective [3+2] cycloaddition of α-nucleobase substituted acrylates. Chem. Commun. 2015, 51, 12451–12454; (d) Niu, H. Y.; Du, C.; Xie, M. S.; Wang, Y.; Zhang, Q.; Qu, G. R.; Guo, H. M. Diversity-oriented synthesis of acyclic nucleosides via ring-opening of vinyl cyclopropanes with purines. Chem. Commun. 2015, 51, 3328–3331; (e) Xie, M. S.; Guo, Z.; Qu, G. R.; Guo, H. M. Regiodivergent Synthesis of Pyrazolines with a Quaternary Carbon Center via Cycloaddition of Diazoesters to N-Purine-Substituted Allenes. Org. Lett. 2018, 20, 5010–5014; (f) Huang, K.-X.; Xie, M.-S.; Zhao, G.-F.; Qu, G.-R.; Guo, H.-M. Synthesis of Chiral Cyclopropyl Carbocyclic Purine Nucleosides via Asymmetric Intramolecular Cyclopropanations Catalyzed by a Chiral Ruthenium(II) Complex. Adv. Synth. Catal. 2016, 358, 3627–3632; (g) Sun, H. L.; Chen, F.; Xie, M. S.; Guo, H. M.; Qu, G. R.; He, Y. M.; Fan, Q. H. Asymmetric Hydrogenation of α-Purine Nucleobase-Substituted Acrylates with Rhodium Diphosphine Complexes: Access to Tenofovir Analogues. Org. Lett. 2016, 18, 2260–2263; (h) Zhang, Q.; Zhang, Y.; Hao, E.; Bai, J.; Qu, G.; Guo, H. Asymmetric Transfer Hydrogenation via Dynamic Kinetic Resolution for the Construction of Carbocyclic N3-Purine Nucleosides. Chin. J. Chem. 2020, 40, 376–383; (i) Zhang, Y. M.; Zhang, Q. Y.; Wang, D. C.; Xie, M. S.; Qu, G. R.; Guo, H. M. Asymmetric Transfer Hydrogenation of rac-α-(Purin-9-yl)cyclopentones via Dynamic Kinetic Resolution for the Construction of Carbocyclic Nucleosides. Org. Lett. 2019, 21, 2998–3002; (j) Thieme, N.; Breit, B. Enantioselective and Regiodivergent Addition of Purines to Terminal Allenes: Synthesis of Abacavir. Angew. Chem. Int. Ed. 2017, 56, 1520–1524; (k) Hao, E. J.; Fu, D. D.; Wang, D. C.; Zhang, T.; Qu, G. R.; Li, G. X.; Lan, Y.; Guo, H. M. Chemoselective asymmetric dearomative [3 + 2] cycloaddition reactions of purines with aminocyclopropanes. Org. Chem. Front. 2019, 6, 863–867; (l) Kang, B.; Zhang, Q. Y.; Qu, G. R.; Guo, H. M. The Enantioselective Synthesis of Chiral Carbocyclic Nucleosides via Palladium-Catalyzed Asymmetric Allylic Amination of Alicyclic MBH Adducts with Purines. Adv. Synth. Catal. 2020, 362, 1955–1960; (m) Xia, C.; Wang, D.; Guo, H. Sc(OT(f)3-Catalyzed Reaction of Purines with o-Hydroxybenzyl Alcohols for Construction of Acyclic Nucleosides. Chin. J. Chem. 2021, 39, 4391–4399.
- 14(a) Leggio, A.; Liguori, A.; Maiuolo, L.; Napoli, A.; Procopio, A.; Siciliano, C.; Sindona, G. Model studies towards the synthesis of 4’-azaerythrofuranosyladenines as analogues of the antiviral drug 2’,3’-dideoxyadenosine (ddA). J. Chem. Soc., Perkin Trans. 1 1997, 3097–3099; (b) ChiaFcchio, U.; Corsaro, A.; Pistara, V.; Rescifina, A.; Iannazzo, D.; Piperno, A.; Romeo, G.; Romeo, R.; Grassi, G. Diastereoselective Synthesis of N,O-Psiconucleosides, a New Class of Modified Nucleosides. Eur. J. Org. Chem. 2002, 2002, 1206–1212; (c) Chiacchio, U.; Corsaro, A.; Iannazzo, D.; Piperno, A.; Pistara, V.; Rescifina, A.; Romeo, R.; Valveri, V.; Mastino, A.; Romeo, G. Enantioselective Syntheses and Cytotoxicity of N,O-Nucleosides. J. Med. Chem. 2003, 46, 3696–3702; (d) Chiacchio, U.; Borrello, L.; Iannazzo, D.; Merino, P.; Piperno, A.; Rescifina, A.; Richichi, B.; Romeo, G. Enantioselective synthesis of N,O-psiconucleosides. Tetrahedron: Asymmetry 2003, 14, 2419–2425; (e) Chiacchio, U.; Corsaro, A.; Iannazzo, D.; Piperno, A.; Pistara, V.; Rescifina, A.; Romeo, R.; Sindona, G.; Romeo, G. Diastereo- and enantioselective synthesis of N,O-nucleosides. Tetrahedron: Asymmetry 2003, 14, 2717–2723.
- 15(a) Cai, B.; Yang, Q.; Meng, L.; Wang, J. Kinetic Resolution of 2-Substituted 1,2-Dihydroquinolines by Rhodium-Catalyzed Asymmetric Hydroarylation. Chin. J. Chem. 2021, 39, 1606–1610;
(b) Yang, Z.; Du, Q.; Jiang, Y.; Wang, J. Asymmetric Access of γ-Amino Acids and γ-Amino Phosphonic Acid Derivatives via Copper-Catalyzed Enantioselective and Regioselective Hydroamination. CCS Chem. 2021, 3, 2507–2517;
10.31635/ccschem.021.202101128 Google Scholar(c) Gu, X.; Meng, L.; Li, M.; Wang, J. Highly enantioselective access to chiral chromanes and thiochromanes via Cu-catalyzed hydroamination with anthranils. Org. Chem. Front. 2021, 8, 1563–1568; (d) Yang, Q.; Li, S.; Wang, J. Asymmetric Synthesis of Chiral Chromanes by Copper-Catalyzed Hydroamination of 2H-Chromenes. ChemCatChem 2020, 12, 3202–3206; (e) Li, S.; Wang, Z.; Xiao, H.; Bian, Z.; Wang, J. Enantioselective synthesis of indole derivatives by Rh/Pd relay catalysis and their anti-inflammatory evaluation. Chem. Commun. 2020, 56, 7573–7576; (f) Jin, M. Y.; Gu, X.; Deng, M.; Wang, C.; Wang, J. Catalytic mutual multicomponent reaction: facile access to α-trifluoromethylthiolated ketones. Chem. Commun. 2020, 56, 10552–10555; (g) Guo, R.; Cai, B.; Jin, M. Y.; Mu, H.; Wang, J. Sulfide-Catalyzed Trifluoromethylthiolation-Cyclization of Tryptamine Derivatives. Asian J. Org. Chem. 2019, 8, 687–690; (h) Yang, Q.; Li, S.; Wang, J. Cobalt-catalyzed cross-dehydrogenative coupling of imidazo[1,2-a]pyridines with isochroman using molecular oxygen as the oxidant. Org. Chem. Front. 2018, 5, 577–581; (i) Li, J.; Yang, Z.; Guo, R.; Jin, M. Y.; Wang, J. Atom-Economical and Stereoselective Difunctionalization of Electron-Withdrawing Alkynes with N-Trifluoromethylthiophthalimide. Asian J. Org. Chem. 2018, 7, 1784–1787; (j) Li, S.; Yang, Q.; Wang, J. Copper(II) triflate-catalyzed highly efficient synthesis of N-substituted 1,4-dihydropyridine derivatives via three- component cyclizations of alkynes, amines, and α,β-unsaturated aldehydes. Tetrahedron Lett. 2016, 57, 4500–4504; (k) Xiao, Q.; He, Q.; Li, J.; Wang, J. 1,4-Diazabicyclo[2.2.2]octane-Promoted Aminotrifluoromethylthiolation of α,β-Unsaturated Carbonyl Compounds: N-Trifluoromethylthio-4-nitrophthalimide Acts as Both the Nitrogen and SCF3 Sources. Org. Lett. 2015, 17, 6090−6093; (l) Lu, Z.; Wang, J.; Han, B.; Li, S.; Zhou, Y.; Fan, B. Palladium-Catalyzed Asymmetric Ring Opening Reaction of Azabenzonorbornadienes with Aromatic Amines. Adv. Synth. Catal. 2015, 357, 3121–3125; (m) Zeng, C.; Yang, F.; Chen, J.; Wang, J.; Fan, B. Iridium/copper-cocatalyzed asymmetric ring opening reaction of azabenzonorbornadienes with amines. Org. Biomol. Chem. 2015, 13, 8425–8428; (n) Li, P.; Fang, F.; Chen, J.; Wang, J. Organocatalytic asymmetric aza-Michael addition of pyrazole to chalcone. Tetrahedron Asymmetry 2014, 25, 98–101.
- 16(a) Aycock, D. F. Solvent Applications of 2-Methyltetrahydrofuran in Organometallic and Biphasic Reactions. Org. Process Res. Dev. 2007, 11, 156–159; (b) Pace, V.; Hoyos, P.; Castoldi, L.; Dominguez de Maria, P.; Alcantara, A. R. 2-Methyltetrahydrofuran (2-MeTHF): A Biomass-Derived Solvent with Broad Application in Organic Chemistry. ChemSusChem 2012, 5, 1369–1379; (c) Farran, A.; Cai, C.; Sandoval, M.; Xu, Y.; Liu, J.; Hernaiz, M. J.; Linhardt, R. J. Green Solvents in Carbohydrate Chemistry: From Raw Materials to Fine Chemicals. Chem. Rev. 2015, 115, 6811–6853; (d) Pace, V.; Hoyos, P.; Fernández, M.; Sinisterraab, J. V.; Alcántara, A. R. 2-Methyltetrahydrofuran as a suitable green solvent for phthalimide functionalization promoted by supported KF. Green Chem. 2010, 12, 1380–1382; (e) Pace, V.; Alcántara, A. R.; Holzer, W. Highly efficient chemoselective N-TBS protection of anilines under exceptional mild conditions in the eco-friendly solvent 2-methyltetrahydrofuran. Green Chem. 2011, 13, 1986–1989; (f) Mamuye, A. D.; Monticelli, S.; Castoldi, L.; Holzer, W.; Pace, V. Eco-friendly chemoselective N-functionalization of isatins mediated by supported KF in 2-MeTHF. Green Chem. 2015, 17, 4194–4197.
- 17(a) Yan, K.; Wu, G.; Lafleur, T.; Jarvis, C. Production, properties and catalytic hydrogenation of furfural to fuel additives and value-added chemicals. Renew. Sust. Energ. Rev. 2014, 38, 663–676; (b) Cai, C. M.; Zhang, T.; Kumar, R.; Wyman, C. E. Integrated furfural production as a renewable fuel and chemical platform from lignocellulosic biomass. J. Chem. Technol. Biotechnol. 2014, 89, 2–10; (c) McElroy, C. R.; Constantinou, A.; Jones, L. C.; Summerton, L.; Clark, J. H. Towards a holistic approach to metrics for the 21st century pharmaceutical industry. Green Chem. 2015, 17, 3111–3121; (d) Xie, Z.; Chen, B.; Wu, H.; Liu, M.; Liu, H.; Zhang, J.; Yang, G.; Han, B. Highly efficient hydrogenation of levulinic acid into 2-methyltetrahydrofuran over Ni–Cu/Al2O3–ZrO2 bifunctional catalysts. Green Chem. 2019, 21, 606–613.
- 18 CCDC 2021789 [for 3ao] contain the supplementary crystallographic data for this paper. These data can be obtained via www.ccdc.cam. ac.uk/data_request/cif.
- 19(a) Murahashi, S.-I.; Imada, Y. Synthesis and Transformations of Nitrones for Organic Synthesis. Chem. Rev. 2019, 119, 4684−4716;
(b) Su, J.; Zhang, K.; Zhuang, M.; Ma, F.; Zhang, W.-Q.; Sun, H.; Zhang, G.; Jian, Y.; Gao, Z. One-Pot Synthesis of Indoles from Aniline and α,β-Ynones through an Iodine-Mediated Transition-Metal-Free Tandem aza-Michael addition/C−H Functionalization. Asian J. Org. Chem. 2019, 8, 482–486;
(c) Meng, L.; Chang, X.; Lin, Z.; Wang, J. Metal-free access to 3-allyl-2-alkoxychromanones via phosphine- catalyzed alkoxy allylation of chromones with MBH carbonates and alcohols. Org. Biomol. Chem. 2021, 19, 2663–2667;
(d) Ahmad, T.; Ullah, N. The oxa-Michael reaction in the synthesis of 5- and 6-membered oxygen-containing heterocycles. Org. Chem. Front. 2021, 8, 1329–1344;
(e) Wang, Y.; Du, D.-M. Recent advances in organocatalytic asymmetric oxa-Michael addition triggered cascade reactions. Org. Chem. Front. 2020, 7, 3266–3283;
(f) Fang, F.; Chen, J.; Wang, J. Organocatalytic asymmetric aza-Michael addition of pyrazole to chalcone. Tetrahedron: Asymmetry 2014, 25, 98–101;
10.1016/j.tetasy.2013.11.012 Google Scholar(g) Nising, C. F.; Bräse, S. The oxa-Michael reaction: from recent developments to applications in natural product synthesis. Chem. Soc. Rev. 2008, 37, 1218–1228; (h) Enders, D.; Wang, C.; Liebich, J. X. Organocatalytic Asymmetric Aza-Michael Additions. Chem. - Eur. J. 2009, 15, 11058–11076; (i) Wu, Y.; Wang, J.; Li, P. F.; Kwong, F. Y. Catalyst-Free Efficient Aza-Michael Addition of Azoles to Nitroalkenes. Synlett 2012, 23, 788–790; (j) Sanchez-Rosello, M.; Acena, J. L.; Simon-Fuentes, A.; del Pozo, C. A general overview of the organocatalytic intramolecular aza-Michael reaction. Chem. Soc. Rev. 2014, 43, 7430–7453; (k) Zhu, Y.; Zhou, J.; Jin, S.; Dong, H.; Guo, J.; Bai, X.; Wang, Q.; Bu, Z. Metal-free diastereoselective construction of bridged ketal spirooxindoles via a Michael addition-inspired sequence. Chem. Commun. 2017, 53, 11201–11204; (l) Guerrero-Corella, A.; Valle-Amores, M. A.; Fraile, A.; Aleman, J. Enantioselective Organocatalyzed aza-Michael Addition Reaction of 2-Hydroxybenzophenone Imines to Nitroolefins under Batch and Flow Conditions. Adv. Synth. Catal. 2021, 363, 3845–3851; (m) Lin, X.; Ge, K. L.; He, N. N.; Chen, X. L.; Li, P. F.; Dong, M. X.; Li, W. J. Organocatalytic Enantioselective Construction of Acyclic N,N-Acetals via Aza-Addition of Arylamines to Ketimines. Adv. Synth. Catal. 2021, 363, 4332–4337; (n) Song, Y. X.; Du, D. M. Recent Advances in Catalytic Asymmetric Aza-Michael Addition Triggered Cascade Reactions. Adv. Synth. Catal. 2021, 363, 4667–4694; (o) Wang, Y.; Chen, B.; He, X.; Gui, J. Development of Biomimetic Synthesis of Propindilactone G. Chin. J. Chem. 2020, 38, 1339–1352; (p) Yu, B.; Gao, B.; Zhang, X.; Zhang, H.; Huang, H. Palladium-Catalyzed Aminomethylation of Nitrodienes and Dienones via Double C—N Bond Activation. Chin. J. Chem. 2021, 39, 566–570; (q) Xu, H.; Han, T.; Luo, X.; Deng, W. Construction of 3-Azabicyclo[3.1.0]hexane Backbone by the Reaction of Allenes with Allylamines via Tandem Michael Addition and Copper-Mediated Oxidative Carbanion Cyclization. Chin. J. Chem. 2021, 39, 666–670.




